Translate this page into:
Bacterial etiology, antibiotic resistance profile and foot ulcer associated amputations in individuals with Type II diabetes mellitus
⁎Corresponding author at: Central Research Laboratory, Meenakshi Academy of Higher Education and Research (Deemed to be University), Chennai, Tamil Nadu, India. n_arunagiri@yahoo.co.in (N. Arunagirinathan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
In this study, bacterial etiology, antibiotic resistance profile and clinical outcomes of foot ulcer associated amputations in persons with diabetes were analyzed.
Methods
A total of 126 persons with Diabetic Foot Ulcer (DFU) admitted in surgical ward from June 2016 to May 2018 were included in this study. Foot ulcers were categorized as per Wagner's classification. Tissue samples obtained from the ulcers were processed for bacterial cultures using conventional techniques. Antibiotic sensitivity of the isolates was done by Kirby-Bauer’s Disc Diffusion method.
Results
Out of 126 persons with diabetes, 74.6% of them had diabetes for 1–5 years. The minimum and maximum levels of HbA1c were 3% and 10.7%, respectively. Majority (58.7%) of the persons with diabetes had foot ulcers for less than a month. Wagner’s grade 3 ulcer was found to be high (38.1%) among the studied patients. Amputation due to foot ulcer was done in 28.6% of patients of which most of them were under insulin therapy. About 75% of amputated patients had DFU which was complicated by Gram-negative bacterial (GNB) infections. Escherichia coli (27.4%) was the predominant bacterium isolated among the GNB where as Staphylococcus aureus (13.4%) was the predominant one among Gram-positive bacteria. S. aureus isolates were highly resistant to penicillin (85%) followed by gentamicin (45.5%) and ciprofloxacin (40.9%). Enterobacteriaceae isolates showed high level of resistance to co-trimoxazole (84.4%). Imipenem was the most sensitive drug (100%) against GNB isolated from DFU.
Conclusion
In this study, the Gram-negative bacteria are the major aetiological agents isolated from the amputated patients with diabetes mellitus. Incidence of multi-drug resistant bacteria associated DFU could be the major burden and limit the availability of antibiotic treatment regimens.
Keywords
Diabetic foot ulcer
Escherichia coli
Imipenem
Amputation
1 Introduction
Diabetes mellitus is a major global non-communical disease (Singh, 2005). As per the WHO report, about 422 million people suffer from diabetes mellitus especially in developing countries and around 1.5 million deaths occur each year worldwide (WHO webpage, last updated on 5th April 2023). The global prevalence of diabetes is estimated to be around 578 million by 2030 and it will be further rising to around 700 million by 2045 (Saeedi et al., 2019). Diabetic foot ulcer (DFU) is one of the serious long-term complications of diabetic mellitus with the life time risk upto 25% (Centers for Disease Control and Prevention (CDC), 2005; Deribe, 2014) and it is the leading cause of hospitalization (20%) in developing countries like India (Goldstein et al., 1996; Shankar et al., 2005). Various clinical factors such as neuropathy, vasculopathy and immunopathy predispose the development of DFU (Alexiadou and Doupis, 2012; Bowering, 2001; Brem et al., 2004; Kumar et al., 1994; Tesfaye et al., 1996). Majority (69–80%) of foot ulcers heal, while 10–15% of them will remain active and 5–24% of them will lead to limb amputation within a period of 6–18 months after the first evaluation (Alexiadou and Doupis, 2012; Moxey et al., 2011). Amputation was required more often for patients with deep soft-tissue infection, either alone or in combination with osteomyelitis (Eneroth et al., 1997; van Baal, 2004).
