Translate this page into:
Bio-efficacy of chloroform crude extracts of chick weed Ageratum conyzoides (Linn.) against the tobacco cutworm Spodoptera litura (Linn.) and their non-toxicity against the beneficial earthworm
⁎Corresponding authors at: Division of Bio-pesticides and Environmental Toxicology, Sri Paramakalyani Centre for Excellence in Environmental Sciences, Manonmaniam Sundaranar University, Alwarkurichi, 627412 Tirunelveli, Tamil Nadu, India (S. Senthil-Nathan). senthil@msuniv.ac.in (Sengottayan Senthil-Nathan), patcharink26@gmail.com (Krutmuang Patcharin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The current study investigates the phyto-chemical screening of chloroform crude extracts of chickweed Ageratum conyzoides (Linn.) (Cm-Ac) and their target and non-target toxicity against the pest Spodoptera litura and beneficial earthworm. Phyto-chemical characterization of Cm-Ac over GC-MS technique revealed thirty four bio-active compounds with highest peak area% observed in Phenol, 2,4-Bis-(1,1-Dimethylethyl)-(6.340 PA%), followed by n-Hexadecanoic acid (5.95 PA%) and 1- Octadecene (5.94 %) respectively. Larval toxicity was observed maximum at the dosage of 1000 ppm at second instar (98.23 %) as compared to other dosages. Lethal dosage (LC50) obtained on 2.467 log ppm respectively. The sub-lethal toxic dosage of Cm-Ac (100–500 ppm) displayed adverse impact on larval-pupal growth and development in dose dependent manner. Also, the sub-lethal dosage of Cm-Ac also decreased the level of mid-gut enzymes in dose dependent manner. The mid-gut tissues including Gut Lumen-(GL), Epithelial Layer-(EL), and Brush Border Membrane-(BBM) severely affected at the prominent concentration of 500 ppm as paralleled with control. Non-target activity against Eudrilis eugeniae revealed that the Cm-Ac treatment is less toxic at the prominent concentration of 1000 ppm as paralleled to Cypermethrin treatment. Overall, the current results are promising in developing botanical based insecticides which are bio-degradable and importantly harmless or less toxic to the non-target earthworms.
Keywords
Chickweeds
Green derivatives
n-Hexadecanoic acid
Tobacco cutworm
Mid-gut
Earthworm
1 Introduction
Biological based insecticides is an effective and alternative method of chemical pesticides to control insect pests causing damage to the agriculture field. As the frequent usage of synthetic pesticides results in minimal effectiveness by development of pest resistance (Selin-Rani et al., 2016; Ponsankar et al., 2016; Vasantha-Srinivasan et al., 2016). Moreover, commercial pesticides in-directly affects the beneficial or non-target animals which generates alternative attention in expending natural products to manage agriculture important pest (Edwin et al., 2016; Ponsankar et al., 2018; Dinesh-Kumar et al., 2018; Yogarajalakshmi et al., 2020). Bio-pesticides are derived from natural materials they includes animals, plants, and plant active compounds (Bhushan et al., 2016). They are easily degradable in the environment and in turn decline the risks to beneficial animals (Isman, 2006).
Plant derived extracts has delivered significant source of bio-active metabolites that are usually synthesized by plants for their acute defense (Vasantha-Srinivasan et al., 2018; Edwin et al., 2021; Sundar et al., 2021). Herbal derived phyto-chemicals widely act as an larvicidal, repellent, ovipositional deterrents, enzyme inhibitors, growth retardants and feeding deterrents against different insect pests and delivered an alternative agent in replacing commercially using synthetic chemicals (Thanigaivel et al., 2018; Amala et al., 2021; Chellappandian et al., 2022). Plant extracts and their active metabolites directly blocks the metabolism of pests by affecting the major enzymes of digestive tract which ensuing in metabolic pathways imbalance, loss of growth and leads to insect pest mortality (Napoleão et al., 2015; Zibaee and Bandani, 2010; Vasantha-Srinivasan et al., 2021). Native plants are considered an excellent resource for wide range of novel chemistries with minimal toxicity to reach market in replacing chemical pesticides (Canter et al., 2005; Capocchi et al., 2022; Loganathan et al., 2022).
The Ageratum genus comprises of more than thirty species and have been widely investigated for its phyto-chemical properties (Okunade, 2002; Yadav et al., 2019). Among, Ageratum conyzoides L. (Asteraceae) commonly known as “Chick Weed” considered as an invasive species it is common in West Africa, Asia and some regions of South America (Kamboj and Saluja, 2008). A. conyzoides has been exploited for the wide range of cure diseases in several countries Africa, Asia, and South America. It is regularly cast-off as a febrifuge, purgative, anti‐ulcer, antimicrobial, anti-dysenteric, and antilithic, and anti‐inflammatory wound dressing (Ahmed et al., 2012; Yadav et al., 2019). A. conyzoides is an annual branched, erect, aromatic plant and grows maximum height of one meter (Kamboj and Saluja, 2008; Nyunaï et al., 2009). A. conyzoides delivers significant ethno-medicinal value to cure diverse ailments including leprosy, rheumatism, skin disorders, sleeping sickness, headaches, toothache, dyspnea, pneumonia and considered as a natural herbal medicine widely applied in primary health care (Yadav et al., 2019). Chemical profile of A. conyzoides delivers various phyto-compounds including sterols, terpenes, flavoides, chromenes etc. (Amadi et al., 2012; Yadav et al., 2019). All these literature supports that, chick weed holds significant phyto-chemical and ethno-pharmacological value. In addition, A. conyzoides delivered significant mosquitocidal action against the mosquito vectors with medical importance (Ramasamy et al., 2021).
The lepidopteran pest Spodoptera litura (Noctuidae) Fab. is considered as major species affecting multiple host and has a diversified range of host crops (>150 crops) and responsible for huge yield losses including groundnut, cotton, tobacco, tomato, soybean and potato (Senthil-Nathan and Kalaivani, 2005). Controlling this pest using synthetic chemicals delivers higher range of resistance and also exhibits significant imbalance in ecology and displays toxic effects to the beneficial non-target species (Chellappandian et al., 2018; Annamalai et al., 2018; Karthi et al., 2019; 2020; Perinelli et al., 2022).
Earthworms are considered to be a significant soil invertebrate’s delivers key role to maintain healthy soil ecosystem. Among, Eudrilis eugeniae belongs to Eudrilidae family is considered as a potential bio-remediate in managing pesticide and herbicide pollution (Murugan et al., 2015). Also, considered as an essential constituents of plants soil systems in agro and natural ecologies (Vasantha-Srinivasan et al., 2018). Chemical insecticides display the risky impact on the non-target animals; plant biodiversity, aquatic and terrestrial food webs. Pimentel (1995) illustrated that >0.1 % of commercial pesticides applied to agriculture field affects the target insects and >99 % have the latent to impact non-target species and it penetrates deep into the soil ecosystems. Thus, effective commercialization of any bio-pesticides are forced to face problems that should be resolved by testing their toxicity against the non-target species by which stability and quantity of any pesticides can be guaranteed (Benelli et al., 2016).
