Translate this page into:
Bioactive compounds extraction of Croton lechleri barks from Amazon forest using chemometrics tools
⁎Corresponding author. carpes@utfpr.edu.br (Solange Teresinha Carpes)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Croton lechleri is a native tree from Amazon Forest and its barks are used as medicine by the indigenous peoples and their descendants. This study aimed to assess the extraction sustainably of bioactive compounds from Croton lechleri barks using chemometric tools.
Methods
Phenolic compounds were extracted and evaluated through 23 factorial design and Response Surface Methodology (RSM). High performance liquid chromatography (HPLC) analysis was carried out, and its chromatographic profile was used for principal component analysis (PCA) to establish relationships between individual phenolic compound concentrations and the best extraction conditions. Global Response (GR) was performed to obtain a unique extraction condition for all dependent Croton lechleri barks’ dependent variables.
Results
The total phenolic compounds (TPC) and antioxidant activity (AA) varied from 8.8 to 46.57 mg GAE/g (Gallic acid equivalent) and from 81.17 to 283 µmol Trolox/g, respectively. These parameters show coefficients determined for TPC and AA models of 99.70% and 99.80%, respectively. Gallic acid, syringic acid, epicatechin and catechin are present in the metabolism of Croton lechleri. According to HPLC analysis, the three first compounds were better extracted in lower temperatures, while catechin proved to be more stable in higher temperatures. Results showed that the best extraction conditions occurred using water as the solvent at 35 °C during 90 min of the extraction. Also, the coefficient determined for GR was 97.05%, indicating a satisfactory fit model.
Conclusion
The extraction of phenolic compounds from Croton lechleri barks could be successfully performed due to the chemometric tools proposed in this research. Thus, the Croton lechleri barks bring prospects for its use as a natural antioxidant and open opportunity to explore new technological applications.
Keywords
Euphorbiaceae
Global response
HPLC
Response surface methodology (RSM)
Principal component analysis
Flavonoids
1 Introduction
The use of the plants by the indigenous peoples of the Amazon and their descendants have aroused researchers' interest to catalogue, characterize and sustainably extract the bioactive compounds to develop new drugs or additive in food products or cosmetics.
Croton lechleri is a native tree from South America (Brazil, Bolivia, Colombia, Ecuador, and Peru). This tree is commonly known as Dragon's blood and is used for the empirical treatment of several diseases in traditional medicines from Latin America (Alonso-Castro et al., 2012). In fact, natives from Amazonia use Croton lechleri parts to treat several illnesses, such as gastric ulcers, diarrhoea, arthritis, insect bites, microbial infections, wound healing, and cancer (Alonso-Castro et al., 2012).
Studies have shown that compounds originated from plants play an essential role in a healthy system since a considerable part of the population relies on traditional medicine (Salatino et al., 2007). This fact is attributed to the low cost of the natural products and difficulty of access to health care systems and the misconception that medicinal herbs do not cause side effects (De Carvalho Nilo Bitu et al., 2015).
Phenolic compounds are secondary metabolites produced by plants that present biological properties, such as antioxidant and antimicrobial activities (De Marino et al., 2008). Antioxidant activity of polyphenols compound from plant material has been attributed to their capacity to scavenge free radicals responsible for disorders such as diabetes, inflammations, Parkinson, Alzheimer, and cancer (Alonso-Castro et al., 2012, Sari et al., 2020). Thus, numerous studies on folk medicines compounds have been performed to explore medicinal plants effect and composition (Xu et al., 2018, Alonso-Castro et al., 2012) to produce alternative drugs to combat resistant microbial strains.