DFU is typically caused by multiple microbial pathogens (polymicrobial) and especially by aerobic Gram-positive cocci (Staphylococcus aureus), Gram-negative bacilli (Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa), and anaerobes (Saseedharan et al., 2018; Zenelaj et al., 2014). Tissue culture from DFU and antibiotic susceptibility testing play important role in the appropriate treatment (Uçkay et al., 2015). DFU is mainly treated with prolonged duration of antibiotic therapy using broad-spectrum antibiotics which was the major clinical risk factor in the development of multidrug-resistant bacteria (Harbarth et al., 2000). Healthcare-associated transmission of drug-resistant bacteria is also likely to happen when DFU patients are hospitalized or under podiatric care in specialized centers (Agostinho et al., 2013). In this study, bacterial etiology, antibiotic resistant profile and clinical outcomes of persons with diabetic foot ulcers in and around Kanchipuram District of Tamil Nadu, India were analyzed.
2 Materials and methods
2.1 Diabetes patients with foot ulcer
In this study, a total of 126 persons with diabetic foot ulcer suffering from type II diabetes mellitus admitted (from June 2016 to May 2018) in surgical ward at Meenakshi Medical College and Research Institute were included. Among 126 patients, 52 (41.3%) were from Kanchipuram, 18 (14.3%) from Arakkonam, 13 (10.3%) from Walaja, 9 (7.1%) from Cheyyar, 8 (6.3%) from Vellore and the remaining 26 (20.6%) were from Sholingur, Arcot, Thiruthani, Arani and Ranipet town areas. This study was approved by the Institutional Ethical Clearance Committee (Approval number 125/MMCH&RI/2016). Informed consent was obtained from all the study patients. As we focused on DFU in diabetic II patients without Peripheral Arterial Disease (PAD) in this study, PAD cases and type 1 diabetes mellitus cases were excluded. A detailed history such as duration of diabetes, its type and onset, duration and progression of DFU were collected. Compliance and control of diabetes details were also obtained. A detailed foot examination was performed and ulcers were categorized as per Wagner's classification (Oyibo et al., 2001). DFU was classified as Grade 0 (intact skin), Grade 1 (superficial ulcer), Grade 2 (deep ulcer to tendon, bone or joint), Grade 3 (deep ulcer with abscess or osteomyelitis), Grade 4 (forefoot gangrene) and Grade 5 (whole foot gangrene).
2.2 Bacterial identification
After DFU grading, deep tissue samples were obtained from the floor of ulcer. Specimens were transported in sterile containers to microbiology laboratory for bacterial culture. Specimen was inoculated on MacConkey agar with 0.5% sodium taurocholate, MacConkey agar with 0.15% bile salts, blood agar (5%) and chocolate agar plates for bacterial isolation. MacConkey agar plates were incubated at 35 ± 2 °C, while blood and chocolate agar plates were incubated at 35 ± 2 °C in a candle jar under CO2 environment. Following incubation for 18 to 24 h, the plates were examined for growth and the colonies isolated were identified by conventional bacterial culture techniques (Collins et al., 2004).
2.3 Antibiotic susceptibility test
Antibiotic susceptibility of bacterial isolates was performed using standard antibiotics such as amikacin, amoxicillin-clavulanic acid, ceftazidime, cefuroxime, cefpodoxime, ciprofloxacin, co-trimoxazole, erythromycin, gentamicin, imipenem, penicillin, and oxacillin by Kirby-Bauer disc (Bauer et al., 1966) diffusion method according to CLSI guidelines (CLSI, 2020). In this method, bacterial lawn culture was made using culture suspension adjusted to 0.5 McFarland’s standards of test bacterial isolates on Mueller-Hinton agar (MHA) plates. Zones of inhibition were measured in millimeter (mm) using Zone Diameter Breakpoints provided in the CLSI guidelines for interpretation of antibiotic susceptibility test results as Sensitive, Intermediate and Resistance.
2.4 Statistical analysis
Statistical analysis was done using SPSS (statistical package for social science), version 21. Chi square test was used to find out the difference in proportion between two groups. p value < 0.05 was considered as significant.
