Translate this page into:
Attenuative effects of poncirin against polyethylene microplastics-prompted hepatotoxicity in rats
⁎Corresponding author. nailaraighafoor357@gmail.com (Naila Ghafoor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polyethylene microplastics (PE-MPs) are of significant concern due to their widespread use, pervasive persistence in the environment that induce multiple organ damage especially in the liver. Poncirin (PON) is a naturally present flavone with conspicuous pharmacological properties. the current investigation was formulated to ascertain the palliative role of PON against PE-MPs-provoked hepatic dysfunction. Twenty-four male albino rats were randomly divided into four groups: control, PE-MPs-treated (1.5 mg/kg), PE-MPs + PON co-treated (1.5 mg/kg and 20 mg/kg), and PON-treated (20 mg/kg). PE-MPs inebriation markedly lowered the expressions of antioxidant genes and Nrf-2, besides escalating Keap-1 expression. It also decreased antioxidants i.e., glutathione (GSH), glutathione S-transferase (GST), catalase (CAT), glutathione peroxidase (GPx), heme oxygenase-1 (HO-1), superoxide dismutase (SOD), glutathione reductase (GSR) activities, while remarkably upsurged reactive oxygen species (ROS) along with malondialdehyde (MDA) contents. Additionally, a notable escalation was observed in the levels of hepatic serum markers i.e., alkaline phosphatase (ALP), alanine transaminase (ALT) and aspartate aminotransferase (AST). Furthermore, PE-MPs exposure increased the levels of inflammatory biomarkers, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), nuclear factor kappa B (NF-kB), interleukin-1β (IL-1β) levels and cyclooxygenase-2 (COX-2) activities. PE-MPs intoxication augmented the expressions of Caspase-3 and Bax along with decreasing the expression of Bcl-2. Nevertheless, PON treatment notably abated PE-MPs prompted liver injuries owing to its hepatoprotective efficacy. Thus, it may be inferred that PON could be a potential therapeutic option for treating hepatic damage caused by PE-MPs.
Keywords
Polyethylene microplastics
Poncirin
Hepatic damage
Nrf-2/Keap-1
Inflammation
Oxidative stress
1 Introduction
Plastics play a crucial role in daily life due to their durability, versatility, affordability as well as convenience. However, their widespread accumulation in the environment has garnered significant concerns (Hou et al., 2021). According to reports of 2017, global plastic production has surged up to 3.48 x 108 tons (Plastics Europe, 2018). Microplastics (MPs) are ubiquitous environmental pollutants that are produced by the fragmentation of large plastic products into tiny plastic particles (diameter < 5 mm). MPs enters into the food chain due to their small size and low-density. Humans and other living organisms are exposed to microplastics (MPs) through dermal contact, inhalation, and ingestion. Numerous human food items, including beer, drinking water, honey, seafood, sugar, and table salt, have been found to contain MPs (Cox et al., 2019). MPs exposure has been reported to retard growth, instigate hormonal disturbance, oxidative stress (OS), immunological dysfunction and genotoxicity (Choi et al., 2018; Ehsan et al., 2023; Ahmad et al., 2023).
One of the most popular forms of plastic is polyethylene (PE). PE is used in the production of composite materials, packaging, and in various other industries, including agriculture (Beg et al., 2016). Polyethylene microplastics (PE-MPs) can cause systematic toxicity in kidney, colon, heart, lung and liver, along with reproductive abnormalities (Kim et al., 2021). PE-MPs also can induce hepatotoxicity by interfering with the lipids metabolism in liver cells (Leal Filho et al., 2019). Exposure to PE-MPs leads to the excessive production of reactive oxygen species (ROS) that results in OS, which leads to metabolism disturbance and DNA damage (Rai et al., 2021). PE-MP exposure may also alter signaling pathways leading to autophagy and apoptosis (Zhao et al., 2020).
Flavonoids are a class of polyphenolic substances that exhibit remarkable biological properties, such as anti-inflammatory properties and the potential to eliminate free radicals and inhibit hydrolases and oxidases. Due to these tremendous therapeutic properties, flavonoids are used to treat multiple ailments (Pourmora et al., 2006). Poncirin (PON) is found in Poncirus trifoliate plant and various citrus fruits such as oranges, tangerines, mandarins, grapefruits, tangelos, and chinotto. PON shows multiple biological characteristics i.e., free radical scavenging, anti-oxidant as well as anti-inflammatory properties (Ullah et al., 2020). Therefore, this research aimed to check the putative efficacy of PON in alleviating liver damage caused by PE-MPs.