Although the genus Croton sap is its most relevant product, other parts of these plants have been studied to validate its traditional uses and describe their chemical composition. However, leaves, bark, stems (wood), seeds, and flowers have biological activities with beneficial effects on people's health (Rossi et al., 2011). Croton lechleri leaves, bark and sap are of pharmaceutical interest due to their potential as antimicrobial, antioxidant, anti-inflammatory and antitumor agents (Alonso-Castro et al., 2012). Regarding the composition, the genus Croton has been studied for its phenolic, terpenoid and alkaloid compositions (De Carvalho Nilo Bitu et al., 2015; Salatino et al., 2007; Xu et al., 2018). According to Rossi et al. (2011), the Croton lechleri essential oil obtained by steam distillation of fresh stem bark, have the potential to be employed as a new flavouring protective ingredient for foods. Probably due to its antimicrobial activity against potential pathogens formed during the food cooking. The author reported the presence of seventy-four chemicals and the most abundant were the sesquiterpenes.
Regardless it is possible to find some reports about the Croton, the antioxidant and antimicrobial activities of the Croton lechleri barks have been less investigated. Additionally, the plant materials have considerable chemical complexity. This diversity is related to the quality and abundance of these compounds, which have different polarity and can lead to dispersed bioactivity results, mainly depending on the extraction method used in the investigation. According to Perin et al. (2020), the study one-factor-at-time approach to determine the best extraction conditions is not suitable due to the interactions between the variables (i.e. time, solvent, temperature, rate), besides that is time-consuming and expensive. Nevertheless, chemometric tools provide the evaluation of variables and their interactions, as well as facilitate the obtention of a single and global response in studies with multiple dependent variables.
Thus, this work investigates the extraction of Croton lechleri bark through the development of a 23 factorial design applying response surface methodology, principal component analysis and global response to determine the best extraction condition to obtain phenolic compounds with antioxidant and antimicrobial activity.
2 Material and methods
2.1 Chemicals
DPPH (2,2-diphenyl-1-picryl-hydrazyl), Folin–Ciocalteu phenol reagents, Trolox, ethanol, gallic acid and others standards were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
2.2 Plant material collection
The Croton lechleri bark was collected in Rondônia State-Brazil in Amazon Forest between January and March 2017. The bark was dried at 40 °C for 24 h and powered using a knife mill (Pulverisette 14, 600 rpm, Fritsch, Idar-Oberstein, Germany).
2.3 Preparation of extracts and factorial design
The factorial design was used to evaluate the effect of the solvent (water and ethanol), time (30 and 90 min) and temperature (35 and 70 °C) on the extraction of the bioactive compounds (Table 1). The extracts were prepared with 3 g of plant material and 30 mL of each solvent using a shaker incubator (SL 222, Solab, Piracicaba, Brazil). The design was carried out with eight runs in triplicate, and the dependent variables were antioxidant activity (AA) and total phenolic compounds (TPC). GAE: Gallic acid equivalent. Values followed by same letters in the same column are not statistically different (p < 0.05). < LOD: Less than limit of detection. LOD: Limit of detection (µg/g); LOQ: Limit of quantification (µg/g).