The present research aims to investigate the chemical profile of chloroform crude extracts of chickweed A. conyzoides (Cm-Ac) and its larvicidal, developmental regulations, enzyme inhibition activity and gut histological actions against the lepidopteran insect pest S. litura and their non-target toxicity against the beneficial earthworm E. eugeniae.
2 Methodology
2.1 Plant harvesting
The healthy Ageratum conyzoides (Asteraceae) plant leaves were collected during morning hours nearby South Western Ghats Area, Tamil Nadu, India. Taxonomist from Department of Plant Conservation authenticated the collected plant samples collected and Biotechnology, Manonmanium Sundaranar University, Tirunelveli, and the voucher specimen no AC18632 was defer to the herbarium.
2.2 Crude extraction
The fresh leaves of chickweed were collected in one day and washed twofold with ordinary tap water and double sterile water to eliminate any related debris. The leaves were shade dried for 7–14 days at room temperature (27–32 °C) in direct to control thermal degradation and photolysis. Dried leaves were sliced into fewer sections and grinded to powder as coarsely with electrical stainless steel blender (made of stainless steel blade). Fine blended powder was kept in airtight container at 3 °C for further extraction procedure.
The powdered sample of 200 g were packed tightly in soxhlet apparatus for extraction. The chloroform extract of A. conyzoides (Cm-Ac) was obtained from the residue of 500 ml of chloroform. It was heated at 70–75 °C for 48 h (2 days). Initially the extract comes from the sample will be dark green. Extraction procedure was stopped until the sample packed remains pale or the extraction comes from the sample remains dark green to colorless. The solvent was distant, evaporated using rotary evaporator, and completely dried under in-vitro temperature. This crude extract (8.93 % total yield) was stored at −5°C and used as a stock for purification process.
2.3 Chemical characterization
The crude extract of Cm-Ac was used for Gas chromatography-Mass spectrometry (GC–MS) examination. Cm-Ac (1 ml) sample was dissolved in High-performance liquid chromatography (HPLC) grade ethanol (≥99.9 % standard for GC) and the crude fraction was exposed to GC and MS JEOL GC mate fortified with secondary electron multiplier. The instrument used for this characterization is Japan Electron Optics Laboratory Co., Ltd. (JEOL) GCmate™ GC/MS Double-Focusing Mass Spectrometer (Agilent Technologies 6890 N Network). The HP5 column (Inner Diameter-0.32 mm; Length: 30 m; Film: 0.25 µm) used was linked with silica (ID: 0.25 mm, film thickness: 0.25 µm length: 50 m) I.D. The running time of the sample is 20 min, Column temperature programmed at 110 °C with 10 °C/min rise to 230 °C, 250 °C for injector temperature, helium (99.99 %) was the carrier gas and split ratio was 4:3. Using a split less injector at the temperature of 280 °C the Cm-Ac (1 μl) was vaporized. Further, the chemicals were recognized through gas chromatography fixed with mass spectrometry with source at 270 °C at electron impact mode 70 eV. The empirical weight, empirical formula and its chemical structure of Cm-Ac were determined by elucidation on mass spectrum of GC–MS utilizing the National Institute Standard and Technology (NIST) database.
2.4 Chemicals
Cypermethrin were purchased (Sigma-Aldrich, PESTANAL®, Analytical standard). All tested chemicals used in the experiments were of spectro-grade quality.
2.5 Insect culture & development
The larvae of lepidopteran pest S. litura composed as of castor plant (Ricinus communis) collected from agriculture field region of Avadi, Chennai, Tamil Nadu, India. The whole culture was conserved according to the adapted procedure Senthil-Nathan et al. (2008). Castor leaves was taken and utilized for rearing S. litura larvae. For larval mass culture, we cast-off mature leaves of castor leaves (125 cm2) were distant from the greater third of the castor leaves as required. Pre-pupae further detached and delivered with clay composed of vermiculture as the site for pupation. Adult moth emerging transported to insect cages and served with a 10 % solution of sucrose solution along with a blend of vitamin mixture for oviposition promotion. Further, the moths moved to the oviposition cages at a ratio of 2 females: 1 male composed of castor leaves and shielded with muslin cloth for laying eggs. The adults were distinguished by the color, shaded grey and brown forewing with a triangular white spots at the tip of the wing usually found in male moth and greyish-brown forewing that is uniform in color in female moths. The eggs hatched in the muslin cloths were detached every day and the surface sterilized for avoiding any contaminations by dropping in formaldehyde (10 %) for two to five minutes and rinsed with sterile water. The muslin cloths containing eggs were moistened and kept in plastic containers to allow hatching. All the procedures and culture conceded with 67 % Relative Humidity (RH) at 27 (±2°C) with a photoperiod cycle of 14-Light: 10-Dark.
2.6 Larval bioassay
Bioassays of larvae were performed with different instars of S. litura utilizing lethal dosages Cm-Ac. Ethanol treated leaves were air dried and used for control. A minimal of 20 larvae/dosage utilized for all the treatments with five replications (N = 100). The castor plant leaves (125 cm2) were treated with different selective dosage of (250, 500, 750 and 1000 ppm) Cm-Ac prepared by diluting the stock solution. All larval instars starved for four hours and independently nourished with different Cm-Ac concentrations. The uneaten leaves were detached for every 24 h, and supplanted with fresh leaves treated with Cm-Ac. Five replications were carried out for each treatment (N = 50). Larval mortality documented for each 24 h and compared with control. The percentage of mortality was estimated through Abbot’s (1925) formula.
The lethal mean dosage (LC50 and LC90) estimated using probit analysis (Finney, 1971).
2.7 Larval-pupal duration
The duration of larval and pupal stages were executed based on the modified protocol of Senthil-Nathan (2006). The laboratory grown third instar of S. litura were utilized for estimating duration assays. Rearing of larvae were separated in sterile containers subjected with fresh castor leaves treated with Cm-Ac 100, 200, and 500 ppm dosage. Ethanol is used to treat the control leaves. Both groups (Treated & Control) containers were conserved at 28 (±2°C) with a photoperiod of 15L:10 D along with Relative Humidity (RH) of 80 %. In a regular 24 h basis records made to distinguish the existing and dead larvae. The larval duration and pupal duration were documented. After pupation of first day, the pupa emouled from all treatments was weighed. Any abnormalities and deformities in the emerging adults were also been recorded.
2.8 Enzyme assay
Fourth instar were utilized for enzyme assays to determine the expression of ACP-Acid phosphatase, ALP-Alkaline Phosphatase and ATP-Adenosine Triphosphatase as per the adapted protocol of Ponsankar et al. (2020). The treated larval instars sedated and the complete digestive tissues separated out (Adhering tissues, Gut contents and Malpighian tubules) in insect ice-cold Ringer’s reagent. The midgut was divided into multiple layers, accuracy weighed in mg and homogenized the tissues using ice cold buffer of citrate–phosphate homogenized (pH-6.7) consuming a tissue centrifuged at 4 °C for 3 min. The homogenate from the centrifuges samples was suspended in to ice cold solution buffer and make to 1 ml and it was spin at 500 rotation per minutes for 10 ‘g’ force minutes and the resulting supernatant were utilized as the source of enzyme for determining ACP, ALP and ATP respectively.
