Translate this page into:
Attenuative role of didymin against paraquat prompted cardiotoxicity in albino rats
⁎Corresponding author. alihamzatabassum7@gmail.com (Ali Hamza)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Paraquat (PQ) an herbicide that is commonly used to remove undesirable grasses. However, PQ also causes cardiac damage through reactive oxygen species (ROS) production. Didymin (DYD) is a dietary flavone, present in citrus fruits and campanula. DYD displays multiple therapeutic activities i.e., anti-oxidant, hepatoprotective, neuroprotective and free radical salvaging. Therefore, the present study was planned to explore the ameliorative effect of DYD against PQ instigated cardiac damage. 24 rats were separated into 4 groups, control, PQ administered PQ + DYD co-administered orally and DYD only administered group. PQ intoxication significantly reduced anti-oxidants including (SOD, GST, GSH, CAT, GPx and GSR activities, while increasing malondialdehyde (MDA) and ROS levels. PQ intoxication escalated the level of cardiac injury marker i.e., creatine kinase myoglobin binding (CK-MB), troponin, creatinine phosphokinase (CPK) and lactate dehydrogenase (LDH). PQ also augmented inflammatory markers i.e., (TNF-α, IL-1β, NF-κB, IL-6 levels and COX-2 activity). Moreover, PQ intoxication escalated the apoptotic proteins levels (Bax, Caspase-3 and Caspase-9), while decreasing Bcl-2 level. PQ intoxication also prompted histomorphological anomalies in the heart of rats. Conversely, DYD therapy restored all the anomalies and structural abnormalities owing to its cardioprotective potentials.
Keywords
Paraquat
Didymin
Cardiac damage
Apoptosis
Anti-oxidant
Inflammation
1 Introduction
Paraquat (PQ) is a persistent organic herbicide, broadly used to eliminate unwanted plantation in agricultural sector. However, PQ is highly toxic to animals as well as humans and associated with high death cases due to the lack of effective treatment (Pezzoli and Cereda, 2013). Death rate ranges from 60 % to 80 % in animals following the PQ exposure (Kuan et al. 2016). The excessive exposure to PQ may result in death in 3.5 h. Exposure to PQ occurs through inhalation and damages the skin, then it is dispersed in all the cells of the body through blood (Nikdad et al., 2009; Qian et al. 2019). PQ intoxication prompts excessive ROS production that disturb the anti-oxidant enzymes as well as cell functions (Cochemé and Murphy, 2008; Colle et al., 2020). Moreover, PQ intoxication leads to multiple organs damage i.e., lung, kidney, liver and heart (Ijaz et al., 2023a; Wang et al., 2017).
According to previous literature PQ has the potential to induce heart damage (Wang et al., 2017). It was reported that the exposure to PQ induces oxidative stress (OS) and ROS production that damage the cellular organelles and eventually leads to cellular death (Lei et al., 2017; Ge et al., 2010). Moreover, the intoxication of PQ induces disruption in myocardial contractive function, suppresses myocardial survival and results in cardiac failure (Vinciguerra et al., 2012; Ge et al., 2010). PQ exposure also induces histomorphological anomalies in heart of rats i.e., swelling in cardiac muscle fibers, edema in the interstitial tissues as well as distension and disorderly alignment of cardiac muscle fibers (Dong et al., 2013).
Flavonoids are plant-based chemicals that are widely used in medicine. They are found in fruits, nuts, vegetables, grains and legumes (Ijaz et al., 2023b). Didymin (DYD) is a dietary glycoside, reported to be present in campanula i.e., orange, bergamot and mandarin as well as citrus fruits. Citrus fruits are the major source of DYD and due to the ease of extraction DYD has been identified as economical, safe as well as effective therapeutic agent as it does not induce damage to normal cells (Singhal et al., 2017). DYD displays anti-oxidant (Calabrò et al., 2004), hepatoprotective (Huang et al., 2017), neuroprotective and free radical salvaging activities (Morelli et al., 2014). Under the consideration of above mentioned attributes, this study was planned to explore the ameliorative effect of DYD on PQ caused cardiac impairments in rats.