Runs
Solvent X1
Time (min) X2
Temperature (°C) X3
TPC (mg GAE/g)
AA (µmol Trolox/g)
Gallic acid (µ/g)
Catechin (µ/g)
Epicatechin (µ/g)
Syringic acid (µ/g)
LOD
1.7
3.3
2.6
2.2
LOQ
5.7
10.9
8.6
7.4
RT (min)
3.2
8.5
13.7
13.1
λ (nm)
272
272
276
274
A
Ethanol(-1)
30(-1)
35(-1)
8.6 ± 0.0 h
49.25 ± 0.6 g
36.6 ± 2.4b
434.4 ± 5.2c
15.84 ± 1.0f
<LOD
B
Water(+1)
30(-1)
35(-1)
35.6 ± 0.2f
193.88 ± 1.08d
78.1 ± 9.9a
131.5 ± 5.8c
25.29 ± 0.9d
26.94 ± 1.0c
C
Ethanol(-1)
90(+1)
35(-1)
13.4 ± 0.0 g
81.17 ± 0.22f
42.6 ± 1.7b
119.7 ± 2.3c
19.77 ± 0.7ef
<LOD
D
Water(+1)
90(+1)
35(-1)
38.0 ± 0.1e
200.66 ± 0.38c
43.6 ± 0.1b
466.4 ± 4.5c
42.22 ± 0.3a
55.02 ± 0.7a
E
Ethanol(-1)
30(-1)
70(+1)
39.5 ± 0.0d
283.11 ± 0.29a
15.1 ± 0.9c
869.2 ± 4.2b
30.89 ± 2.5c
<LOD
F
Water(+1)
30(-1)
70(+1)
41.2 ± 0.1b
206.62 ± 0.28b
47.2 ± 1.0b
414.7 ± 2.0c
24.37 ± 0.8de
26.62 ± 2.0c
G
Ethanol(-1)
90(+1)
70(+1)
46.6 ± 0.1a
280.12 ± 2.29a
<LOD
1241.8 ± 14.8a
36.33 ± 0.7b
<LOD
H
Water(+1)
90(+1)
70(+1)
40.4 ± 0.1c
152.65 ± 2.21e
19.7 ± 0.5c
377.8 ± 5.7c
29.26 ± 2.1 cd
44.46 ± 2.3b
2.4 Total phenolic compounds
The total phenolic content (TPC) of Croton lechleri bark extracts was performed according to Folin-Ciocalteau method (Singleton et al., 1999). The reaction was carried out through 2.5 mL of Folin Ciocalteau reagent 10%, 0.5 mL of extract and 2 mL of sodium carbonate 4%. The tubes were kept in the dark for 2 h until reading at 740 nm. A gallic acid standard curve (2.5 to 125 mg/kg) was used, and the results were expressed as mg GAE/g (Gallic acid equivalent).
2.5 Antioxidant activity
The Croton lechleri bark extract's antioxidant activity was determined by DPPH (2,2-diphenyl-1-picryl-hydrazyl) radical scavenging to the methodology described by Brand-Williams et al. (1995). The result was expressed in µmol Trolox/g.
2.6 HPLC profiling
The extract was analyzed trough a reversed-phase high performance liquid chromatography system (RP-HPLC) (Varian Inc. Walnut Creek, C.A US) equipped with a C-18 column (250 mm × 4.6 mm, 5 μm) and a photodiode array detector. The extracts were filtered through a 0.45 µm membranes (Millipore, Massachusetts, USA). An aliquot of 10 µL of the extracts and standard were injected into the chromatograph using a binary gradient system formed by water acidified with 2% of acetic acid (phase A) and water: acetonitrile: acetic acid (58:40:2 v/v/v) (phase B). The column was kept at 30 °C and the solvent flow rate of 1 mL/min. The identification was performed by comparison of retention times and absorption in the ultraviolet. The quantification of the compounds was carried out by external standardization, using a calibration curve prepared with a mix of the standards: gallic acid, coumaric acid, syringic acid, ferulic acid, catechin, and epicatechin. The limits of detection (LOD) and quantification (LOQ) of the method were also determined, and the contents of phenolic compounds identified were expressed as µg/g (Oldoni et al., 2019).
2.7 Statistical analysis
The experimental data were performed by analysing variance using one-way ANOVA, Tukey’s and Qui-square test (X2) at a 5% significance level. Besides a Principal Component Analysis (PCA) was employed to evaluate the extracted phenolic compounds, their similarities and differences in the extraction process according to the temperature, time and solvent treatments. Global response (GR) was applied to obtain the best extraction conditions for all dependent variables, TPC, epicatechin, syringic acid, gallic acid, catechin and DPPH. Statistic 12 Software (StatSoft Inc., USA) was employed in the statistical analysis. GR was calculated according to Eq. (1). Where: R(xn) is the response for each dependent variable, MR (xn) is the maximum value of response for each dependent variable.