2.9 Gut-Histology
The sectioning of mid gut procedure was implemented as per the adapted technique of Selin-Rani et al. (2016). Treated and control mid gut samples from fourth instar utilized for tissues sectioning. The mid gut sections further observed and shattered using fluorescent microscope (HBO Optika Flow series, B-600, Ponteranica, TiFl, Italy).
2.10 Earthworm toxicity
Eudrilis eugeniae earthworm stock was preserved at Bio-pesticides and Eco-toxicology lab, Manonmaniam Sundaranr University, Tirunelveli, India. It was initially purchased and authenticated by Tamil Nadu Agriculture University (TNAU), India. The earthworm maintained at an average ambient temperature of 27.5 ± 0.36 °C by means of crop residues amended with cattle dung as food with our adapted methodology of Vasantha-Srinivasan et al. (2018). Worms and cocoons produced were isolated from the soil and the debris present in the worms were removed by washing in sterile water, then consequently weighed, live weight basis and individual earthworms were kept in separate glass vessels. Also, the separated cocoons were totaled and hosted in separate bedding with the same materials in which their parents were nurtured.
2.11 Contact filter paper test (FPT)
A section of filter paper was located in a petridish and exposed with Cm-Ac (1000, 1500 and 2000 ppm) and standard Cypermethrin (99.1 % purity) (0.1 ppm) were used for our study. Water is used as a control for comparison of results obtained from treated experiment. Earthworm was kept at the top of the filter paper was moisturized with distilled water. Further, the petridish was incubated at 26 (±2°C) for 48 h and the rate of mortality documented.
2.12 Artificial soil test (AST)
As per the adapted protocol of OECD (1984) 15 %- kaolinite clay, 15 %-ground sphagnum peat, and fine sand-70 % used to cover the artificial soils for the assay. A minimal quantity (0.001 mg) calcium carbonate included to maintain the 6.5 ± 0.5 pH. The remaining 35 % of the weight were adjusted with the water content of the dry weight in artificial soil toxicity assay. Additional testing were adapted as per the protocol (Vasantha-Srinivasan et al., 2018).
2.13 Data analysis
Result obtained through the mortality was visible to (ANOVA of arcsine) analysis of variance and data presented as five replicates mean value. In addition, the statistical changes between the treatments evaluated through Tukey’s multiple range method (P < 0.05) through Minitab®18 software. The Sigma-plot 11.0 (Microcal Software) was utilized for determining the mid gut enzyme assay. The larval lethal Concentration (LC50) observed at 24 h were determined by with a 95 % dependability interval using Probit analysis.
3 Results
3.1 Chemical characterization of Cm-Ac
Phyto-chemical characterization of Cm-Ac over GC–MS analysis exposed total of thirty four chemicals with total Peak-area (PA) percentage of 97.04 % (Fig. 1B). The peak area was maximum observed in Phenol, 2,4-Bis(1,1-Dimethylethyl)- (6.34 PA%), followed by n-Hexadecanoic acid (5.95 PA%) and 1- Octadecene (5.94 %). The identified chemicals were confirmed through NIST library. Retention Time (RT) of the screened compound, molecular formula and weight of major peaks attained from reliable spectral library using GC–MS was displayed (Table 1).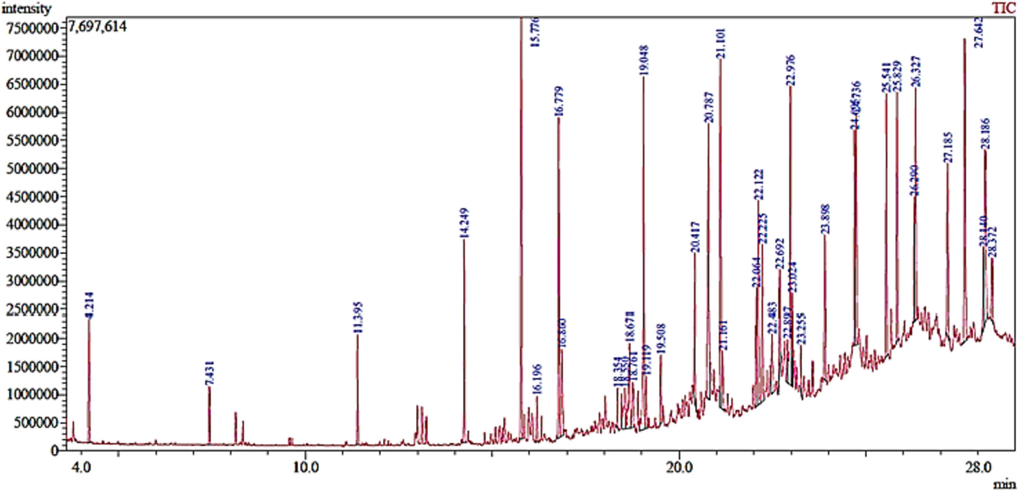
(A) Physical appearance of plant Ageratum conyzoides and (B) GC–MS chromatogram of Chloroform extract of Ageratum conyzoides (Cm-AC).