2 Materials and Methods
2.1 Chemicals
PQ and DYD were procured from Germany (Sigma Aldrich).
2.2 Animals
24 rats (170 ± 35 g) were selected from the Animal Room of University of Agriculture, Faisalabad (UAF). Rats were imprisoned in cages with normal circadian rhythms (12 hrs light/dark cycle), standard (21–25 °C), temperature as well as adequate amount of moisture (40–50 %). Rats were handled in compliance with the instructions of the European union of animal care and experimentation (CEE Council 86/ 609).
2.3 Experimental design
The animals (n = 24) were categorized into 4 groups (n = 6). Different doses of PQ and DYD were administrated to the following groups orally; Control, PQ exposed (5 mgkg−1), DYD + PQ exposed group (1 mgkg−1 and 5 mgkg−1 respectively) and DYD exposed group (1 mgkg−1). Rats were make sedative after 30 days of trial using ketamine (60 mg/kg) and xylazine (6 mg/kg), beheaded and trunk blood was drawn into sterilized cylinders using sterile syringes. To separate plasma, the sample of blood was underwent centrifugation for 10 min at 3000 rpm and kept at −20 °C for later evaluation. The heart was excised and cut into 2 halves. One was stored in 10 % formalin for histomorphological investigation and other half was kept at −80 °C in zipper bag for biochemical assessment.
2.4 Evaluation of biochemical parameters
CAT activity was quantified by using the protocol deliberated by Chance and Maehly (1955). Kakkar et al. (1984) technique was used to analyze SOD activity. GPx activity was appraised by following Jollow et al. (1974) approach. The level of GSR was examined via the protocol of Carlberg and Mannervik (1975). GST level was estimated by following the approach of Habig et al. (1974). Sedlak and Lindsay (1968) method was used to measure GSH activity. ROS content was measured through the procedure devised by Hayashi et al. (2007). MDA level was evaluated through the method of Ohkawa et al. (1978).
2.5 Evaluation of cardiac markers
Lactate dehydrogenase (LDH) level was assessed via the procedure of Bais and Philcox (1994). Whereas, CPK and CK-MB activities were appraised via enzymatic assay and immune inhibition assay respectively by using automated analyzer Cobas-Integra 400, (Tietz et al., 1983). Moreover, the technique stated by Panteghini et al. (2004) was followed to estimate troponin level.
2.6 Evaluation of inflammatory indices
Inflammatory indices were accessed using ELISA kits according to manufacturer's guidelines. The analyses were completed by using ELISA Plate as per the manufacturer’s directions.
2.7 Evaluation of apoptotic proteins
ELISA kits were employed to measure Caspase-3, Bax, Caspase-9 and Bcl-2 levels in compliance with the manufacturer’s directions (Cusabio Technology Llc, USA).
2.8 Histopathological study
For histomorphological examination the cardiac tissues were fixed in formalin (10 %) for 24 h, desiccated in alcohol & inserted in paraffin wax. Using a microtome, 4–5 μm thin slices were made and stain (H & E) was applied. Then the slides were examined under a light microscope (400X).
2.9 Statistical analysis
Data were shown as Mean ± SEM. One-way ANOVA and Tukey’s test was used to compare the result of different groups. The level of significance was set at P < 0.05.
3 Results
3.1 Therapeutic potential of DYD on oxidative stress parameters
PQ intoxication markedly (P < 0.05) suppressed the activity of anti-oxidants, while MDA & ROS levels were increased relative to the control. However, the co-treatment of PQ + DYD considerably augmented the anti-oxidants activities and lowered MDA and ROS contents relative to PQ poisoned rats. Additionally, in DYD only administered rats the parameters were almost near to the control group (Table 1). Values are shown on the basis of Mean ± SEM. The values with different superscripts in a row are significantly (p < 0.05) different from other groups. All the values in this table are based on 12 biological replicates per group with 3 technical replicates of each.