3 Results
Out of 126 persons with diabetic foot ulcer included in this study, 69.8% (n = 88) were males and 30.1% (n = 38) were females. The mean age of DFU patients was 55 years (both males and females). It was noted that 74.6% (n = 94) of patients had diabetes mellitus for 1–5 years. The minimum and maximum levels of HbA1c were 3% and 10.7%, respectively. Those patients with the age limit between 50 and 60 years had high incidence of foot ulcers. Further, DFU was developed spontaneously without any definite history of trauma among 65.9% (n = 83) of patients and 34.1% (n = 43) of patients with DFU had history of trauma. It was noted that 16.7% (n = 21) had systemic hypertension and 27.8% (n = 35) had anemia (Table 1). Also, 58.7% (n = 74) of diabetic patients had foot ulcer complications for less than a month. Majority of the patients had Wagner’s grade 3 ulcer (38.1%; n = 48) followed by Grade 4 ulcer (22.2%; n = 28). About 71.4% (n = 90) of the foot ulcers were treated conservatively of which 34.4% (n = 31) underwent split skin grafting (SSG) (Table 1). Off-loading of the foot was done with Plaster of Paris (POP) cast and a window over the ulcer site. Male Female 16–30 31–45 46–60 61–75 76–90 Less than a month 1–6 months More than 6 months Less than a year 1–5 years More than 5 years Oral Hypoglycaemic Agents Insulin Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 Conservative Amputation
Parameter
Percentage
Gender
70 (n = 88)
30 (n = 38)
Age
1.6 (n = 2)
19 (n = 24)
53.2 (n = 67)
23 (n = 29)
3.2 (n = 4)
Duration of ulcer
58.7 (n = 74)
27.8 (n = 35)
13.5 (n = 17)
Duration of Diabetes Mellitus
15.9 (n = 20)
74.6 (n = 94)
9.5 (n = 12)
Treatment of Diabetes mellitus
51.6 (n = 65)
48.4 (n = 61)
Wagner Grading
4 (n = 5)
35.7 (n = 45)
38.1(n = 48)
22.2 (n = 28)
0 (n = 0)
Treatment of Ulcer
71.4 (n = 90)
28.6 (n = 36)
3.1 Amputation due to foot ulcer
Amputation was done in 28.6% (n = 36) of patients and among amputated patients, high percentage (87.8%; n = 28) of DFU belongs to Wagner grade 4 followed by Wagner grade 3 ulcer (22.2%; n = 8). Statistical analysis revealed that higher proportion (80.6%; n = 29) of amputated patients were found to be on insulin therapy (p value < 0.001) (Fig. 1). Furthermore, ray amputation was done in more number (80.5%; n = 29) of patients followed by forefoot amputation (5.5%; n = 2) and below knee amputation (13.9%; n = 5) (Fig. 2).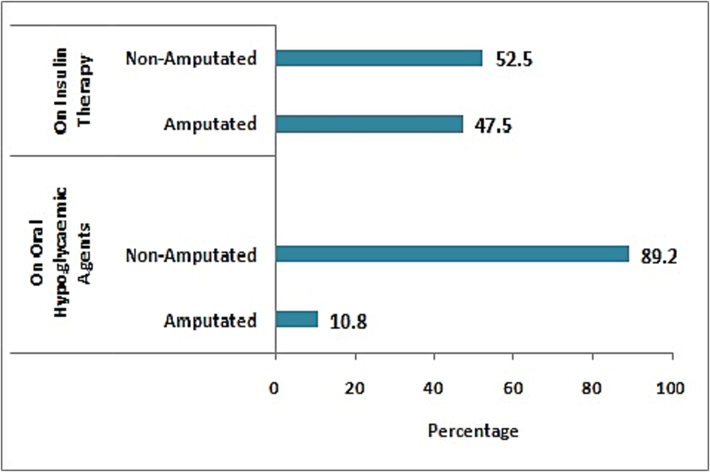
Anti-diabetic therapies given to diabetic patients.