3 Results and discussion
The TPC and AA of Croton lechleri bark extract varied from 8.60 to 46.57 mg GAE/g and from 49.25 to 283.11 μmol Trolox/g of dry bark, respectively (Table 1). The highest TPC values (G run) and the highest AA (E run) were observed in the ethanol extract at 70 °C during 90 min and 30 min of the extraction, respectively. However, no significant difference (p < 0.05) was found among the AA values obtained from the G and E extraction condition (Table 1).
In this study, all factors: solvent, time, and temperature, were statistically significant (p < 0.05) for both the dependent variable (TPC and AA). According to F-values, temperature, solvent, and the interaction between these two factors were the most significant factors for TPC. In contrast, the interaction temperature and solvent were the most important factors for AA (Table 2). Additionally, the ANOVA obtained in the dataset adjusted to the model showed a high coefficient of determination R2 = 0.99 for both variables (TPC and AA), which indicates that the model fits well with the experimental data. Factors as solvent and temperature presented positive coefficients TPC and AA, suggesting that variations in these factors significantly increase the TPC and AA. However, time and all effects interactions had negative coefficients, decreasing AA when the extraction time increases. * significant at p < 0.05; ns: not significant at p > 0.05; Fratio: Fvalue/Fstatistic table.
Source
TPC
AA
Global Response
Gallic acid
Catechin
Epicatechin
Syringic acid
β0
32.90
180.93
0.00
35.36
506.94
28.00
19.13
β1
5.91
7.52
1.13
11.79
−159.33
2.29
19.13
β2
1.69
−2.28
0.31
−8.89
0.00
3.90
5.74
β3
9.01
49.69
0.79
−14.87
218.93
2.22
−1.36
β12
1.29
−9.52
0.00
−6.64
0.00
1.56
5.74
β13
7.02
−58.51
−1.28
0.00
−190.30
−5.69
−1.34
β23
0.00
−11.96
−0.18
0.00
0.00
0.00
−1.28
R2
0.99
0.99
0.97
0.96
0.83
0.94
0.99
Main effects
Solvent (X1)
*
*
*
*
*
*
*
Time (X2)
*
*
*
*
ns
*
*
Temperature (X3)
*
*
*
*
*
*
*
Fvalue
2956.85
5077.27
248.07
69.83
19.86
33.34
321.27
Fstatistic table
4.45
4.45
4.03
3.36
3.49
3.33
3.37
Fratio
664.46
1140.96
61.55
20.78
5.69
10.01
95.33
RP-HPLC performed on the extracts confirmed four phenolic compounds in Croton lechleri bark after being extracted in different conditions (A to H). Table 1 presents the results of gallic acid, catechin, epicatechin and syringic acid. Their correlation coefficients (R2) obtained above 0.83 indicate that the models fit well with the experimental data.
According to B extraction conditions, Gallic acid was better extracted using water at 35 °C for 30 min (78.13 μg/g). The solvent's effect was positive, indicating that a change in its value in the positive solvent direction (water) would increase the content of gallic acid. Besides, the solvent and time interaction was significant (p < 0.05), denoting that the extraction of gallic acid from Croton lechleri bark was favoured by water with shorter extraction times. Gallic acid is a phenolic acid comprising a benzoic acid aromatic ring with three hydroxyl substituents. The presence of OH groups increases the polarity of the molecule, which in turn will have a preference for more polar solvents. Moreover, a steric hindrance that electronic clouds set around the gallic acid molecule prevent the interaction of the hydrophobic part (aromatic ring) with the non-polar part of the molecule of ethanol, causing the gallic acid to have more interaction with water (Perin et al., 2020; Casagrande et al., 2019).
Epicatechin and syringic acid were extracted more efficiently after 90 min of extraction, presenting stability for longer times at 35 °C (condition D, 42.22 and 55.02 μg/g, respectively) (Table 1). All factors were significant in the content of epicatechin and syringic acid, as well as the solvent and temperature interactions, and solvent and time (Table 2). Thus, the positive effects of solvent and time indicate that a change for the solvent's positive direction (water) and time (90 min) would increase both compounds' content. The presence of the methyl ether and hydroxyl groups in these molecules gives greater polarity, increasing its interaction with the water compared to ethanol.