Parameters
Groups
Control
PQ
PQ + DYD
DYD
CAT (Umg−1 protein)
9.77 ± 0.98a
4.82 ± 0.41c
7.48 ± 0.15b
9.74 ± 0.97a
SOD (Umg−1 protein)
9.28 ± 0.19a
3.86 ± 0.22c
7.60 ± 0.17b
9.34 ± 0.20a
GPx (Umg−1 protein)
26.01 ± 2.02a
7.66 ± 0.41c
14.89 ± 0.84b
26.09 ± 2.02a
GSR (nM NADPH oxidized/min/mg tissue)
7.23 ± 0.36a
2.49 ± 0.24c
5.44 ± 0.18b
7.19 ± 0.36a
GST (nM/min/mg protein)
33.93 ± 1.10a
12.66 ± 1.28c
25.04 ± 1.46b
35.29 ± 0.83a
GSH (μM/g tissue)
17.41 ± 1.28a
4.45 ± 0.14c
12.26 ± 0.93b
17.39 ± 1.28a
ROS (Umg−1 tissue)
0.57 ± 0.03a
8.42 ± 0.06c
2.98 ± 0.08b
0.54 ± 0.04a
MDA (nmol/mg protein)
0.78 ± 0.03a
7.08 ± 0.13c
2.61 ± 0.11b
0.76 ± 0.03a
3.2 Therapeutic potential of DYD on cardiac markers
The poisoning of PQ considerably (P < 0.05) augmented CK-MB, LDH, CPK and troponin levels. Conversely, PQ + DYD administration considerably decreased these levels as matched with PQ poisoned group. Furthermore, in DYD only administered rats these parameters were close to the control group (Table 2). Values are shown on the basis of Mean ± SEM. The values with different superscripts in a row are significantly (p < 0.05) different from other groups. All the values in this table are based on 12 biological replicates per group with 3 technical replicates of each.
PARAMETERS
GROUPS
Control
PQ
PQ + DYD
DYD
LDH (mg/dl)
13.63 ± 0.58a
48.16 ± 1.64c
21.85 ± 1.12b
13.39 ± 0.55a
CPK (mcg/L)
183.07 ± 8.33a
365.78 ± 0.74c
252.08 ± 5.23b
181.27 ± 7.94a
CK-MB (ng/mL)
36.55 ± 1.64a
84.79 ± 1.30c
59.93 ± 2.70b
35.78 ± 1.52a
Troponin (pg/ml)
0.57 ± 0.04a
3.58 ± 0.06c
1.53 ± 0.09b
0.55 ± 0.04a
3.3 Therapeutic potential of DYD on inflammatory indices
PQ poisoning considerably (P < 0.05) augmented the inflammatory indices relative to control animals. Nevertheless, in PQ + DYD administered animals inflammatory indices were decreased relative to PQ exposed rats. Additionally, in DYD only administered rats inflammatory indices were almost near to control group (Table 3). Values are shown on the basis of Mean ± SEM. The values with different superscripts in a row are significantly (p < 0.05) different from other groups. All the values in this table are based on 12 biological replicates per group with 3 technical replicates of each.