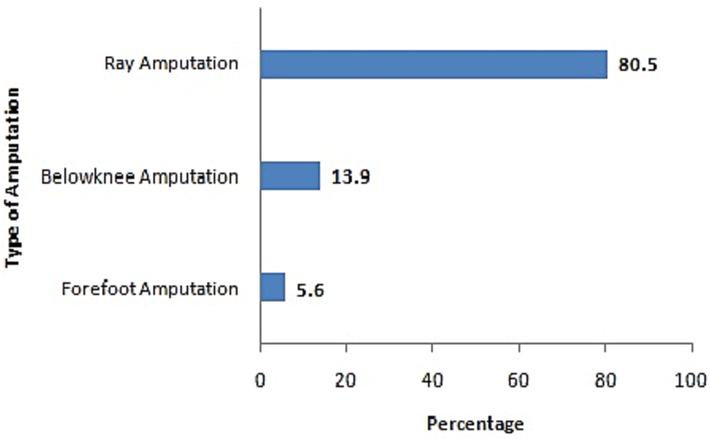
Percentage of amputations performed in diabetic patients with foot ulcer.
3.2 Bacterial isolates from foot ulcer
A total of 164 bacterial strains were isolated from 126 tissue samples collected from DFU. Of 164 bacterial isolates, 65.9% (n = 108) were Gram-negative bacteria (GNB) and among them E. coli (27.4%; n = 45) was the predominantly isolated GNB. Gram-positive bacteria (GPB) were 34.1% (n = 56) and among them S. aureus was (13.4%; n = 22) the most commonly isolated GPB. Other bacterial species isolated from DFU are given in Table 2. Out of 36 amputated patients, GNB were isolated from tissue samples of 27 (75%) patients and GPB from 9 (25%) patients. It was found that among amputated patients, E. coli (33.3%) was the major bacterial isolate causing DFU followed by K. pneumoniae (13.9%), coagulase negative Staphylococcus spp. (13.9%) and S. aureus (11.1%). Other bacterial species isolated from persons with DFU that led to amputation are given in Fig. 3. It was observed that more than one bacterial pathogens were isolated from 38 DFU tissue samples (Table 3).
Organisms
Positivity (Total number: 164)
Number (n)
Percentage (%)
Gram-negative bacteria
108
65.9
Escherichia coli
45
27.4
Klebsiella sp.
20
12.2
Pseudomonas aeruginosa
19
11.6
Proteus sp.
19
11.6
Acinetobacter baumannii
4
2.4
Enterobacter sp.
1
0.6
Gram-positive bacteria
56
34.1
Staphylococcus aureus
22
13.4
Coagulase negative Staphylococcus sp.
22
13.4
Streptococcus pyogenes
6
3.7
Enterococci sp.
5
3.0
Diptheroids
1
0.6
Isolated Bacterial Pathogens
Percentage (n)
Non-amputated Persons with Diabetes
Escherichia coli and Staphylococcus aureus
18.4 (n = 7)
Escherichia coli and Proteus sp.
10.5 (n = 4)
Klebsiella sp. and Proteus sp.
7.9 (n = 3)
Klebsiella sp. and Pseudomonas aeruginosa
10.5 (n = 4)
Escherichia coli and Enterococcus sp.
5.3 (n = 2)
Escherichia coli and Proteus sp.
5.3 (n = 2)
Pseudomonas aeruginosa and Streptococcus pyogenes
2.6 (n = 1)
Acinetobacter sp. and Coagulase negative staphylococcus
5.3 (n = 2)
Escherichia coli and Klebsiella sp.
2.6 (n = 1)
Escherichia coli and Pseudomonas aeruginosa
5.3 (n = 2)
Klebsiella sp. and Staphylococcus aureus
2.6 (n = 1)
Coagulase negative staphylococcus and Klebsiella sp.
2.6 (n = 1)
Coagulase negative staphylococcus and Pseudomonas aeruginosa
2.6 (n = 1)
Amputated Persons with Diabetes
Escherichia coli and Enterococcus sp.