The best extraction condition for catechin was obtained using ethanol at 70 °C for 90 min (condition G, 1241.83 μg/g) with a significant difference (p < 0.05) with other conditions. The temperature and solvent factors and the solvent and temperature interaction were statistically significant on the quantified compound. The positive effect of temperature indicates that a change in its value in the positive sense would lead to an increase in the catechin content (Tables 1 and Table 2). Catechin belongs to the flavanol class with five OH groups. This compound's medium polarity is due to the interactions between the aromatic rings and the hydrophobic part of the solvent that explains catechin's better interaction with ethanol.
Principal component analysis (PCA) was applied to the results to reduce the dimensionality of data amount and determine the extracts pattern as a function of their independent variables (solvent, extraction time, and temperature). Fig. 1 shows the plot of PCA data for phenolic compounds extracted from Croton lechleri bark. As shown, the first (PC1) and second (PC2) principal components were able to describe 92.59% total variance of the data. The group formed by A and C experimental runs located in the first quadrant presented the lowest yield of the extracted compounds, proving that ethanol at 35 °C was not efficient in removing large amounts of phenolic compounds. The second group comprises the G and E conditions of extractions (ethanol, 70 °C) and gave the highest catechin quantities. Epicatechin was better extracted in conditions D and H (water, 90 min.). It required more time to be extracted, as indicates its position on the third quadrant. On the other hand, gallic and syringic acids were better extracted from Croton lechleri bark in B and F runs (water, 30 min).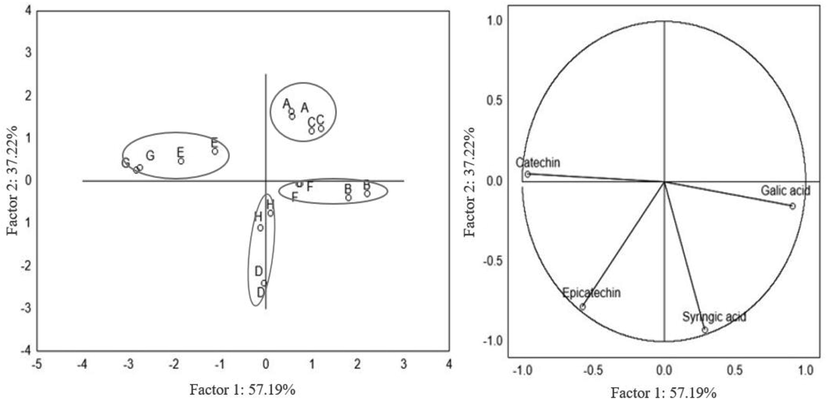
First principal component (PC1) versus second principal component (PC2) (a): Distribution of samples on scores plot; (b) distribution of variables on loadings plot.
The limited number of studies on the extraction of phenolic compounds on Croton lechleri barks makes it difficult to compare previous studies' values. They are often from different species of the genus Croton or other parts of the plant. Nevertheless, Cordeiro et al. (2016) found catechin, epicatechin, and gallocatechin on the methanolic extract from Croton urucurana. At the same time, Nascimento et al. (2017) identified catechin and epicatechin from the ethyl acetate fraction of the Croton cajucara leaves.