2 Materials and methods
2.1 Chemicals
PON (Cas No. 14941–08-3) and PE-MPs (Cas No. 9002–88-4) were retrieved from Merck (United States of America).
2.2 Experimental animals
The current experiment was conducted on 24 male albino rats weighing 200 ± 20 g. Animals were confined in animal care facility of University of Agriculture Faisalabad, Pakistan under controlled environmental conditions (Humidity 45 ± 5 %, temperature 23–26 °C, and 12 h. day/dark light cycles). The animals were granted complete access to pelleted food as well as water. The animals were treated and handled according to the guidelines of EU Directive 2010/63/EU for animal experiments.
2.3 Experimental layout
The rats were categorised into four groups of equal size, with each group including six rats; control, a group exposed to PE-MPs (1.5 mg/kg), a group co-treated with PE-MPs and PON (1.5 mg/kg + 20 mg/kg) respectively and the last group supplemented with only PON (20 mg/kg). The dose of PE-MPs and PON were administered according to the previous studies of Ijaz et al. (2022) and Kang and Kim (2016), respectively. Following a 30-day treatment period, the rats were anesthetized with ketamine and xylazine and decapitated, and their cardiac blood was taken using heparinized syringes. The collected blood samples were then placed at a temperature of −20℃ for biochemical testing. The liver was carefully excised, rinsed with a saline solution and stored in a zippered bag at a temperature of −80 °C for further analysis.
2.4 Evaluation of antioxidant profile
Chance and Maehly’s (1955) protocol was followed to assess CAT activity. The methodology of Kakkar et al. (1984) was employed for the analysis of SOD activity. GPx activity was quantified via following the protocols of Lawrence and Burk (1976). GSR activity was appraised with the approach of Carlberg and Mannervik (1975). The method of Couri and Abdel-Rahmans (1979) was applied to quantify GST activity. GSH activity was ascertained using the method of Sedlak and Lindsay (1968), while the activity of HO-1 was quantified by using the technique outlined by Magee et al. (1999). The contents of ROS were accessed using the method of Hayashi et al. (2007). Moreover, the level of MDA was appraised via following the method of Ohkawa et al. (1978).
2.5 qRT-Polymerase chain reaction (qRT-PCR)
The expressions of Nrf-2/Keap-1, apoptotic markers (Caspase-3, Bax and Bcl-2) and antioxidant genes were appraised by qRT-PCR. The total RNA of the cell was obtained with the help of TRI-zol reagent. RNA was transcribed to produce cDNA via using Fast Quant reverse transcription kit (Takara, China). Alterations in the expressions of these parameters were determined by 2-ΔΔCT, employing β-actin as internal control. Table 1 depicted the primer of the genes (Ijaz et al., 2022).
Gene
Primers 5′ −> 3′
Accession number
Nrf-2
F: ACCTTGAACACAGATTTCGGTG
NM_031789.1
R: TGTGTTCAGTGAAATGCCGGA
Keap-1
F: ACCGAACCTTCAGTTACACACT
NM_057152.1
R: ACCACTTTGTGGGCCATGAA
CAT
F: TGCAGATGTGAAGCGCTTCAA
NM_012520.2
R: TGGGAGTTGTACTGGTCCAGAA
SOD
F: AGGAGAAACTGACAGCTGTGTCT
NM_017051.2
R: AAGATAGTAAGCGTGCTCCCAC
GPx
F: TGCTCATTGAGAATGTCGCGTC
NM_030826.4
R: ACCATTCACCTCGCACTTCTCA
GSR
F: ACCAAGTCCCACATCGAAGTC
NM_053906.2
R: ATCACTGGTTATCCCCAGGCT
HO-1
F: AGGCTTTAAGCTGGTGATGGC
NM_012580.2
R: ACGCTTTACGTAGTGCTGTGT
Bax
F: GGCCTTTTTGCTACAGGGTT
NM_017059.2
R: AGCTCCATGTTGTTGTCCAG
Bcl-2
F: ACAACATCGCTCTGTGGAT
NM_016993.1
R: TCAGAGACAGCCAGGAGAA
Caspase-3
F: ATCCATGGAAGCAAGTCGAT
NM_012922.2
R: CCTTTTGCTGTGATCTTCCT
β-actin
F: TACAGCTTCACCACCACAGC
NM_031144
R: GGAACCGCTCATTGCCGATA
2.6 Assessment of hepatic serum indices
The levels of hepatic markers i.e., AST (ab263883), ALP (ab287823) and ALT (ab285264) were analyzed with the help of commercially available ELISA kits (Wiesbaden, Germany). The company’s guidelines were followed to access these parameters.