2.6 (n = 1)
Escherichia coli and Staphylococcus aureus
2.6 (n = 1)
Escherichia coli and Proteus sp.
5.3 (n = 2)
Klebsiella sp. and Pseudomonas aeruginosa
2.6 (n = 1)
Klebsiella sp. and Proteus sp.
2.6 (n = 1)
Pseudomonas aeruginosa and Streptococcus pyogenes
2.6 (n = 1)
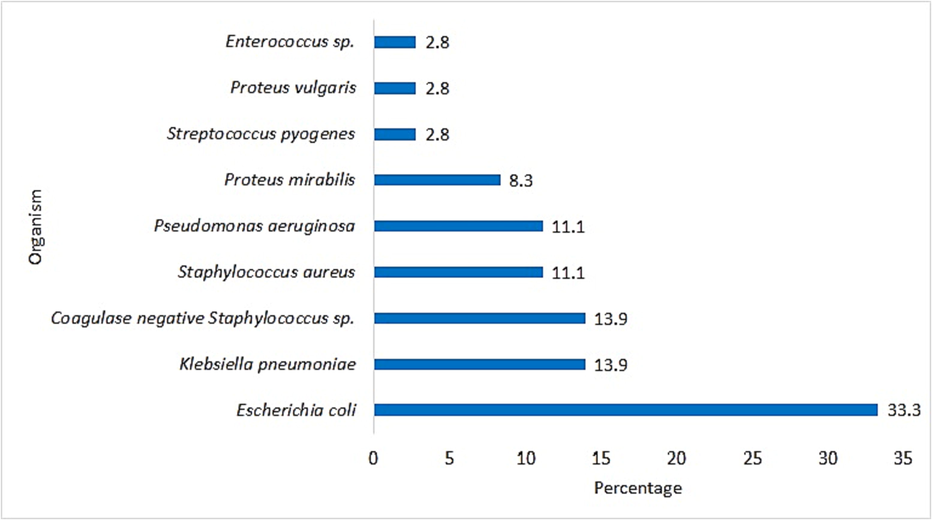
Percentage of bacterial species isolated from diabetic foot ulcers in amputated patients.
3.3 Antibiotic susceptibility of the bacterial isolates
Among Enterobacteriaceae isolates, E. coli showed high level of resistance to co-trimoxazole (90.2%) followed by amoxicillin-clavulanic acid (73.2%), ciprofloxacin (70.7%) and ceftazidime (58.5%). K. pneumoniae showed high-level of resistance to co-trimoxazole (80%) followed by amoxicillin-clavulanic acid (75%), ceftazidime (55%), ciprofloxacin and gentamicin (50%). P. mirabilis exhibited high level of resistance to gentamicin (78.6%) followed by co-trimoxazole and amoxicillin-clavulanic acid (71.4%), ceftazidime (64.3%) and ciprofloxacin (50%). Imipenem showed 100% sensitivity to E. coli and P. vulgaris, 95% to K. pneumoniae and 85.7% to P. mirabilis. S. aureus showed high level of resistance to penicillin (68.2%) followed by gentamicin (45.5%). P. aeruginosa exhibited high level of resistance to co-trimoxazole (63.2%) followed by amoxicillin-clavulanic acid (57.9%) and ceftazidime (52.6%) and 100% sensitivity to imipenem (Fig. 4). Furthermore, 13.6% of the bacterial isolates were found to be Methicillin-Resistant Staphylococcus aureus (MRSA).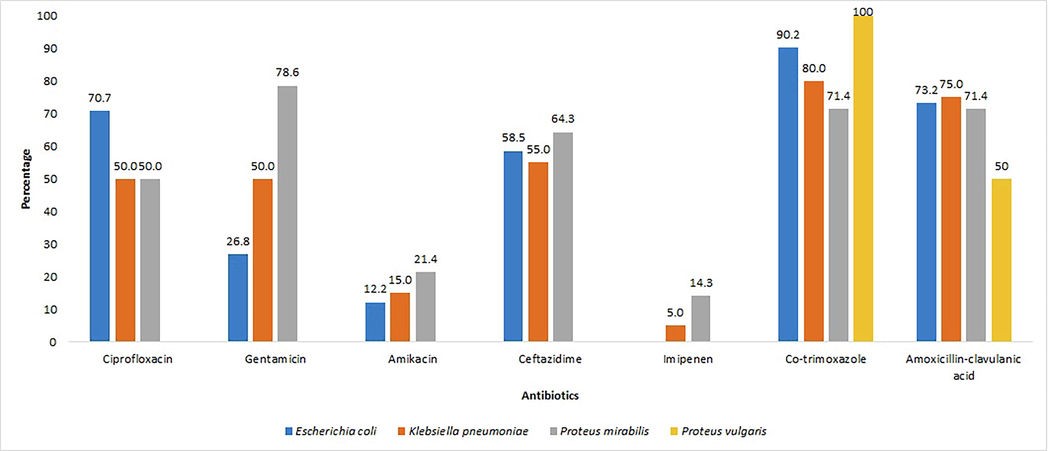
Antibiotic resistance profile of Enterobacteriaceae isolates from diabetic patients with foot ulcer.
4 Discussion
Diabetes mellitus is the most serious chronic disease and it has high global prevalence (8.8%) and it is still on the rise (Standl et al., 2019). Foot ulcers in diabetes mellitus patients cause high rate of morbidity and mortality which lead to non-traumatic amputations worldwide (Ogba et al., 2019). In this study, the bacterial etiology and clinical outcomes of foot ulcers among persons with diabetes in and around Kanchipuram district, Tamil Nadu, India were studied. In the present study, diabetes mellitus was found in higher level in