Translate this page into:
Aucubin mitigates nonylphenol-induced renal damage by attenuating apoptosis, oxidative stress and histopathological profile
⁎Corresponding author. nailaraighafoor357@gmail.com (Naila Ghafoor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nonylphenol (NP) is potent noxious pollutant which is documented to induce nephrotoxicity via escalating the levels of oxidative stress in renal tissues. Aucubin (AUC) is a novel phytochemical having tremendous pharmacological abilities. This research aimed to estimate the efficacy of AUC against NP intoxicated renal impairment in male albino rats. 48 albino rats were randomly assigned into four different groups viz. control group, NP administered group (50 mg/kg), NP + AUC administrated (50 mg/kg + 40 mg/kg) group and AUC treated (40 mg/kg) group. Our results showed that NP intoxication raised urinary proteins, creatinine, urea, urobilinogen, NGAL and KIM-1 levels. Besides, NP exposure lowered the levels of creatinine clearance and albumin. Furthermore, NP treatment lowered level of total protein, activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione S-transferase (GST), glutathione reductase (GSR) & glutathione (GSH) contents along with the level of total antioxidant status (TAS) however, raising the oxidative stress markers levels Thiobarbituric acid reactive substance (TBARS), reactive oxygen species (ROS), hydrogen peroxide (H2O2) & total oxidant status (TOS). Additionally, following the NP administration, the levels of Bax, caspase-9, & caspase-3 were augmented however the Bcl-2 level was decreased. Furthermore, rats exposed to NP showed various histopathological disruptions. Whereas treatment with AUC showed a palliative effect against NP-induced renal damage, restoring all alterations to normal levels. Taken together, AUC administration convalesced NP-induced kidney impairment.
Keywords
Aucubin
Curative potential
Renal damage
Nonylphenol
1 Introduction
Nonylphenol (NP) is a man-made xenobiotic with the potential to generate toxicity in various organs (Lalonde and Garron; 2021). NP is reported as a volatile, hazardous, and persistent chemical (Hong et al., 2020). NP is commonly used in various industries to manufacture surfactants, dyes, plastic products, rubbers, resins, fibers as well as pesticides (Noorimotlagh et al., 2020). Eye contact, skin contact, absorption, inhalation, and intake of vegetables, milk, fruits, and cereals are all plausible routes of NP exposure. Because of its hydrophobicity and prolonged half-life, NP can persist and build up in the environment, posing several health risks to both animals and human beings (Shen et al., 2014).
Biotransformation and accumulation of NP are critical elements in developing NP-induced kidney impairment (Perazella, 2012). NP can cause severe toxicities in the body, including immunotoxicity, neurotoxicity, embryotoxicity, reproductive toxicity, and nephrotoxicity (Soares et al., 2008). The primary mechanism through which NP provokes toxicity is through excessive production of ROS, which causes an increase in LPO as well as an imbalance of oxidants and antioxidants (Rehman et al., 2022). Antioxidant-oxidant imbalance destroys the DNA, lipids, and proteins resulting in oxidative stress in different organs, including kidney (Ratliff et al., 2016). Kidney is well-known for being the centre of filtration and regulatory processes of fluid maintenance, as well as a place for detoxification and removal of metabolic wastes (Mori et al., 2005). It is documented that NP accumulates in the kidneys causing a rapid disruption in KIM-1and NGAL. NP interacts with estrogen receptors on kidney endothelial cells to cause renal tubular injury and nephropathy (Ding et al., 2007). NP has tendency to induce inflammation and mitochondrial dysfunction in renal tissues, which subsequently leads to hyperbilirubinemia (Kruger et al., 2015).
Plants are often considered an active and effective source of medicine, so, to cure various ailments, scientists are looking for phytochemicals with antioxidant capabilities (Alsharari et al., 2016). Aucubin (AUC) is an important member of iridoids that are found in variety of plants with diverse pharmacological effects (Regginato et al., 2020). It is extracted from many plants, including the rubber tree and the traditional Chinese plant Aucuba japonica (Shen et al., 2019). AUC has been demonstrated to exhibit anti-apoptotic, neuroprotective, anti-inflammatory, anti-carcinogenic, & antioxidant potentials (Yang et al., 2018). Therefore, this investigation was conducted to ascertain the curative potential of AUC against NP instigated renal toxicity.