Peak No
Compound Name
MS
MF
RT
PA%
1
Benzene, Methyl-
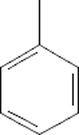
C6H5CH3
4.214
1.56
2
Butane, 1,1-diethoxy-3-methyl-
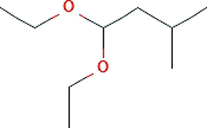
C9H20O2
7.431
0.75
3
1-Dodecene

C12H24
11.395
1.43
4
1-Hexadecene

C16H32
14.249
2.70
5
Phenol, 2,4-Bis(1,1-Dimethylethyl)-
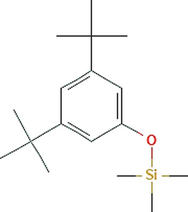
C17H30OSi
15.776
6.34
6
1-Decanol, 2-hexyl-
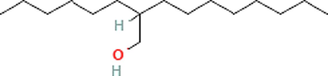
C16H34O
16.196
0.71
7
1-Hexadecanol
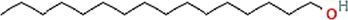
C16H34O
16.779
4.49
8
Limonen-6-ol, pivalate
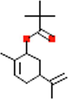
C15H24O2
16.860
1.96
9
1-Decanol, 2-hexyl-
C16H34O
18.354
0.51
10
1-Tridecanol
C13H28O
18.671
2.06
11
1-Octadecene

C18H36
19.048
5.94
12
Heneicosane

C21H44
19.119
1.89
13
Neophytadiene
C20H38
19.508
0.94
14
Hexadecanoic acid, Methyl Ester
C17H34O2
20.417
1.97
15
n-Hexadecanoic acid
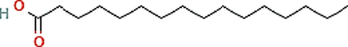
C16H32O2
20.787
5.95
16
Behenic alcohol
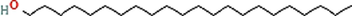
C22H46O
21.101
5.17
17
Pentatriacontane
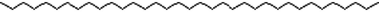
C35H72
21.161
1.05
18
9,12-Octadecadienoic Acid (Z,Z)-, Methyl
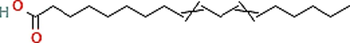
C18H32O2
22.064
1.65
19
11,14,17-Eicosatrienoic acid, methyl ester
C21H36O2
22.122
3.12
20
Phytol
C20H40O
22.225
3.86
21
Dichloroacetic acid, tridec-2-ynyl ester
C15H24C12O2
22.483
1.24
22
Octadecanoic acid
C18H36O2
22.692
4.21
23
Hexadecanoic acid, butyl ester
C20H40O2
22.897
0.82
24
1-Heptacosanol
C27H56O
22.976
4.89
25
Phytane
C20H42
23.024
1.32
26
Hexatriacontane
C36H74
23.898
3.13
27
Octacosanol
C28H58O
24.695
3.06
28
Dotriacontane
C32H66
24.763
3.23
29
Bis(2-ethylhexyl) phthalate

C24H38O4
25.829
3.52
30
Hexadecanedinitrile

C16H28N2
26.290
1.84
31
1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester
C24H38O4
27.642
5.69
32
Cyclohexane, 1,1′-Hexylidenebis-
C18H34
28.140
1.52
33
Tetrapentacontane
C54H110
28.186
3.97
34
Squalene
C30H50
28.372
4.57
Total PA (%)
97.04
3.2 Cm-Ac larval toxicity
Cm-Ac against the II, III and IV instars of lepidopteran pest S. litura displayed dose dependent mortality with maximum percentage of mortality was obtained in Cm-Ac 1000 ppm across all the instars as compared to the other treatment concentrations (Cm-Ac 250–750 ppm) and control respectively. The larval mortality was maximum displayed at second instar 98.23 %- (F4,20 = 23.62, P ≤ 0.001) followed by third instar 95.23 %-(F4,20 = 18.34, P ≤ 0.001) and 92.31 %-%-(F4,20 = 14.22, P ≤ 0.001) at the prominent concentration of Cm-Ac 1000 ppm respectively (Fig. 2). All dosage treatment was significant with control in second (5.43 %- F4,20 = 12.66, P ≤ 0.001), third (7.13 %- F4,20 = 18.88, P ≤ 0.001) and fourth instar (8.99 %- F4,20 = 10.21, P ≤ 0.001). The lethal concentration (LC50 and LC90) was observed at 2.467 and 2.981 log concentration (ppm) respectively (Fig. 3).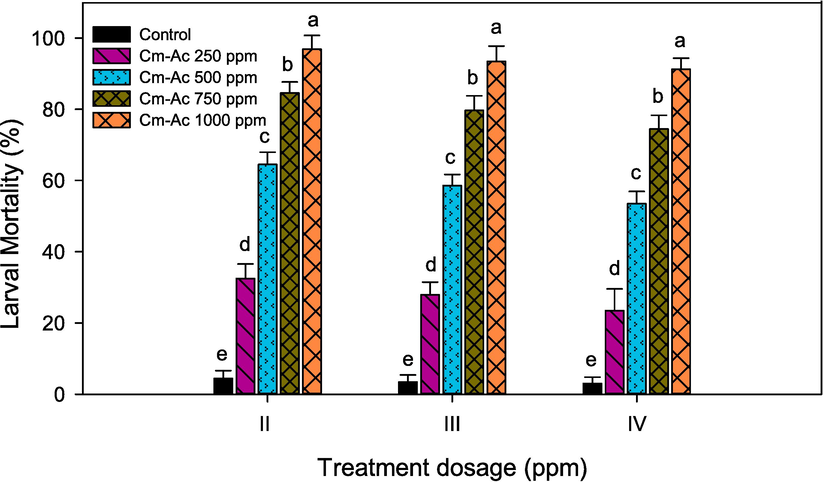
Percentage mortality of second, third and fourth instar larvae of S. litura post treatment with chloroform extract of Ageratum conyzoides (Cm-AC). Means (SEM ± ) followed by the same letters above bars indicate no significant difference (P ≤ 0.05) according to a Tukey’s test.
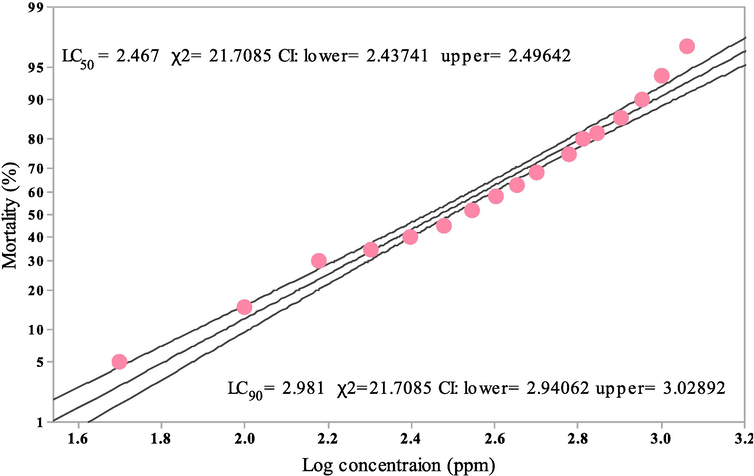
Median Lethal Concentration (LC50) and (LC90) of Chloroform extract of Ageratum conyzoides (Cm-AC) post treatment with fourth instar larvae of S. litura using Probit analysis.
3.3 Cm-Ac developmental activity
Sub-lethal dosage of Cm-Ac (100–500 ppm) treated larvae displayed extended larval and pupal durations and also showed adversarial impact on larval development. The larval and pupal growth was extended significantly in dose dependent manner. The sub-lethal Cm-Ac treatment displayed inert growth and development and it was significant at the profound dosage of 500 ppm in larva (32 Days-F4,20 = 12.88, P ≤ 0.001) and pupal (26 Days-F4,20 = 12.45, P ≤ 0.001) respectively. (Fig. 4). Despite all the treatment dosage was significant with control larvae (7 Days- F4,20 = 12.88, P ≤ 0.001) and pupae (18 days- F4,20 = 12.45, P ≤ 0.001) respectively.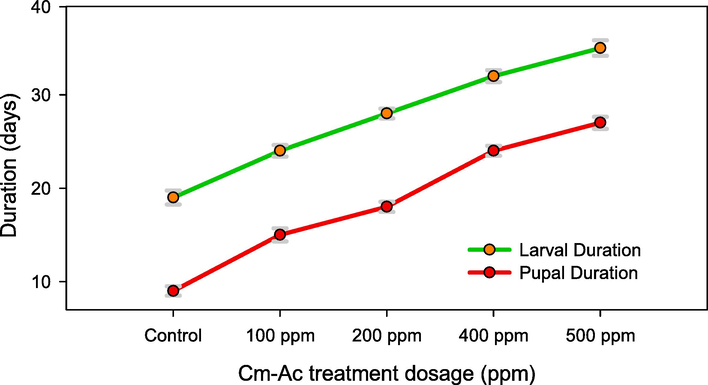
Developmental time in days for fourth instar larvae and pupae, post treatment with Chloroform extract of Ageratum conyzoides (Cm-AC). Means (± (SE) standard error) bars indicate no significant difference (P ≤ 0.0 5) according to a Tukey’s test.