PARAMETERS
GROUPS
Control
PQ
PQ + DYD
DYD
NF-κB (ngg−1 tissue)
14.92 ± 1.24a
82.11 ± 1.39c
37.54 ± 0.98b
13.79 ± 1.23a
TNF-α (ngg−1 tissue)
8.37 ± 0.49a
23.08 ± 1.25c
13.44 ± 0.86b
8.25 ± 0.49a
IL-1β (ngg−1 tissue)
23.75 ± 1.31a
72.52 ± 1.82c
46.21 ± 1.12b
23.65 ± 1.27a
IL-6 (ngg−1 tissue)
7.17 ± 0.27a
46.19 ± 1.63c
15.95 ± 0.96b
7.11 ± 0.28a
COX-2 (ngg−1 tissue)
15.49 ± 0.94a
54.86 ± 0.76c
25.91 ± 0.82b
15.47 ± 0.94a
3.4 Therapeutic potential of DYD on apoptotic markers
PQ administration considerably (P < 0.05) upregulated Caspase-3, Bax and Caspase-9 levels, besides Bcl-2 level was decreased in contrast to the control animals. Nevertheless, PQ + DYD treatment significantly increased Bcl-2, besides suppressed Caspase-9, Bax and Caspase-3 levels as matched with PQ exposed animals. Furthermore, the above-mentioned levels were comparable in only DYD administered and the rats (Table 4). Values are shown on the basis of Mean ± SEM. The values with different superscripts in a row are significantly (p < 0.05) different from other groups. All the values in this table are based on 12 biological replicates per group with 3 technical replicates of each.
PARAMETERS
GROUPS
Control
PQ
PQ + DYD
DYD
Bcl-2 (ng/mg Protein)
15.29 ± 0.92a
4.24 ± 0.27c
8.37 ± 3.36b
15.48 ± 1.03a
Bax (ng/mg Protein)
1.73 ± 0.05a
7.61 ± 0.15c
2.47 ± 0.25b
1.71 ± 0.04a
Caspase-3 (pg/mL)
1.48 ± 0.14a
11.86 ± 0.76c
2.68 ± 0.12b
1.44 ± 0.13a
Caspase-9 (pg/mL)
2.54 ± 0.16a
16.73 ± 1.18c
3.37 ± 0.15b
2.51 ± 0.15a
3.5 Therapeutic potential of DYD on histopathology
The poisoning of PQ considerably prompted histopathological anomalies in heart i.e., inflammation, oedema and degeneration in myofibrils structure relative to the control animals. However, supplementation of DYD + PQ remarkably improved the histological profile of the heart and restored all the damages. Furthermore, in only DYD administered rats histological profile was comparable to the control rats (Fig. 1).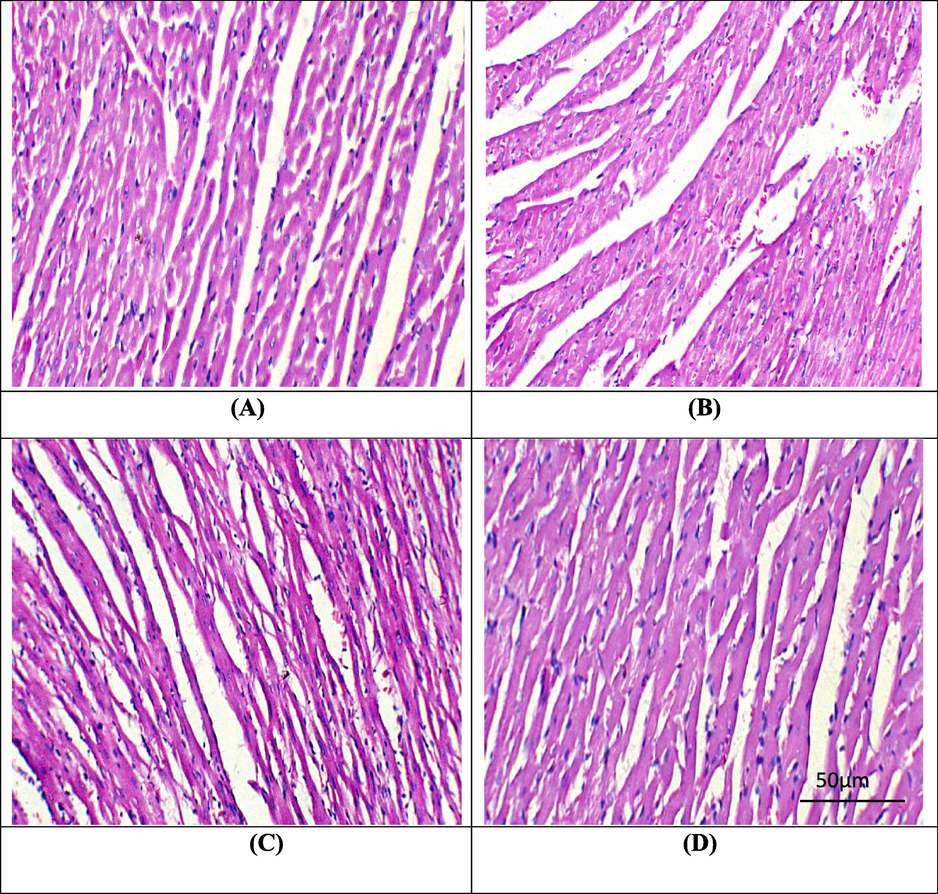
Microphotographs of cardiac tissues. A) Control group showing normal histomarphology. B) PQ administration prompted hazardous damages in cardiac tissues. C) PQ + DYD supplemented group presenting restored histomarphology of cardiac tissues. D) DYD supplemented group displaying normal histomarphology almost similar to control. PQ: Paraquat; DYD: Didymin.