2 Materials and methods
2.1 Chemicals
NP and AUC were obtained from Merch (USA).
2.2 Animals
Albino rats (200 ± 20, age 10–12 weeks) were caged at animal house of University of Agriculture, Faisalabad. Tap water & chaw feed was provided to all animals. The animals were given standard laboratory conditions (25 ± 2 °C temp.), humidity (55 ± 10) as well as equal period of light and dark (12 h) and acclimatized for 7 days. The protocol provided by “European Union for animal Care and Experimentation” was followed to handle the rats.
2.3 Experimental protocol
Forty-eight rats (190–210 g) were categorized into four groups (n = 12). Only water and food were supplied to group I, which served as control group. 50 mg/kg of NP was orally given to group II. Group III was treated orally with the NP and AUC (50 and 40 mg/kg, respectively). Group IV was given 40 mg/kg of AUC orally. Rats were anesthetized by using ketamin (60 mg/kg) + xylazine (5 mg/kg) and dissected at the end of the experiment. The estimation of the serum profile was determined by drawing blood. Following dissection, one kidney from each group was isolated and preserved at −80 degrees Celsius for histopathological examination while other was packed in zipper bag for the measurement of other parameters (biochemical). Before analysis, renal tissues were homogenized in PBS 3 mL having pH 7.4 and centrifugation was performed at 12000 rpm for 15 min.
2.4 Biochemical study
Activity of CAT was estimated by using approach given by Chance and Maehly (1955). To determine SOD activity, the procedure stated by Kakkar et al. (1984) was used. The protocol delineated by Habig et al. (1974) was employed to assess GST activity. GSR activity was observed by using the approach demonstrated by Carlberg and Mannervik (1975). The GSH contents were evaluated by following the process outlined by Jollow et al. (1974) was applied. The GPx activity was measured using the technique elucidated by Rotruck et al. (1973). The total protein levels were evaluated using the Bradford assay kit (BioRad, USA). TAS & TOS levels were evaluated by using the Rel Assay Diagnosing kit as well as the procedure delineated by Erel, (2005) & Erel, (2004).
2.5 Evaluation of TBARS, ROS and H2O2
The level of TBARS was observed as per the methodology given by Iqbal et al. (1996). ROS and H2O2 levels were measured using the (Pick and Keisari, 1981) technique.
2.6 Evaluation of kidney function markers
To assess the renal function markers including albumin, urobilinogen, urea, creatinine, creatinine clearance, and urinary proteins, standard diagnostic kits were used. KIM-1 & NGAL were measure by using standard ELISA kits manufacture by R & D system Chine Company limited.
2.7 Evaluation of apoptotic markers
The levels of Bax, caspase-3, caspase-9 and Bcl-2 were measured by using commercially available ELISA kits (Cusabio Technology Llc, Houston, TX, USA) as per the directions provided in manual book.
2.8 Histopathological assay
For fixation, renal tissues were fixed in a buffer solution of 10 % formalin. With the increasing alcohol concentrations (80 %, 90 %, and 100 %), dehydration was carried out. The tissues were entrenched in paraplast, and a microtomy was done. Using microtome, 3–4 μm thin sections were made and stained with haematoxylin/eosin. By using a light microscope, microphotography was performed at 40X.
2.9 Statistical analysis
The mean values obtained from the were displayed as Mean ± SEM. One-way ANOVA and the Tucky’s test were used to compare various groups using Minitab (V17). The level of significance was set at p < 0.05.
3 Results
3.1 Effects of AUC on antioxidant enzymes
NP inebriation noticeably (p < 0.05) diminished the level of total protein and antioxidant enzymes activities including SOD, CAT, GSH, GPx, GSR, & GST content as matched with the control. Combination of NP and AUC considerably (p < 0.05) increased total protein and antioxidant enzymes activities. Nevertheless, only AUC and control group showed almost same result with no significant difference (Table 1). Distinct superscripts on resulted values exhibiting notable difference from other groups.