2.7 Evaluation of inflammatory indices
NF-κB (CSB-E13148r), IL-1β (CSB-E08055r), IL-6 (CSB-E04640r), TNF-α (CSB-E07379r) levels and COX-2 activity (CSB-E13399r) was evaluated via ELISA kit (YL Biotech Co. Ltd., Shanghai, China). The analyses were performed as per the directions of manufacturers.
2.8 Statistical analysis
The data were reported as the mean value ± SEM. A one-way ANOVA followed by Tukey’s test was used for group comparisons. The level of statistical significance was set at p < 0.05.
3 Results
3.1 Efficacy of PON on the expression of Nrf-2/Keap-1
The group intoxicated with PE-MPs lead to a remarkable (p < 0.05) decline in the expression of Nrf-2 and antioxidant genes, whereas increasing the expression of Keap-1, in relation to the control. Nonetheless, PON + PE-MPs co-treatment regulated the expressions of these parameters in contrast to PE-MPs treated rats. Moreover, the rats subjected to PON only exhibited these expressions close to control animals (Figs. 1,2).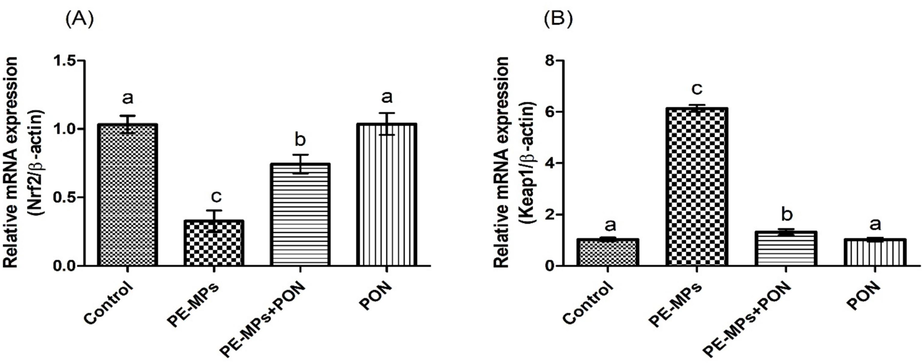
Protective role of PON on (a) Nrf-2, (b) Keap-1 expression. Values are depicted as mean ± standard error of mean. Different superscripts on bars are presenting significant variation.
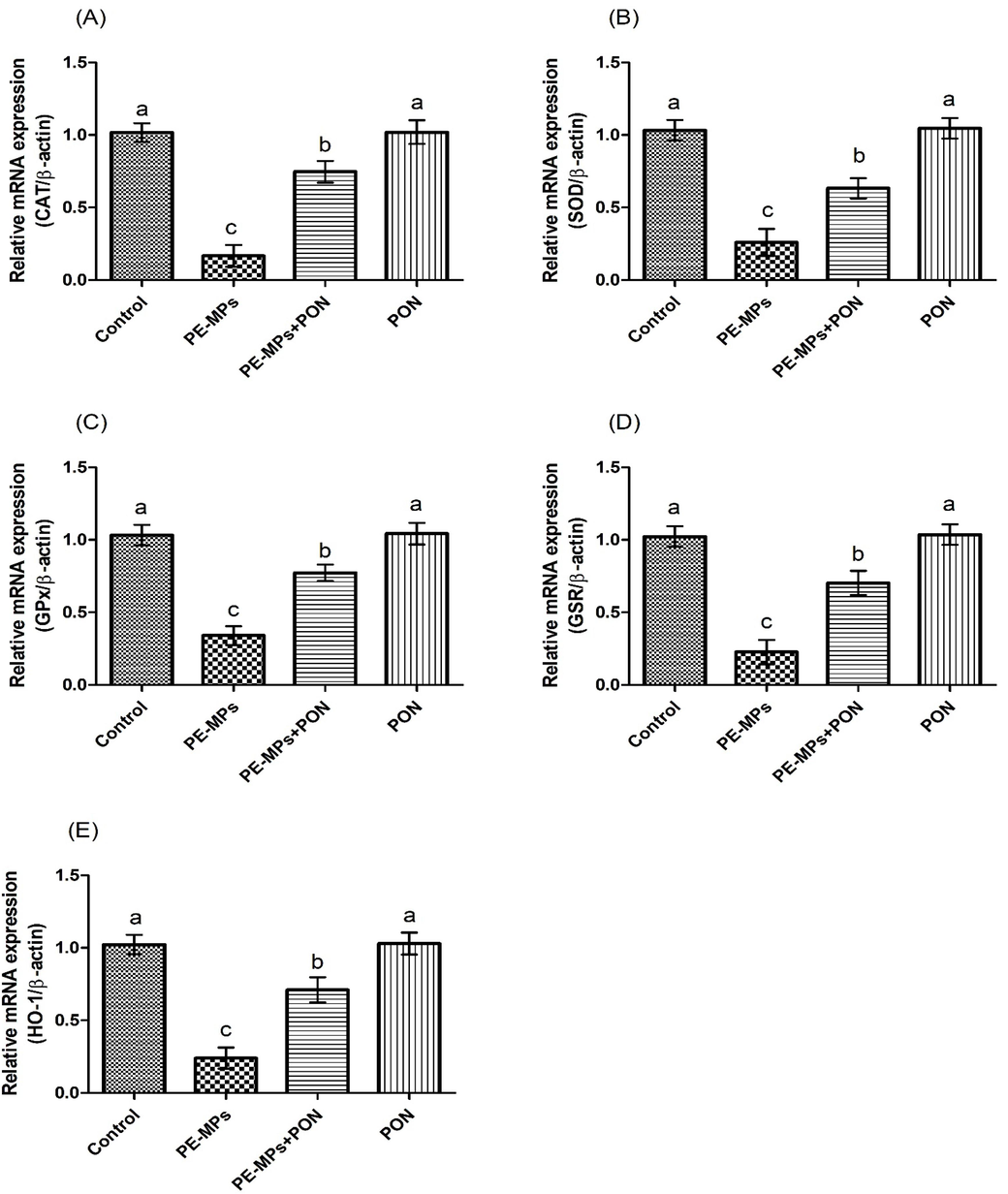
Protective role of PON on (a) CAT, (b) SOD, (c) GPx, (d) GSR, (e) HO-1 expression. Values are depicted as mean ± standard error of mean. Different superscripts on bars are presenting significant variation.
3.2 Efficacy of PON on biochemical indices