males as compared to females. In this study, glycated hemoglobin (HbA1c) was assessed in all the diabetic patients for screeneing their glucemic control. The minimum and maximum levels of HbA1c were 3% and 10.7%, respectively. HbA1c is an indicator of long-term glycemic control and its frequent increase is an independent risk factor for coronary heart disease and stroke in persons with diabetes (Martín-Timón, 2014; Sherwani et al., 2016). In this study, a very minimal difference was observed in the types of treatment such as oral hypoglycemic drugs (51.6%) and insulin therapy (48.4%) given to patients with DFU.
Increased prevalence of diabetes accelerates the complications such as foot ulcers and lower extremity amputations (Centers for Disease Control and Prevention (CDC), 2006; Hobizal and Wukich, 2012). In our study, more of spontaneously developed (59.5%) foot ulcer was noted among persons with diabetes when compared to that of post traumatic ulcers (34.1%). Split skin graft was done in patients (34.9%) who had large size ulcers and for ulcers which might take a longer duration for secondary healing. Grading of foot ulcers revealed that Wagner grade 3 was mostly noted in this study population followed by Wagner grade 2 and Wagner grade 4. Early identification of foot ulcers and prompt treatment may optimize the patient’s outcome and provide limb salvage (Hobizal and Wukich, 2012).
Amputation was done in 28.6% of persons with diabetes who had DFU of Wagner grade 4 ulcers (77.8%) followed by Wagner grade 3 ulcers (22.2%). Wagner grading plays a crucial role in the decision making for amputation. The indication for amputation was overwhelming infection of the ulcer with impending septicemia which was life threatening. In this study, it was also observed that majority of amputated patients (80.55%) were under insulin therapy. It indicated that there was no proper monitoring of the insulin intake with poor glycemic control resulting in DFU and amputation. Non-amputated patients were advised to have regular follow up for glycemic control and foot care. DFU causes negative impact on patient’s life quality and economic burden; hence prevention of DFU is crucial (Prompers et al., 2008).