4 Discussion
PQ exposure markedly lowered anti-oxidants activity, besides augmented ROS and MDA contents. The endogenous-anti-oxidants protect the biological molecules via reducing the ROS level. The major primary enzymes CAT aids in the conversion of H2O2 into O2 and H2O (Safhi et al., 2016). SOD plays an important role in the conversion of O2 to H2O2. GSR keeps the normal concentration of GSH that helps to maintain GPx activity, GPx protects from OS by decreasing H2O2 (Stinghen et al., 2014; Deponte, 2013), besides GST facilitates the process of detoxification (Allocati et al., 2018). MDA is a direct indicator of LP that is produced in high concentration as a result of free radicals. According to previous literature anti-oxidants possesses strong potentials for the prevention of multiple diseases. Over the last 10 years, studies have concentrated on the importance of anti-oxidants generated from plants as possible treatments for the toxicities related disorders (Alvi et al., 2022; Ishtiaq et al., 2022). The outcomes of the present study revealed that DYD supplementation escalated anti-oxidants activity, while reducing the MDA and ROS levels owing to its anti-oxidative property.
PQ intoxication considerably increased the levels of CK-MB, troponin, CPK and LDH. Cardiac injury markers are discharged into the blood following the damage to heart. CK-MB diagnostic test is performed to check multiple coronary disorders i.e., acute myocardial infraction. Moreover, the level of above-mentioned parameters also revealed the strength of myocardia injury (Christenson et al., 1997). However, the co-treatment of PQ + DYD significantly reduced the level of above-mentioned parameters that may be attributed to its cardioprotective potential.
The administration of PQ augmented the levels of inflammatory markers in PQ poisoned rats. According to Kandemir et al. (2018), a major transcription factor NF-κB encourages IL-6, TNF-α, COX-2 and IL-1β through the upregulation of genes that are linked to severe inflammation. It was demonstrated by Gandhi et al. (2017), COX-2 is a critical indicator that also plays a crucial function in inflammation. Nevertheless, the co-treatment of PQ + DYD lowered inflammatory indices that might be credited to its anti-inflammatory property.
PQ poisoning suppressed Bcl-2 level, while increasing Caspase-9, Bax and Caspase-3 levels. Bax is an apoptotic marker that boosts apoptosis; on the other hand Bcl-2 prevents the apoptosis and acts as anti-apoptotic protein. Reduction in Bcl-2 level and an escalation in Bax changes mitochondrial membrane permeability and prompts an increased discharge of cytochrome C (Cyto C) into the cytosol (Opferman and Kothari, 2018; Yang et al., 2017). The increased Cyto C stimulates Caspase-9, which triggers Caspase-3, resulting in cellular proteins apoptosis as well as degradation (Chipuk and Green, 2009). However, DYD supplementation resulted in a substantial reduction in Caspase-9, Bax as well as Caspase-3 level besides escalating Bcl-2 level.