A substantial (p < 0.05) down-regulation in the activities of antioxidants activities (GSH, GST, CAT, GPx, HO-1, SOD, and GSR), while an escalation in the levels of ROS and MDA was observed in rats administered with PE-MPs, relative to control group. Nevertheless, the co-supplementation of PON + PE-MPs substantially augmented the activities of antioxidant enzymes, whereas MDA and ROS contents were significantly decreased, as compared to the PE-MPs treated rats. In addition to this, no marked variations were noted in the level of these biomarkers in PON only supplemented and control animals (Table 2). The values with different superscript are significantly distinct from other groups.
PARAMETERS
GROUPS
Control
PE-MPs
PE-MPs + PON
PON
CAT (Umg−1 protein)
14.14 ± 1.08a
6.96 ± 0.20c
11.58 ± 0.93b
14.95 ± 1.138a
SOD (Umg−1 protein)
10.77 ± 1.02a
4.23 ± 0.35c
8.01 ± 0.48b
11.06 ± 1.22a
GPx (Umg−1 protein)
24.80 ± 1.35a
8.27 ± 0.99b
22.49 ± 1.02a
25.74 ± 1.58a
GSR (nM NADPH oxidized/min/mg tissue)
8.41 ± 0.34a
2.28 ± 0.36c
4.86 ± 0.50b
8.750 ± 0.72a
GST (nM/min/mg protein)
35.54 ± 2.71a
9.86 ± 1.69c
27.52 ± 1.32b
34.32 ± 2.86a
GSH (μM/g tissue)
17.27 ± 1.26b
6.62 ± 0.62c
25.25 ± 2.00a
16.72 ± 1.02b
HO-1(pmoles bilirubin/mg protein/h)
285.60 ± 11.44 a
53.72 ± 10.25c
214.67 ± 12.75b
293.62 ± 14.33a
ROS (Umg−1 tissue)
1.36 ± 0.35b
9.41 ± 1.09a
2.49 ± 0.42b
1.28 ± 0.37b
MDA (nmol/mg protein)
0.54 ± 0.29b
3.38 ± 0.47a
1.38 ± 0.27b
0.47 ± 0.33b
3.3 Efficacy of PON on hepatic function markers
The levels of ALT, ALP and AST were markedly (p < 0.05) escalated in rats exposed to PE-MPs, as matched with the control. While PON + PE-MPs treatment reduced these levels relative to animals subjected to PE-MPs. In addition to this, only PON treated group displayed the levels of these markers almost in line with control (Table 3). The values with dissimilar letters are notably distinct from other groups.