3.4 Cm-Ac enzyme activity
Cm-Ac sub-lethal dosage significantly affected the ACP, ALP and LDH rate in dose reliant manner (Fig. 5). Level of ACP gets declined maximum to 6.770 µmol/mg at the prominent concentration of 500 ppm (F4,20 = 18.44, P ≤ 0.001) in parallel to other dosages 400 ppm (8.470 µmol/mg), 200 ppm (10.11 µmol/mg), 100 ppm (12.31 µmol/mg) and control (15.800 µmol/mg) respectively. In parallel, ALP level also gets declined maximum to 8.470 µmol/mg at the profound dosage of 500 ppm and significant with treated other dosages and control (23.450 µmol/mg-F4,20 = 26.11, P ≤ 0.001). In addition, LDH gets declined significant at the profound dosage of 500 ppm (11.43-µmol/mg- F4,20 = 22.10, P ≤ 0.001) in parallel with control and treated other dosages (27.45 µmol/mg).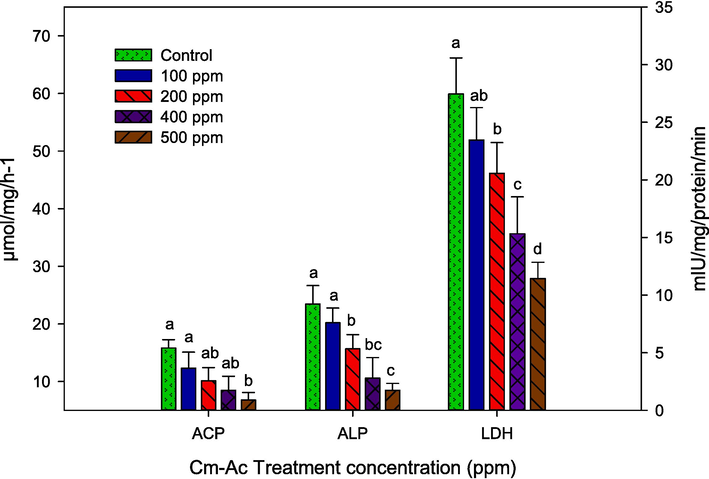
Enzyme activity of Acid phosphatase (ACP), Alkaline phosphatase (ALP) and Adenosine Triphosphatase (ATP) of fourth instar larvae S. litura post treatment with Chloroform extract of Ageratum conyzoides (Cm-AC). The data were fitted on polynomial (regression) model, whereas vertical bars indicate standard error (±SEM).
3.5 Cm-Ac Gut-histological activity
Gut-histological activity of Cm-Ac (500 ppm) displayed major cellular and morphological reparations in the tissues of midgut. The midgut tissues treated with Cm-Ac displayed major change in the epithelial layer (EL) alignment (Fig. 6B). However, the control display well preserved epithelial layer. Likewise, there was a substantial modification in the shape of brush border membrane (BBM) and gut lumen (GL) compare to control (Fig. 6A).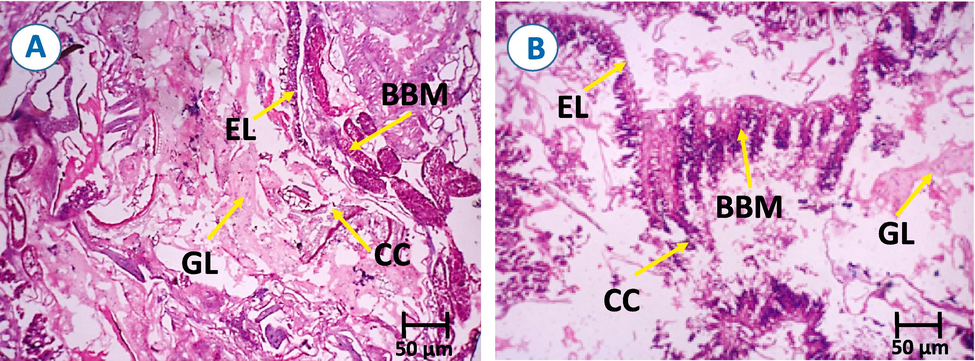
Mid-gut histological section of fourth instar larvae of S. litura (A) Control and (B) Cm-Ac Treatment (500 ppm) were CC-columnar cells; EL- epithelial layer; BBM- brush border membrane; and GL- gut lumen.
3.6 Cm-Ac non-target activity
Acute toxicity of E. eugeniae earthworm showed significance in the mortality with the standard chemical (Cypermethrin) and Cm-Ac treatments in FPT and AST. The mortality rate was significant between 24 hr and 48 hr of treatment across different dosages of Cm-Ac and cypermethrin. The rate of mortality was significant for cypermethrin treatments at (0.1 ppm) at 24 hrs (28.22 %- F4,20 = 22.10, P ≤ 0.001), as compared to 48 hrs (38.33 %- F4,20 = 22.10, P ≤ 0.001). Despite the larval mortality was lesser in Cm-Ac (2000, 1500 and 1000 ppm) treatment and control with 14.32 %, 11.23 %, 7.21 % and 3.41 % respectively in 24hrs. Similar trends were observed in the 48 hrs as the mortality rate was lesser in control 3.12 % as compared to Cm-Ac treatment (Fig. 7). Moreover, the E. eugeniae exposed to AST was also showed dose dependent mortality rate in Cm-Ac treatment and the control at all exposure periods. Despite cypermethrin (0.1 ppm) treatment showed prominent mortality rate at 2d (46 %- F4,20 = 18.12, P ≤ 0.001), 7d (42 %-F4,20 = 21.21, P ≤ 0.001) and 14d (28 %-F4,20 = 14.55, P ≤ 0.001) respectively.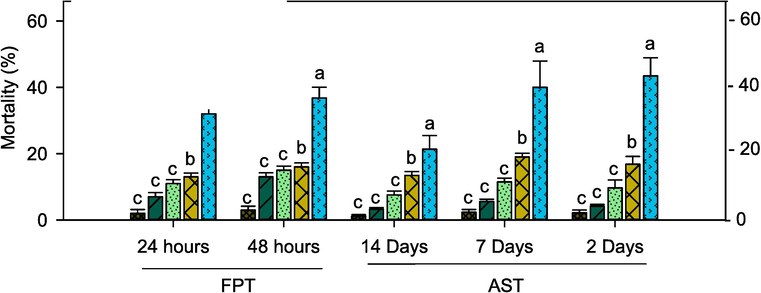
Percentage mortality of E. eugeniae in filter paper test (FPT) and artificial soil test (AST) post treatment with Chloroform extract of Ageratum conyzoides (Cm-AC) and commercial pesticide cypermethrin. Means (± (SEM) standard error) indicate no significant difference (P ≤ 0.05) according to a Tukey’s test.