PQ administration provoked considerable structural anomalies in the heart of rats i.e., myocardial damage, alterations in cellular morphology as well as inflammation, fibrosis. Regular cardiac histomarphology is responsible for retaining the structural and functional reliability of heart. These structural irregularities disrupt cardiac contractility and myocardial perfusion that eventually lead to multiple heart related anomalies. According to Vinciguerra et al. (2012) PQ induced damage heart, predominantly myocardial infarction by inducing OS that eventually results in heart failure. However, the co-treatment of PQ + DYD significantly decreased these histopathological damages and restored the histology of heart due to its cardioprotective property.
5 Conclusion
PQ induced cardiotoxicity through the generation of OS, disturbing the levels of cardiac injury, apoptotic and inflammatory markers as well as histological profile of heart. However, DYD therapy improved all these anomalies & histomorphological injuries owing to its cardioprotective, anti-apoptotic, anti-oxidant and anti-inflammatory nature. Based on these findings, it can be assumed that DYD can be used as a drug to remedy heart related disorders in future.
CRediT authorship contribution statement
Ali Hamza: Conceptualization, Investigation, Methodology, Writing – original draft. Moazama Batool: Data curation, Formal analysis, Software, Visualization. Mudassar Yaseen: Formal analysis, Methodology, Validation, Writing – original draft. Naila Ghafoor: Data curation, Formal analysis, Validation, Visualization. Mukhtar Ahmed: Funding acquisition, Resources, Software, Writing – original draft. Mian Nadeem Riaz: Formal analysis, Visualization, Writing – review & editing.
Acknowledgement
The authors are grateful to the Researchers Supporting Project Number (RSPD2024R984), King Saud University, Riyadh, Saudi Arabia for the support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7:11-15.
- [Google Scholar]
- Nephroprotective Effects of Delphinidin against Bisphenol A Induced Kidney Damage in Rats. Pak. Vet. J.. 2022;43(1):189-193.
- [Google Scholar]
- IFCC methods for the measurement of catalytic concentration of enzymes. Part 8. IFCC methods for lactate dehydrogenase (L-lactate: NAD oxidoreductase, EC 1.1.1.27) J. Autom. Chem.. 1994;16:167-182.
- [Google Scholar]
- Study of the extraction procedure by experimental design and validation of a LC method for determination of flavonoids in Citrus bergamia juice. J. Pharm. Biomed. Anal.. 2004;35:349-363.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chemis.. 1975;250:5475-5480.
- [CrossRef] [Google Scholar]
- Chance, B. and Maehly, A.C., 1955. [136] Assay of catalases and peroxidases. DOI: 10.1016/S0076-6879(55)02300-8.
- PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis. Cell Cycle.. 2009;28:2692-2696.
- [CrossRef] [Google Scholar]
- Cardiac markers in the assessment of acute coronary syndromes. Md. Med. J.. 1997;1985:18-24.
- [Google Scholar]
- Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem.. 2008;283:1786-1798.
- [Google Scholar]
- Early postnatal exposure to paraquat and maneb in mice increases nigrostriatal dopaminergic susceptibility to a Re-challenge with the same pesticides at adulthood: implications for Parkinson’s disease. Neurotox. Res.. 2020;37:210-226.
- [Google Scholar]
- Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. Gen. Sub.. 2013;1830:321732-321766.
- [Google Scholar]
- Toll-like receptor 4 is involved in myocardial damage following paraquat poisoning in mice. Toxicology.. 2013;312:115-122.
- [Google Scholar]
- Role of modulator of inflammation cyclooxygenase-2 in gammaherpesvirus mediated tumorigenesis. Front. Microbiol.. 2017;8:538.
- [CrossRef] [Google Scholar]
- Cardiac-specific overexpression of catalase attenuates paraquat-induced myocardial geometric and contractile alteration. role of ER stress. Free Radic. Biol. Med.. 2010;49:2068-2077.
- [Google Scholar]
- Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [CrossRef] [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutation Research/genetic Toxicol. Environ. Mutagenesis.. 2007;631:55-61.