However, two-way joining analysis was applied to confirm the clustering of samples related to dependent variables. In this analysis, the intensity of the colour shows of the dependent variables has a higher relation to the extracts produced (Fig. 2). In fact, the extracts from D, F, E, and G runs showed the highest antioxidant activity by the DPPH test, while, A and C conditions showed the lowest activity. The highest amount of catechin was found in the G run extracts, while the C and B extracts showed the compound's lowest content. Besides TPC, epicatechin, syringic acid, and gallic acid did not lead differences on the Two-way joining analysis. However, according to Sari et al. (2020), each phenolic compound demands an individual approach for extraction and optimization in natural products.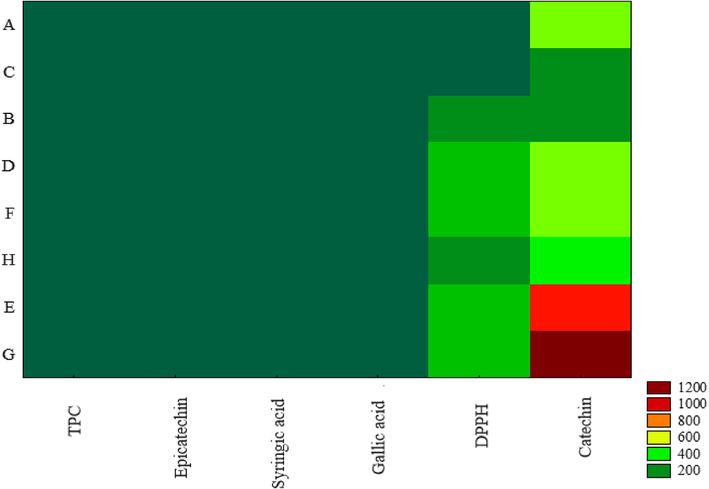
Two-way joining results for C. lechleri bark extract. Total phenolic compounds (TPC), epicatechin, syringic acid, gallic acid, antioxidant activity (DPPH), and catechin.
Global response (GR) was performed to obtain the best extraction condition of Croton lechleri bark extract for all dependent variables (TPC, AA, epicatechin, syringic acid, gallic acid, and catechin) (Fig. 3). According to ANOVA results, all factors, as well as the interactions of the temperature with solvent and time, were significant (p < 0.05). The R2 = 0.97 proved that it is possible to obtain a single and global response in research with multiple dependent variables (Table 2). Additionally, the predicted means for Global Response in Fig. 3 show that the water was the best solvent for extracting natural compounds from Croton lechleri barks at 35 °C for 90 min of extraction.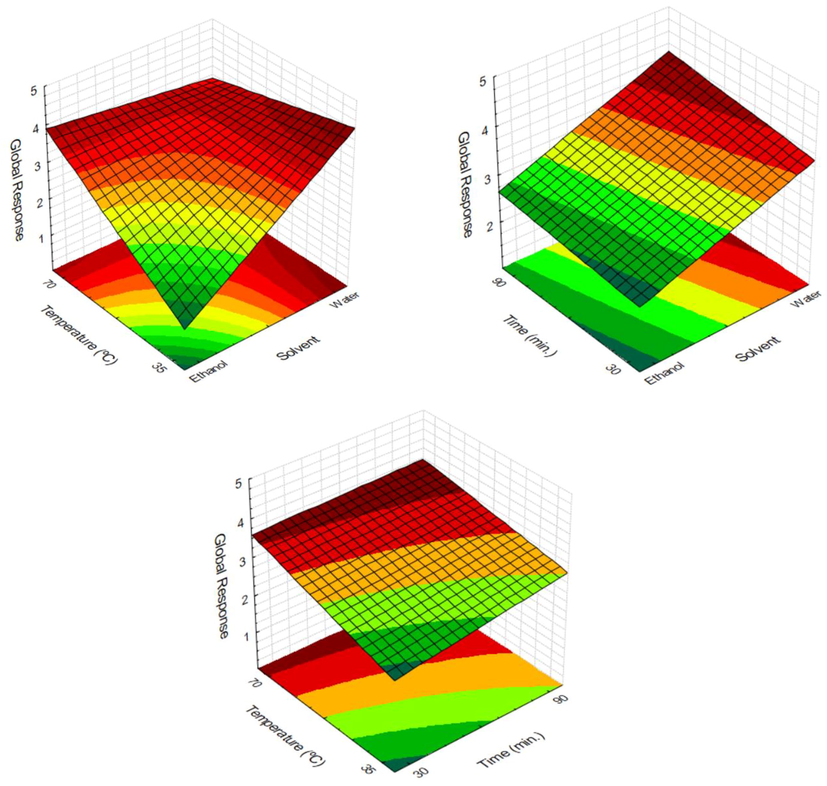
Response Surface for Global Response on the C. lechleri bark extraction.