4 Discussion
Commercial pesticide usage has driven a significant impact to the agronomic crop production by aggregating the crops protection against the agriculture pests and disease (Murfadunnisa et al., 2019; Benelli et al., 2020). Synthetic pesticides acts on wide-ranging host insects and they are appropriate for its dynamic part in managing pests in agricultural field (Zhang et al., 2013; Edwin et al., 2016; Pradeepa et al., 2016). Cypermethrin is an active commercial substance with an explicit inherent toxicity with measured substantial sources of drawn-out pollutants that can cause harmful impact on non-targets (Dinesh-Kumar et al., 2018; Karthi et al., 2019). Present research been floated on harvesting the herbal chemistry derived from bio-rational plants with target specific insecticidal actions and harmless to beneficial species (Ponsankar et al., 2020; Giongo et al., 2016).
Previous investigation of number of phyto-constituents have been examined such as flavonoids, terpenes, alkaloids, sterols and chromenes from nearly every part of this plant (Ahmed et al., 2012). Correspondingly, Mary and Giri (2016) evaluated the phyto-chemical profile of ethanolic leaf extract of A. conyzoides and delivered Twenty eight major bio-active compounds and the peak area was prominent in (27.24 %)-9-octadecanamide, Phenol,4,6-di(1,1-dimethylethyl)2-methyl-(5.73 %), Phytol (5.92 %), Cyclopropane,1-methyl,2-octyl-(4.62 %), Dibutyl phthalate (4.92 %), á-Amyrin (4.06 %), Squalene (4.37 %) and Stigmasterol (3.08 %) respectively. Similar to their findings the present research also displayed 34 major bio-active compounds in Cm-Ac and the peak area percentage was maximum at Phenol, 2,4-Bis(1,1-Dimethylethyl)- (6.34 %).
Previous investigation of essential oil derived from A. conyzoides and their aerial parts delivered thirty two major bio-active compounds with major peak area percentage in Precocene II (45.75 %) and Precocene I (14.09 %) respectively. Also, their larvicidal screening against Asian tiger mosquito showed LC50 value of 61.22 μg/ml and their peak area compounds (Precocene I and Precocene II) showed 43.54 and 41.61 μg/ml respectively (Liu and Liu, 2014). Similar to the above findings, Cm-Ac also exhibited strong larvicidal activity on S. litura larvae with LC50 value of 2.467 log ppm. Plant derived crude extract has delivered a significant larval toxicity against the tobacco cutworm (Torres et al., 2003; Karthi et al., 2019; Ammar et al., 2020; Amala et al., 2021). Previously crude fruit pulp extracts of Citrullus colocynthis (L.) commercially known as bitter apple and their major bio-active chemical cucurbitacin E delivered significant larval mortality rate (93.8 %) against the tobacco cutworm S. litura with the discriminating dosage of 50 ppm (Ponsankar et al., 2020).
In addition, the sub-lethal dosage of Cm-Ac also showed significant larval and pupal duration against S. litura. The duration of larvae get extended in response to the sub-lethal concentration of Cm-Ac. The reduced feeding on leaves treated with Cm-Ac discriminating dosages may have subsidized to the extended period larval and pupal duration. Similar research were witnessed in the sub-lethal treatment of Yucca periculosa (commonly known as izote) crude extract tested against larval and pupa S. frugiperda and revealed that the pupal and larval weight gets declined significantly in dose dependent manner (Selin-Rani et al., 2016). Similarly, the methanolic crude extracts derived from the Alangium salvifolium (commonly known as sage-leaved alangium) showed significant larval and pupal growth rate reduction in S. litura. Our results are well paralleled with the previous findings Mahmoud and Hassan (2022) showed that the seed acetone and methanol extracts of Annona squamosa (commonly known as sugar-apples) have affected the pupae length, weight and pupal period of S. littoralis.
The major gut enzymes such as ACP, ALP and LDH are the major bio-markers which are most sensitive for the pesticide exposure (Murfadunnisa et al., 2019). The gut enzyme expression was considered as a crucial biomarker agent to spot the insect resistance exposed to toxic compounds (Vasantha-Srinivasan et al., 2018). Decreased level of ACP, ALP and LDH level in Cm-Ac treated larvae displays that the bioactive compounds of Cm-Ac had dropped the release of phosphorous for energy metabolism in insect results in metabolism rate reduction. In parallel, Senthil-Nathan et al. (2005) also found that the crude extract of Melia azedarach (chinaberry tree) declined the rate of gut enzymes of S. litura in dose dependent manner. Edwin et al. (2016) stated that the bio-active molecules are in direct to block the feeding rate of insect pests which in direct down-regulates the level of gut enzymes of insect pests. The above statement was well match with our results that the bio-active compounds of Cm-Ac significantly reduced the major gut enzymes (ACP, ALP and LDH) major digestive and detoxifying functions.
Botanical extracts and their active compounds plays a vital role in damaging mid-gut epithelium layer (EL) of insect pests (Thanigaivel et al., 2017). In parallel to the above statement, sub-lethal concentration of Cm-Ac significantly affected the mid-gut tissues like columnar cells, epithelial layer, brush border membrane and gut lumen of S. litura. These statements suggest that the major metabolites of Cm-Ac blocks the mid-gut cells and downregulates the development and growth rate of lepidopteran pest.
Eco-toxicological valuation of any chemical toxins in the environment was generally investigated using earthworm and considered as a vital endpoint (Wu et al., 2011). In general, chemical pesticides sprayed in the agriculture field to control pests has non-target toxicity on earthworms. These chemical pesticides has arrested the earthworm activity in soil composition (Tripathi et al., 2010). Our present study reveals that lethal dosage of Cm-Ac was harmless or less toxic as compared to the chemical toxin cypermethrin. In parallel to our study, Vasantha-Srinivasan et al. (2016) showed that crude volatile oil (CVO) derived from Piper betle (Linn.) delivered less toxic against Eudrilus eugeniae (Kinberg) as compared to chemical toxin cypermethrin. It is well established that, the toxicological screening may be supportive for screening the pesticide dosage acted upon the metabolic enzymes of earthworms (Pelosi et al., 2013). Moreover, Wang et al. (2012) stated that the artificial soil test will aid to determine the chemicals which are absorbed in the gut of earthworms during feeding. Our results, evident that the plant derived extracts Cm-Ac are harmless to soil-dwellers as compared to the commercial insecticides.
5 Conclusion
Overall, the present investigation delivers a chemical profile screening and baseline toxicity of Cm-Ac against the lepidopteran pest. The results are evident that the Cm-Ac are effective as larval toxicity, growth retardant, gut enzyme inhibition activity against the target tobacco worm and harmless against the beneficial earthworms and delivers a suitable pest management approach that declines the chemical burden of our society. Future perspectives will be directed to screen the toxicity of individual chemistries of Cm-Ac and novel formulations for better commercialization of herbal-based pesticides.