- [CrossRef] [Google Scholar]
- Didymin ameliorates hepatic injury through inhibition of MAPK and NF-κB pathways by up-regulating RKIP expression. Int. Immunopharmacol.. 2017;42:130-138.
- [Google Scholar]
- Sciadopitysin attenuates paraquat induced renal toxicity by modulating Nrf 2/Keap-1 pathway in male albino rats. Asian J. Agric. Biol.. 2023;(4),:2023110.
- [Google Scholar]
- Tectochrysin attenuates cisplatin-induced hepatotoxicity by restoring biochemical, inflammatory and histological profile in rats. Pak. Vet. J. 2023
- [Google Scholar]
- Therapeutic effect of oroxylin a against bisphenol A-induced kidney damage in rats: a histological and biochemical study. Pak. Vet. J.. 2022;42(4):511-516.
- [CrossRef] [Google Scholar]
- Bromobenzene-induced liver necrosis. protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology.. 1974;11:151-169.
- [CrossRef] [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [CrossRef] [Google Scholar]
- Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed. Pharmacother.. 2018;105:981-991.
- [Google Scholar]
- Toll-like receptor 4 ablation rescues against paraquat-triggered myocardial dysfunction: role of ER stress and apoptosis. Environ. Toxicol.. 2017;32:656-668.
- [Google Scholar]
- Neuroprotective effect of didymin on hydrogen peroxide-induced injury in the neuronal membrane system. Cells. Tissues. Organs.. 2014;199:184-200.
- [Google Scholar]
- Antioxidative effects of nano-curcumin on liver mitochondria func-tion in Paraquat-induced oxidative stress. J. Cell. Mol. Med.. 2009;8:37-42.
- [Google Scholar]
- Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J. Lipid Res.. 1978;19:1053-1057.
- [CrossRef] [Google Scholar]
- Anti-apoptotic BCL-2 family members in development. Cell Death Differ.. 2018;25:37-45.
- [CrossRef] [Google Scholar]
- Standardization of immunoassays for measurement of myoglobin in serum. Clin. Chim. Acta.. 2004;341:65-72.
- [Google Scholar]
- Exposure to pesticides or solvents and risk of Parkinson disease. Neurology.. 2013;80:2035-2041.
- [Google Scholar]
- Cadmium-induced nephrotoxicity via oxidative stress in male Wistar rats and capsaicin protects its toxicity. Bull. Environ. Pharmacol. Sci.. 2016;5:5-11.
- [Google Scholar]
- Estimation of total protein-bound, and nonprotein sulfhydryl groups in tissue with ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [CrossRef] [Google Scholar]
- Didymin: an orally active citrus flavonoid for targeting neuroblastoma. Oncotarget.. 2017;8:29428-29441.
- [Google Scholar]
- Differential effects of indoxyl sulfate and inorganic phospShate in a murine cerebral endothelial cell line (bEnd. 3) Toxins. 2014;6:1742-1760.
- [Google Scholar]
- Tietz, N., Rinker, A. and Shaw, L., 1983. IFCC methods for the measurement of catalytic concentration of enzymes. Part 5. IFCC method for alkaline phosphatase (orthophosphoricmonoester phosphohydrolase, alkaline optimum, EC 3.1 3.1). IFCC Document Stage 2, Draft 1, 1983-03 with a view to an IFCC Recommendation. Clin. Chim. Acta. 135, 339F.
- mIGF- 1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell.. 2012;11:139-149.
- [Google Scholar]
- Ablation of Akt2 prevents paraquat-induced myocardial mitochondrial injury and contractile dysfunction: role of Nrf2. Toxicology Letters.. 2017;269:1-14.
- [CrossRef] [Google Scholar]
- Assessment of doxorubicin-induced mouse testicular damage by the novel second-harmonic generation microscopy. Am. J. Transl. Res.. 2017;9:5275.
- [Google Scholar]