Groups
CAT (U/mg protein)
GPx (U/mg protein)
SOD (U/mg protein)
GSR (nM NADPH
oxidized/min/mg tissue)
GST (nM/min/mg protein)
GSH (μM/g tissue)
Total
protein (µg/mg tissues)
Control
9.77 ± 1.05a
17.67 ± 1.60a
6.85 ± 0.05a
3.95 ± 0.09a
28.22 ± 0.87
18.7 ± 0.57ab
258.68 ± 3.74a
NP
4.63 ± 1.07b
7.46 ± 0.17a
3.10 ± 0.11c
1.40 ± 0.13c
10.42 ± 0.93
7.73 ± 0.66c
101.1 ± 4.76c
AUC + NP
8.39 ± 1.13a
12.81 ± 0.54b
5.48 ± 0.18b
2.66 ± 0.12b
23.36 ± 1.01
15.60 ± 0.66b
190.89 ± 4.76b
AUC
9.83 ± 1.47a
0.97 ± 0.56c
6.87 ± 0.08a
3.99 ± 0.09a
28.31 ± 0.95
18.98 ± 1.97a
263.77 ± 4.06a
3.2 Effects of AUC on oxidants
In NP-intoxicated group, TBARS, ROS, H2O2, and TOS levels were notably (p < 0.05) higher while the level of TAS was lowered in contrast to control group. Conversely, co-treatment with NP + AUC brought a prominent (p < 0.05) reduction in TBARS, ROS, H2O2, and TOS levels while the level of TAS was increased in renal tissues. Only AUC-treated group showed a significant change in TBARS, ROS, H2O2, TOS, and TAS levels. However, no prominent discrepancies were found in the values of only AUC supplemented and untreated group (Table 2). Distinct superscripts on resulted values exhibiting notable difference from other groups.
Groups
TBARS
(nM/min/mg tissue)
ROS
(U/mg tissue)
H2O2 (nM/min/mg protein)
TOS
(µmol/L)
TAS
(mmol/L)
Control
1.06 ± 0.10c
0.85 ± 0.09c
1.25 ± 0.11c
8.10 ± 0.08c
3.02 ± 0.06a
NP
7.12 ± 0.32a
3.76 ± 0.07a
7.60 ± 1.02a
18.60 ± 0.25a
1.12 ± 0.05c
AUC + NP
2.44 ± 0.10b
1.71 ± 0.13b
2.54 ± 0.16b
12.95 ± 0.08b
2.62 ± 0.03b
AUC
1.02 ± 0.12c
0.79 ± 0.08c
1.22 ± 0.09c
8.97 ± 0.08c
3.05 ± 0.06a
3.3 Effects of AUC on renal function profile
NP provision brought a prominent (p < 0.05) rise in urea, creatinine, urobilinogen, urinary protein, NGAL and KIM-1 levels. Besides, albumin and creatinine clearance were lowered considerably (p < 0.05) compared to the control group. Nevertheless, AUC + NP exposure demonstrated a counter-effect to NP by decreasing urinary proteins, creatinine, urobilinogen, urea, KIM-1, and NGAL levels however restored creatinine clearance and serum albumin concentration. However, there was no discernible difference kidney function marker levels between the only AUC treated and control group (Table 3). Distinct superscripts on resulted values exhibiting notable difference from other groups.