AUTHOR CONTRIBUTIONS:
Conceptualization, Methodology, PV and SK.; Software, RG and SK.; Validation, MC and NR; formal analysis, PV and MC.; Investigation, PV.; resources, SS and RG.; Data curation, SK.; writing-original draft preparation, PV and SK.; writing-review and editing, SS and KP.; supervision and project administration, SS; funding acquisition, KP. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Chiang Mai University, Thailand. The authors gratefully acknowledge the Researchers Supporting Project Number (RSP2023R46 5), King Saud University, Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Prabhakaran Vasantha-Srinivasan: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Pavana K. Sivadasan Unni: . Sengodan Karthi: Conceptualization, Methodology, Software, Data curation, Writing – original draft. Raja Ganesan: Software, Resources. Sengottayan Senthil-Nathan: Resources, Writing – review & editing, Supervision, Project administration. Muthiah Chellappandian: Validation, Formal analysis. Narayanaswamy Radhakrishnan: Validation. Rajakrishnan Rajagopal: . Krutmuang Patcharin: Writing – review & editing, Funding acquisition.
Acknowledgements
We would like to thank the Sri Paramakalyani Centre for Excellence in Environmental Sciences, Manonmaniam Sundaranar University and Saveetha Institute of Medical and Technical Sciences (SIMATS), for providing the infrastructural facility for carrying out this research work. In addition, this research work was partially supported by Chiang Mai University, Thailand.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A method of computing the effectiveness of an insecticide. J. Econ. Entomol.. 1925;18:265-267.
- [Google Scholar]
- Antihyperglycemic and antinociceptive activity of Fabaceae family plants–an evaluation of Mimosa pigra L. stems. Adv. Natural Appl. Sci.. 2012;6(8):1490-1495.
- [Google Scholar]
- Chemical profiles of leaf, stem, root and flower of Ageratum conyzoides. Asian J. Plant Sci. Res.. 2012;2(4):428-432.
- [Google Scholar]
- Larval and gut enzyme toxicity of n-hexane extract Epaltes pygmaea DC. against the arthropod vectors and its non-toxicity against aquatic predator. Toxin Rev.. 2021;40(4):681-691.
- [Google Scholar]
- Essential oils from three Algerian medicinal plants (Artemisia campestris, Pulicaria arabica, and Saccocalyx satureioides) as new botanical insecticides? Environ. Sci. Pollut. Res.. 2020;27(21):26594-26604.
- [Google Scholar]
- Effect of thiamethoxam on growth, biomass of rice varieties and its specialized herbivore, Scirpophaga incertulas Walker. Physiol. Mol. Plant Pathol.. 2018;101:146-155.
- [Google Scholar]
- Mosquito vectors and the spread of cancer: an overlooked connection? Parasitology Research. 2016;115:2131-2137.
- [Google Scholar]
- Arthropod-borne disease control at a glance: what’s new on drug development? Molecules. 2020;25(21):5175.
- [Google Scholar]
- Assessment of insecticidal action of 3-Isothiocyanato-1-propene on the growth and development of Spodoptera litura (Fab.)(Lepidoptera: Noctuidae) J. Entomol Zool. Stud.. 2016;4(5):1068-1073.
- [Google Scholar]
- Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol.. 2005;23(4):180-185.
- [Google Scholar]
- A new monomeric α-amylase inhibitor from the tetraploid emmer wheat is mostly active against stored product pests. J. Pest. Sci.. 2022;95(3):1401-1412.
- [Google Scholar]
- Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ. Int.. 2018;113:214-230.
- [Google Scholar]
- Volatile toxin of Limonia acidissima (L.) produced larvicidal, developmental, repellent, and adulticidal toxicity effects on Aedes aegypti (L.) Toxin Rev.. 2022;41(1):119-128.
- [Google Scholar]
- Target and non-target response of Swietenia Mahagoni Jacq. chemical constituents against tobacco cutworm Spodoptera litura Fab. and earthworm, Eudrilus eugeniae Kinb. Chemosphere.. 2018;199:35-43.
- [Google Scholar]
- Effects of temperature and nonionizing ultraviolet radiation treatments of eggs of five host insects on production of Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) for biological control applications. J. Asia Pac. Entomol.. 2016;19(4):1139-1144.
- [Google Scholar]
- Toxicity of Bioactive Molecule Andrographolide against Spodoptera litura Fab and Its Binding Potential with Detoxifying Enzyme Cytochrome P450. Molecules. 2021;26(19):5982.
- [Google Scholar]
- Statistical logic in the monitoring of reactions to therapeutic drugs. Methods Inf. Med.. 1971;10(04):237-245.
- [Google Scholar]
- Toxicity of secondary metabolites from Meliaceae against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) Neotrop. Entomol.. 2016;45(6):725-733.
- [Google Scholar]
- Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol.. 2006;51:45-66.
- [Google Scholar]
- Ageratum conyzoides L.: A review on its phytochemical and pharmacological profile. Int. J. Green Pharmacy.. 2008;2(2)
- [Google Scholar]
- Comparative efficacy of two mycotoxins against Spodoptera litura Fab. And their non-target activity against Eudrilus eugeniae Kinb. Ecotoxicol. Environ. Saf.. 2019;183:109474
- [Google Scholar]
- Larvicidal enzyme inhibition and repellent activity of red mangrove Rhizophora mucronata (Lam.) leaf extracts and their biomolecules against three medically challenging arthropod vectors. Molecules. 2020;25(17):3844.
- [Google Scholar]
- Evaluation of larvicidal activity of the essential oil of Ageratum conyzoides L. aerial parts and its major constituents against Aedes albopictus. Journal of Entomology and Zoology. Studies.. 2014;2(4):345-350.
- [Google Scholar]
- Phytosynthesis of silver nanoparticle (AgNPs) using aqueous leaf extract of Knoxia sumatrensis (Retz.) DC and their multi-potent biological activity: an eco-friendly approach. Molecules. 2022;27(22):7854.
- [Google Scholar]
- Insecticidal Activity of seed extracts of Annona squamosa L., against the cotton leafworm Spodoptera littoralis (Boisd.) Egypt. J. Chem.. 2022;65(6):1-2.
- [Google Scholar]
- Phytochemical screening and GC-MS analysis in ethanolic leaf extracts of Ageratum conyzoides (L.). World J. Pharm. Res.. 2016;5(7):1019-1029.
- [Google Scholar]
- Larvicidal and enzyme inhibition of essential oil from Spheranthus amaranthroids (Burm.) against lepidopteran pest Spodoptera litura (Fab.) and their impact on non-target earthworms. Biocatal. Agric. Biotechnol.. 2019;21:101324.
- [Google Scholar]
- Toxicity studies of antibiotics in earthworm, Eudrilus eugeniae. Int. J. Pure App. Biosci.. 2015;3:241-255.