4 Conclusion
This research supports that Croton lechleri bark aqueous extracts at low temperature had significant amounts of phenolic compounds, especially gallic acid, catechin, epicatechin, and syringic acid, proving the effectiveness of this plant's popular use. Besides, RSM, PCA, and GR chemometric tools employed proved to be efficient in analyzing the data obtained. Moreover, Global Response showed a powerful technique to determine a single response that represents a set of attributes. Our results also revealed the importance of investigating Amazonian natural products, yielding novel findings on antioxidant and antimicrobial agents and can be used in the food sector. These results represent a relevant contribution to the Croton lechleri tree's bioactivity knowledge grown in Brazil, an important source of phenolic compounds with antioxidant activity. This study can support natural additive development with potential food technology, pharmaceutical, and chemical industries. Besides, considering the interest in finding natural antioxidants, it is possible to suggest that Croton lechleri barks have biological activity, mainly because of reducing compounds, free radical scavengers, and hydrogen donors plant material. Nevertheless, for consumers' safety, the alternative plant-based additive needs to be evaluated first to present possible toxic and mutagenic components. Additionally, it will be necessary to display a complete profile of the risks and benefits of using these products.
Acknowledgements
The project was funded by CAPES (Coordination of Superior Level Staff Improvement) and CNPq (National Council for Scientific and Technological Development). Authors want to thank the Department of Chemistry, Analysis Center at UTFPR-Pato Branco/PR.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antitumor effect of Croton lechleri Mull. Arg. (Euphorbiaceae) J. Ethnopharmacol.. 2012;140(2):438-442.
- [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol.. 1995;28(1):25-30.
- [Google Scholar]
- Optimization of the extraction of antioxidant phenolic compounds from grape pomace using response surface methodology. J. Food Meas. Charact.. 2019;13(2):1120-1129.
- [Google Scholar]
- Anti-inflammatory and antinociceptive activities of Croton urucurana Baillon bark. J Ethnopharmacol.. 2016;183:128-135.
- [Google Scholar]
- Ethnopharmacological study of plants sold for therapeutic purposes in public markets in Northeast Brazil. J. Ethnopharmacol.. 2015;172:265-272.
- [Google Scholar]
- Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity. Molecules. 2008;13(6):1219-1229.
- [Google Scholar]
- Phytochemical analysis and anti-inflammatory evaluation of compounds from an aqueous extract of Croton cajucara Benth. J. Pharm. Biomed. Anal.. 2017;145:821-830.
- [Google Scholar]
- Bioguided extraction of phenolic compounds and UHPLCESI-Q-TOF-MS/MS characterization of extracts of Moringa oleifera leaves collected in Brazil. Food Res. Int.. 2019;125:108647.
- [CrossRef] [Google Scholar]
- RGB pattern of images allows rapid and efficient prediction of antioxidant potential in Calycophyllum spruceanum barks. Arab. J. Chem.. 2020;13(9):7104-7114.
- [Google Scholar]
- Chemical fingerprinting and bioactivity of Amazonian Ecuador Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil: A new functional food ingredient? Food Chem.. 2011;126(3):837-848.
- [Google Scholar]
- Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae) J. Braz. Chem. Soc.. 2007;18(1):11-33.
- [Google Scholar]
- Extraction of phenolic compounds from Tabernaemontana catharinensis leaves and their effect on oxidative stress markers in diabetic rats. Molecules. 2020;25(10):2391.
- [CrossRef] [Google Scholar]
- Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol.. 1999;299:152-178.
- [Google Scholar]
- Chemical constituents from Croton species and their biological activities. Molecules. 2018;23(9):2333.
- [CrossRef] [Google Scholar]