Groups
Urea
(mg/dl)
Creatinine
(mg/dl)
Creatinine Clearance
(ml/min)
Albumin
(mg/dl)
Urobilinogen
(mg/dl)
Urinary
Proteins
(mg/dl)
KIM-1
(mg/ml)
NGAL
(ng/day)
Control
19.77 ± 0.60c
1.52 ± 0.08bc
1.56 ± 0.05a
8.36 ± 0.26a
1.65 ± 0.07b
10.73 ± 1.19
0.37 ± 0.02c
0.71 ± 0.80c
NP
75.42 ± 1.74a
7.62 ± 0.15a
0.57 ± 0.08c
1.86 ± 0.11c
11.11 ± 0.86a
39.22 ± 1.87
3.74 ± 0.07a
5.48 ± 0.16a
AUC + NP
29.49 ± 0.71b
2.14 ± 0.21b
1.01 ± 0.05b
6.51 ± 0.06b
2.70 ± 0.23b
20.79 ± 0.92
1.14 ± 0.06b
1.43 ± 0.07b
AUC
18.48 ± 0.70c
1.44 ± 0.87c
1.53 ± 0.05a
7.43 ± 0.25a
1.60 ± 0.06b
10.72 ± 1.06
0.36 ± 0.02c
0.69 ± 0.07c
3.4 Effects of AUC on apoptotic biomarkers
NP inebriation cause a prominent (p < 0.05) rise in caspase-3, Bax, and caspase-9 levels while decreasing Bcl-2 level in the renal tissues. Whereas co-treatment with AUC + NP brought a noteworthy (p < 0.05) escalation in level of Bcl-2 but diminishing the Bax, caspase-9 & caspase-3 levels in comparison to control. Furthermore, values of apoptotic marker levels in control group were near to the values of AUC only supplemented group as illustrated in Table 4. Distinct superscripts on resulted values exhibiting notable difference from other groups.
Groups
Bax (pg/mL)
Bcl-2 (ng/mL)
Caspase-3 (pg/mL)
Caspase-9 (pg/mL)
Control
2.21 ± 0.23a
21.27 ± 1.16a
1.18 ± 0.22a
2.25 ± 0.26a
NP
9.76 ± 0.57b
7.00 ± 0.26b
14.40 ± 0.68b
24.71 ± 1.08b
AUC + NP
3.21 ± 0.19c
15.59 ± 0.72c
3.18 ± 0.35c
3.89 ± 0.21c
AUC
2.13 ± 0.26a
22.23 ± 1.57a
1.16 ± 0.17a
2.21 ± 0.23a
3.5 Effect of AUC on the architecture of renal tissues
The control and AUC (only) treated groups both had normal renal tissue histology. In NP-intoxicated group, histopathological abnormalities were found. Damaged corticular sections and necrotic cell aggregates were seen in the malpighian body. Inflammatory cell penetration, tubular stretching, Bowman capsule constriction, and blood vessel packing were also seen in the corticular and medullary sections. However, co-administration of NP and AUC decreased the renal damage by restoring glomerular deterioration, interstitial fibrosis, and normal renal tissues (Fig. 1).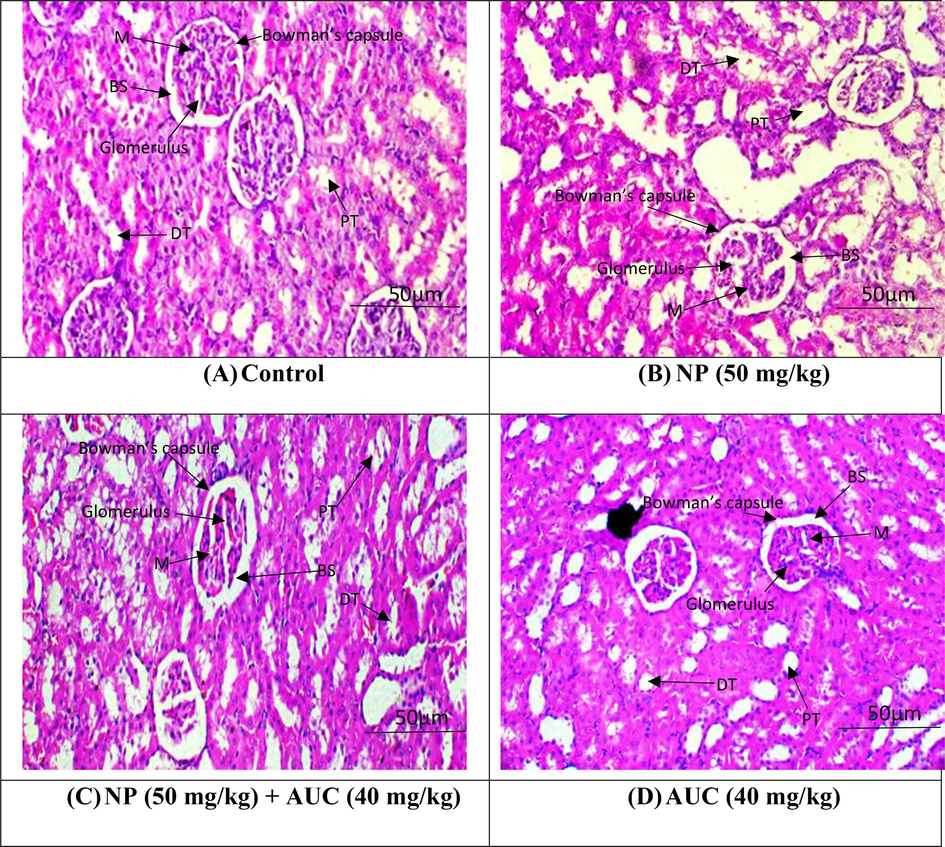
Nephroprotective effect of ACU on NP induced alterations in kidney histology. A) Control group B) NP group (50 mg/kg b.wt) C) NP (50 mg/kg b.wt) + AUC (40 mg/kg b.wt) group D) AUC group (40 mg/kg b. wt).