- [Google Scholar]
- Biology, ecology and strategies for control of stored-grain beetles: a review. In: Beetles: Biodiversity, Ecology and Role in the Environment. New York: Nova Science Publishers Inc.; 2015. p. :105-122.
- [Google Scholar]
- Hypoglycaemic and antihyperglycaemic activity of Ageratum conyzoides L. in rats. Afr. J. Tradit. Complement. Altern. Med.. 2009;6(2)
- [Google Scholar]
- Searching for a more sensitive earthworm species to be used in pesticide homologation tests A meta-analysis. Chemosphere. 2013;90:895-900.
- [Google Scholar]
- Development, characterization, insecticidal and sublethal effects of Bunium persicum and Ziziphora clinopodioides-based essential oil nanoemulsions on Culex quinquefasciatus. Ind. Crop. Prod.. 2022;186:115249
- [Google Scholar]
- Amounts of pesticides reaching target pests: Environmental impacts and ethics. J Agric Environ Ethics. 1995;8:17-29.
- [CrossRef] [Google Scholar]
- Target and non-target toxicity of botanical insecticide derived from Couroupita guianensis L. flower against generalist herbivore, Spodoptera litura Fab. and an earthworm, Eisenia foetida Savigny. Ecotoxicol. Environ. Saf.. 2016;133:260-270.
- [Google Scholar]
- Response of Spodoptera litura Fab. (Lepidoptera: Noctuidae) larvae to Citrullus colocynthis L. (Cucurbitales: Cucurbitaceae) chemical constituents: larval tolerance, food utilization and detoxifying enzyme activities. Physiol. Mol. Plant Pathol.. 2018;101:16-28.
- [Google Scholar]
- Toxicity and developmental effect of cucurbitacin E from Citrullus colocynthis L. (Cucurbitales: Cucurbitaceae) against Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Environ. Sci. Pollut. Res.. 2020;27(19):23390-23401.
- [Google Scholar]
- Potential mode of action of a novel plumbagin as a mosquito repellent against the malarial vector Anopheles stephensi,(Culicidae: Diptera) Pestic. Biochem. Physiol.. 2016;134:84-93.
- [Google Scholar]
- Chemical characterization of billy goat weed extracts Ageratum conyzoides (Asteraceae) and their mosquitocidal activity against three blood-sucking pests and their non-toxicity against aquatic predators. Environ. Sci. Pollut. Res.. 2021;28(22):28456-28469.
- [Google Scholar]
- Toxicity of Alangium salvifolium Wang chemical constituents against the tobacco cutworm Spodoptera litura Fab. Pestic. Biochem. Physiol.. 2016;126:92-101.
- [Google Scholar]
- Alessandro, R.T.; Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere. 2016;165:257-267.
- [Google Scholar]
- Effects of Melia azedarach on nutritional physiology and enzyme activities of the rice leaffolder Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae) Pestic. Biochem. Physiol.. 2006;84(2):98-108.
- [Google Scholar]
- Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stål) Ecotoxicol. Environ. Saf.. 2008;70(2):244-250.
- [Google Scholar]
- Effect of biopesticides applied separately or together on nutritional indices of the rice leaffolder Cnaphalocrocis medinalis. Phytoparasitica. 2005;33(2):187-195.
- [Google Scholar]
- Efficacy of nucleopolyhedrovirus and azadirachtin on Spodoptera litura Fabricius (Lepidoptera: Noctuidae) Biol. Control. 2005;34(1):93-98.
- [Google Scholar]
- Efficacy of Precocene I from Desmosstachya bipinnata as an Effective Bioactive Molecules against the Spodoptera litura Fab. and Its Impact on Eisenia fetida Savigny. Molecules. 2021;26(21):6384.
- [Google Scholar]
- Chemicals isolated from Justicia adhatoda Linn reduce fitness of the mosquito, Aedes aegypti L. Arch. Insect Biochem. Physiol.. 2017;94(4):21384.
- [Google Scholar]
- Development of an eco-friendly mosquitocidal agent from Alangium salvifolium against the dengue vector Aedes aegypti and its biosafety on the aquatic predator. Environ. Sci. Pollut. Res.. 2018;25(11):10340-10352.
- [Google Scholar]
- Antioxidant and insect growth regulatory activities of stilbenes and extracts from Yucca periculosa. Phytochemistry. 2003;64(2):463-473.
- [Google Scholar]
- Comparative studies on carbofuran-induced changes in some cytoplasmic and mitochondrial enzymes and proteins of epigeic, anecic and endogeic earthworms. Pestic. Biochem. Physiol.. 2010;96:30-35.
- [Google Scholar]
- Developmental response of Spodoptera litura Fab. to treatments of crude volatile oil from Piper betle L. and evaluation of toxicity to earthworm, Eudrilus eugeniae Kinb. Chemosphere.. 2016;155:336-347.
- [Google Scholar]
- novel herbal product based on Piper betle and Sphaeranthus indicus essential oils: Toxicity, repellent activity and impact on detoxifying enzymes GST and CYP450 of Aedes aegypti Liston (Diptera: Culicidae) J. Asia Pac. Entomol.. 2018;21(4):1466-1472.
- [Google Scholar]
- Acute toxicity of chemical pesticides and plant-derived essential oil on the behavior and development of earthworms, Eudrilus eugeniae (Kinberg) and Eisenia fetida (Savigny) Environ. Sci. Pollut. Res.. 2018;25(11):10371-10382.
- [Google Scholar]
- The efficacy of methanolic extract of Swietenia mahagoni Jacq. (Meliaceae) and a commercial insecticide against laboratory and field strains of Aedes aegypti (Linn.) and their impact on its predator Toxorhnchites splendens. Biocatal. Agric. Biotechnol.. 2021;31:101915
- [Google Scholar]
- Comparative acute toxicity of twenty-four insecticides to earthworm, Eisenia fetida. Ecotoxicol. Environ. Saf.. 2012;79:122-128.
- [Google Scholar]
- Effects of phenanthrene on the mortality, growth, and anti-oxidant system of earthworms (Eisenia fetida) under laboratory conditions. Chemosphere. 2011;83(4):429-434.
- [Google Scholar]
- Phytochemical constituents and ethnopharmacological properties of Ageratum conyzoides L. Phytother. Res.. 2019;33(9):2163-2178.
- [Google Scholar]
- Toxicological screening of marine red algae Champia parvula (C. Agardh) against the dengue mosquito vector Aedes aegypti (Linn.) and its non-toxicity against three beneficial aquatic predators. Aquat. Toxicol.. 2020;222:105474
- [Google Scholar]
- Feasibility, limitation and possible solutions of RNAi-based technology for insect pest control. Insect Sci.. 2013;20(1):15-30.
- [Google Scholar]
- Effects of Artemisia annua L. (Asteracea) on the digestive enzymatic profiles and the cellular immune reactions of the Sunn pest, Eurygaster integriceps (Heteroptera: Scutellaridae), against Beauveria bassiana. Bull. Entomol. Res.. 2010;100(2):185-196.
- [Google Scholar]