Most of the persons with diabetes would have received antimicrobial treatment prior to tissue sampling and some samples may yield false negative results. Clinicians may have to rely solely on their clinical considerations (Turhan et al., 2013). In this study, more of GNB (65.9%) as compared to GPB (34.1%) were isolated from DFU. In a prospective study by Banu et al. (2015), 75.6% of the DFU were caused by GNB and only 24.4% were by GPB. They reported that E. coli (24.4%) was the most predominantly isolated bacterium. In our study, majority of amputated patients had DFU caused by GNB (75%) and among them E. coli was the predominant (33.3%) organism followed by K. pneumoniae (13.9%), P. aeruginosa (11.1%), P. mirabilis (8.3%), and P. vulgaris (2.8%). Furthermore, among GPB, coagulase negative Staphylococcus (13.9%) was the predominant organism followed by S. aureus (11.1%), Streptococcus pyogenes (2.8%), and Enterococcus sp. (2.8%). Banu et al. (2015) reported that all the specimens from DFU yielded monomicrobial isolates. Many studies showed that DFUs were mostly caused by polymicrobial infections (Garg et al., 2008; Pathare et al., 1998). The monomicrobial nature of infection is associated with the duration of the DFU and antimicrobial treatment. Earlier in the infection, the monomicrobial state prevails and as the infection progresses with time, a polymicrobial state arises. Sasikumar et al. (2018) reported that all the persons with diabetes were on antimicrobial treatment during sampling and only the multidrug-resistant organisms not responding to the treatment would have been cultured. In our current study, antibiotic susceptibility of isolates revealed that imipenem (100%) was highly sensitive antibiotic followed by amikacin (85.7%) against bacterial isolates belonging to Enterobacteriaceae and imipenem exhibited 100% sensitivity against P. aeruginosa isolates. Cervantes-García et al. (2015) reported that 34% of S. aureus isolated from DFU of type 2 diabetes mellitus patients were resistant to Methicillin. In this study, only 13% of S.aureus isolates showed resistance to Methicillin. Treatment for DFU was mainly conservative while 71.4% were treated by wound debridement. Appropriate diabetic care is required to reduce the amputations and the detrimental effects of amputations found in diabetic patients with foot ulcer can be avoided by maintaining good glycemic control and high standard of foot care (Sasikumar et al., 2018).
5 Conclusion
In this study, the Gram-negative bacteria are the major aetiological agents isolated from the amputated patients with diabetic mellitus. Incidence of multi-drug resistant bacteria associated diabetic foot ulcers could be the major burden and threaten the treatment by limiting the availability of proper antibiotic regimens. Therefore, besides personal hygiene and foot care, good glycemic control is required for treatment of patients with diabetic foot ulcers in order to reduce the risk of amputations.
Acknowledgement
The authors extend their appreciation to the Researchers Supporting Project Number RSP2023R190, King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Epidemiology and acquisition of extended-spectrum beta-lactamase-producing Enterobacteriaceae in a septic orthopedic ward. Springerplus. 2013;2:91.
- [CrossRef] [Google Scholar]
- Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas. Med. J. 2015;8:280-285.
- [CrossRef] [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45:493-496.
- [Google Scholar]
- Diabetic foot ulcers. Pathophysiology, assessment, and therapy. Can. Fam. Physician. 2001;47:1007-1016.
- [Google Scholar]
- Protocol for treatment of diabetic foot ulcers. Am. J. Surg.. 2004;187:S1-S10.
- [CrossRef] [Google Scholar]
- Lower extremity disease among persons aged > or =40 years with and without diabetes–United States, 1999–2002. MMWR Morb. Mortal. Wkly Rep.. 2005;54:1158-1160.
- [Google Scholar]
- Geographic disparities in diabetes-related amputations–Texas-Mexico border, 2003. MMWR Morb. Mortal. Wkly. Rep.. 2006;55:1251-1253.
- [Google Scholar]
- Infections of diabetic foot ulcers with methicillin-resistant Staphylococcus aureus. Int. J. Low. Extrem. Wounds. 2015;14:44-49.
- [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing; Thirtieth Informational Supplement (M100–S30) Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
- [Google Scholar]
- Collins & Lyne’s Microbiological Methods (8th ed.). London: Edward Arnold; 2004.