4 Discussion
Our investigation was aimed to evaluate the nephroprotective impacts of AUC against NP-prompted kidney tissue damage in male albino rats. In this investigation, NP provision diminished the level of total protein & antioxidant enzymes activities in renal tissues. SOD, GPx and CAT are the key antioxidant enzymes with a great potential to reduce the production of ROS (Bennett et al., 2008). The SOD enzyme can substantially prevent harmful OH• ions formation by converting O−2 into H2O2 (Ighodaro and Akinloye; 2018). CAT is a vital antioxidant enzyme that facilitates GSH and GPx in converting H2O2 into H2O and O2 (Bennett et al., 2008). AUC provision led to an escalation in level of total protein & activities of antioxidant enzymes Jeoung stated that the hydroxyl groups of AUC are the key elements, which may possibly contribute to its antioxidant characteristic to scavenge ROS (Jeong et al., 2002).
NP administration elevated the levels of ROS, TBARS, TOS, H2O2 while TAS level was downregulated. The elevation in the levels of lipid peroxidation was reflected by the increase in TBARS level. NP causes kidney damage by raising H2O2, which increases TBARS and ROS levels, resulting in a lower antioxidant index (Afsar et al., 2016). Increased lipid peroxidation, which is caused by decreased activity of cellular antioxidant enzymes & an increase in ROS, is a contributing factor to a number of adverse effects brought on by NP exposure in the body. Overall oxidation status is indicated by TOS (Sahreen et al., 2015). Whereas TAS reflects the total antioxidant state of the body (Erel, 2004). Reduction in the antioxidant enzyme was also reflected by the decrease in the value of TAS. While OS was revealed by the increase in TOS. However, co-treatment AUC brought down the levels of ROS, TBARS, TOS, H2O2 while increased the level of TAS, which may be ascribed its therapeutic potential.
Our study revealed that NP inebriation substantially increased the concentration of creatinine, urea, albumin, urinary proteins, creatinine clearance, and urobilinogen. According to Sahreen et al. (2015), urobilinogen is not a component of urine normally; the presence of a high concentration of urobilinogen in urine indicates renal damage. Kotb et al. (2018) reported that NP augmented blood urea and serum creatinine concentrations that indicated renal damage. Khan et al. (2010) elucidated that higher urea, creatinine, and urinary protein contents, along with a considerable diminution in albumin and creatinine clearance, are biomarkers of acute oxidative damage. The results of this research are in conformity with the outcomes of by Korkmaz et al. (2010) who reported that NP-intoxicated rats had elevated levels of urea, urobilinogen, and creatinine levels than the control group. However, AUC therapy reversed the altered concentrations of these markers, owing to the therapeutic impacts of AUC against NP-prompted kidney damage.