- Prevalence and factors influencing diabetic foot ulcer among diabetic patients attending arbaminch hospital, South Ethiopia. J. Diabetes Metab.. 2014;05:1000322.
- [CrossRef] [Google Scholar]
- Clinical characteristics and outcome in 223 diabetic patients with deep foot infections. Foot Ankle Int.. 1997;18:716-722.
- [CrossRef] [Google Scholar]
- Spectrum of microbial flora in diabetic foot ulcers. Indian J. Pathol. Microbiol.. 2008;51:204.
- [CrossRef] [Google Scholar]
- Diabetic Foot Infections: Bacteriology and activity of 10 oral antimicrobial agents against bacteria isolated from consecutive cases. Diabetes Care. 1996;19:638-641.
- [CrossRef] [Google Scholar]
- Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-2921.
- [CrossRef] [Google Scholar]
- Diabetic foot infections: current concept review. Diabetic Foot & Ankle. 2012;3:18409.
- [CrossRef] [Google Scholar]
- The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet. Med.. 1994;11:480-484.
- [CrossRef] [Google Scholar]
- Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? WJD. 2014;5:444.
- [CrossRef] [Google Scholar]
- Lower extremity amputations - a review of global variability in incidence: Lower extremity amputations-a global review. Diabet. Med.. 2011;28:1144-1153.
- [CrossRef] [Google Scholar]
- Aerobic bacteria associated with diabetic foot ulcers and their susceptibility pattern. Biomed. Dermatol.. 2019;3:1.
- [CrossRef] [Google Scholar]
- A comparison of two diabetic foot ulcer classification systems. Diabetes Care. 2001;24:84-88.
- [CrossRef] [Google Scholar]
- Diabetic foot infections: a study of microorganisms associated with the different Wagner grades. Indian J. Pathol. Microbiol.. 1998;41:437-441.
- [Google Scholar]
- Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia. 2008;51:1826-1834.
- [CrossRef] [Google Scholar]
- Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract.. 2019;157:107843
- [CrossRef] [Google Scholar]
- Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz. J. Microbiol.. 2018;49:401-406.
- [CrossRef] [Google Scholar]
- Clinico-microbiological profile of septic diabetic foot with special reference to anaerobic infection. Cureus 10, e2252. 2018
- [CrossRef] [Google Scholar]
- Bacterial etiology of diabetic foot infections in South India. Eur. J. Intern. Med.. 2005;16:567-570.
- [CrossRef] [Google Scholar]
- Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights. 2016;11:95-104.
- [CrossRef] [Google Scholar]
- Preventing foot ulcers in patients with diabetes. J. Am. Med. Assoc.. 2005;293:217.
- [CrossRef] [Google Scholar]
- The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur. J. Prev. Cardiolog.. 2019;26:7-14.
- [CrossRef] [Google Scholar]
- Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39:1377-1384.
- [CrossRef] [Google Scholar]
- Increasing incidence of Gram-negative organisms in bacterial agents isolated from diabetic foot ulcers. J. Infect. Dev. Ctries.. 2013;7:707-712.
- [CrossRef] [Google Scholar]
- Diabetic foot infections: what have we learned in the last 30 years? Int. J. Infect. Dis.. 2015;40:81-91.
- [CrossRef] [Google Scholar]
- Surgical treatment of the infected diabetic foot. Clin. Infect. Dis.. 2004;39(Suppl 2):S123-S128.
- [CrossRef] [Google Scholar]
- World Health Organization. Diabetes. WHO. Geneva. (last updated on 5th April 2023, accessed on September 17, 2023) https://www.who.int/health-topics/diabetes#tab=tab_1.
- Do diabetic foot infections with methicillin-resistant Staphylococcus aureus differ from those with other pathogens? Int. J. Low. Extrem. Wounds. 2014;13:263-272.
- [CrossRef] [Google Scholar]