NP administration resulted in a considerable increase in NGAL and KIM-1 levels, reflecting serious renal injury. The measurement of the aforementioned kidney function biomarkers in the blood is a reliable method for analysing the effects of toxicants on kidney functions (Lalonde and Garron, 2021). Renal proximal tubular disruption causes a loss of membranous integrity that results in kidney dysfunction (Khan et al., 2010). Previous research has demonstrated that excessive production of ROS damages the membrane integrity of renal tissues, increasing the levels of renal biological markers such as KIM-1 and NGAL (Lalonde and Garron, 2021). Besides, AUC treatment reduced the levels of renal markers in blood serum. Therefore, it was deduced that AUC might exert palliative impact on the membranous structure of the kidney, which was evinced by the low levels of KIM-1 and NGAL (Mali and Dhake, 2011).
Our findings showed that exposure to NP elevated the levels of caspase-3, Bax, & caspase-9 while diminishing the Bcl-2 level. Bax is an apoptosis-promoting protein, while Bcl-2 is an anti-apoptosis protein (Subbaramaiah and Dannenberg, 2003). Apoptotic cell death results due to the relative imbalance of these proteins. Apoptosis is caused by a number of signals that change the mitochondrial membrane permeability which enhance the liberation of cytochrome C from the mitochondria (Klanova et al., 2022). Cytochrome C is a pro-apoptosis factor that is liberated from the outer membrane of mitochondria into the cytoplasm, where it links to Apaf-1 & pro-caspase-9 to activate caspase-9. Furthermore, caspase-9 provokes caspase-3 activation which ultimately induces various apoptotic events in cell (Budihardjo et al., 1999). Nonetheless, AUC treatment remarkably restored abovementioned apoptotic dysregulations owing to its antiapoptotic nature.
Histopathological analyses revealed that NP administration significantly caused structural damages such as degenerated proximal tubules, glomerular atrophy and annihilated Bowman’s capsule in the kidney tissues. The histopathological damages caused due to NP administration in our study are in consistent with the previous investigations in which NP exposure resulted in structural degenerations in kidney (Arya et al., 2011). NP exposure reduced the antioxidant enzymes activities and elevated the level of oxygen free radicals, resulting in OS subsequently causing lipid peroxidation and disruption of biomolecules such as DNA, lipids and protein that eventually resulted in renal toxicity. Ijaz et al. (2023) elucidated that excessive generation of ROS disrupts the normal architecture of renal tissues in albino rats. In a prior investigation, it was found that 4-NP intoxication produced glomerular degeneration, an increase in Bowman's space, and congestion (Shirdel et al., 2020; Kandemir et al., 2018). However, AUC administration remarkably reduced the NP induced histological damages in rat’s kidney which is attributed to its reno-protective abilities.
5 Conclusion
Our study elucidated the therapeutic potential of AUC to treat NP-induced kidney impairment. AUC exhibited significant protective effects against OS, inflammation, and apoptosis, which are the key aspects of NP prompted kidney toxicity. The alteration in the levels of antioxidants, kidney function markers, inflammatory indices, apoptotic markers, & histological disruptions were successfully alleviated by AUC treatment. The antioxidant, anti-apoptotic, & anti-inflammatory properties of AUC are linked to its nephroprotective efficacy.
Acknowledgement
The authors are grateful to the Researchers Supporting Project (RSPD2023R984), King Saud University, Riyadh, Saudi Arabia for the support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: involvement of multiple signal transduction pathways. Sci. Rep.. 2016;6(1):23077.
- [Google Scholar]
- Rutin attenuates Hepatotoxicity in high-cholesterol-diet-fed rats. Oxid. Med. Cell. Longev.. 2016;2016:5436745.
- [Google Scholar]
- Relationship between oxidative stress and apoptotic markers in lymphocytes of diabetic patients with chronic non healing wound. Diabetes. Res. Clin. Pract.. 2011;94(3):377-384.
- [Google Scholar]
- Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin. J. Am. Soc. Nephrol.. 2008;3(3):665.
- [Google Scholar]
- Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol.. 1999;15(1):269-290.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250(14):5475-5480.
- [Google Scholar]
- Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin. Immunol.. 2007;123(2):227-234.
- [Google Scholar]
- A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem.. 2004;37(4):277-285.
- [Google Scholar]
- A new automated colorimetric method for measuring total oxidant status. Clin. Biochem.. 2005;38(12):1103-1111.
- [Google Scholar]
- Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249(22):7130-7139.
- [Google Scholar]
- Nonylphenol occurrence, distribution, toxicity and analytical methods in freshwater. Environ. Chem. Lett.. 2020;18:2095-2106.
- [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria. Med. J.. 2018;54(4):287-293.
- [Google Scholar]
- Sciadopitysin attenuates paraquat induced renal toxicity by modulating Nrf-2/Keap-1 pathway in male albino rats. Asian J. Agric. Biol.. 2023;2023(4):2023110
- [Google Scholar]
- Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Rep.. 1996;2(6):385-391.
- [Google Scholar]
- Inhibition of TNF-α and IL-6 production by aucubin through blockade of NF-κB activation in RBL-2H3 mast cells. Cytokine. 2002;18(5):252-259.
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21(2):130-132.
- [Google Scholar]
- Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed. Pharmacother.. 2018;105:981-991.
- [Google Scholar]
- Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food. Chem. Toxicol.. 2010;48(8–9):2469-2476.
- [Google Scholar]
- Anti-apoptotic MCL1 protein represents critical survival molecule for most Burkitt lymphomas and BCL2-negative diffuse large B-cell lymphomas. Mol. Cancer. Ther.. 2022;21(1):89-99.
- [Google Scholar]
- Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food. Chem. Toxicol.. 2010;48(10):2865-2871.
- [Google Scholar]
- Protective effect of Nigella sativa on 4-nonylphenol-induced nephrotoxicity in Clarias gariepinus (Burchell, 1822) Sci. Total Environ.. 2018;619:692-699.
- [Google Scholar]
- Neutrophils: between host defence, immune modulation, and tissue injury. PLoS. Pathog.. 2015;11(3):e1004651
- [Google Scholar]
- Nonylphenol, octylphenol, and nonylphenol ethoxylates dissemination in the Canadian freshwater environment. Arch. Environ. Contam. Toxicol.. 2021;80(2):319-330.
- [Google Scholar]
- Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Invest.. 2005;115(3):610-621.
- [Google Scholar]
- Environmental exposure to nonylphenol and cancer progression Risk–A systematic review. Environ. Res.. 2020;184:109263
- [Google Scholar]
- Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin. J. Am. Soc. Nephrol.. 2012;7(10):1713-1721.
- [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytic stimuli. Cell. Immunol.. 1981;59(2):301-318.
- [Google Scholar]
- Oxidant mechanisms in renal injury and disease. Antioxid. Redox. Signal.. 2016;25(3):119-146.
- [Google Scholar]
- Antidiabetic and hypolipidemic potential of Campomanesia xanthocarpa seed extract obtained by supercritical CO 2. Braz. J. Biol.. 2020;81:621-631.
- [Google Scholar]
- Protective effects of aucubin against nonylphenol-induced liver toxicity by improving biochemical, inflammatory and histopathological indices. J. King. Saud. Univ. Sci.. 2022;34(4):102033
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588-590.
- [Google Scholar]
- Protective effects of Carissa opaca fruits against CCl4-induced oxidative kidney lipid peroxidation and trauma in rat. J. Food. Nutr. Res.. 2015;59(1):28438.
- [Google Scholar]
- Does nonylphenol promote the growth of uterine fibroids? Eur. J. Obstet. Gynecol. Reprod. Biol.. 2014;178:134-137.
- [Google Scholar]
- Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell. Mol. Med.. 2019;23(6):4063-4075.
- [Google Scholar]
- Disruptive effects of nonylphenol on reproductive hormones, antioxidant enzymes, and histology of liver, kidney and gonads in Caspian trout smolts. Comparative Biochem. Physiol. Part c: Toxicol. Pharmacol.. 2020;232:108756
- [Google Scholar]
- Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int.. 2008;34(7):1033-1049.
- [Google Scholar]
- Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends. Pharmacol. Sci.. 2003;24(2):96-102.
- [Google Scholar]
- Enrichment and purification of aucubin from Eucommia ulmoides ionic liquid extract using macroporous resins. J. Mater. Sci.. 2018;11(9):1758.
- [Google Scholar]