PARAMETERS
GROUPS
Control
PE-MPs
PE-MPs + PON
PON
ALT (U/L)
54.92 ± 2.12c
92.63 ± 2.59a
67.79 ± 2.44b
53.82 ± 2.38c
AST (U/L)
75.75 ± 2.65c
249.83 ± 10.53a
159.24 ± 8.27b
73.70 ± 1.39c
ALP (U/L)
98.41 ± 3.79c
414.58 ± 12.43a
187.78 ± 9.76b
97.38 ± 3.54c
3.4 Efficacy of PON on apoptotic markers
PE-MPs treatment led to a remarkable (p < 0.05) increased in the expressions of Caspase-3 and Bax, besides down-regulating the expression of Bcl-2, as matched with control group. Nevertheless, concurrent treatment of PON + PE-MPs remarkably regulated these expressions, relative to PE-MPs administered rats. Additionally, the rats supplemented with PON only exhibited the aforementioned parameters comparable to the control (Fig. 3).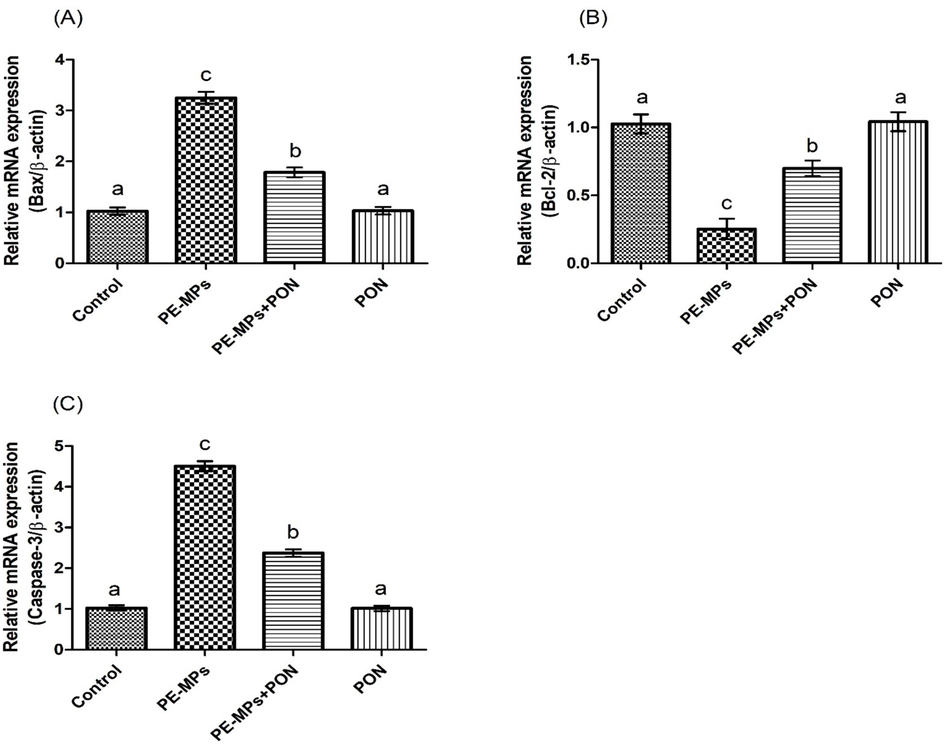
Protective role of PON on (a) Bax, (b) Bcl-2 and (c) Caspase-3 expression. Values are depicted as mean ± standard error of mean. Significant variation is depicted by different superscripts each bar.
3.5 Efficacy of PON on inflammatory indices
PE-MPs markedly (p < 0.05) elevated the levels of inflammatory biomarkers, as compared to the control. However, PON + PE-MPs supplementation lowered the levels of inflammatory indices, compared to the PE-MPs treated rats. However, the levels of inflammatory indices in PON only treated animals were presented to be almost similar to the control (Table 4). The values with dissimilar letters are significantly different from other groups.
PARAMETERS
GROUPS
Control
PE-MPs
PE-MPs + PON
PON
NF-κB (ngg−1 tissue)
23.89 ± 1.55c
84.31 ± 2.06a
33.82 ± 2.13a
22.96 ± 1.26c
TNF-α (ngg−1 tissue)
16.70 ± 1.66c
65.59 ± 1.82 a
28.07 ± 2.30 a
15.71 ± 1.54c
IL-1β (ngg−1 tissue)
7.48 ± 1.00c
58.39 ± 3.88 a
17.76 ± 1.68b
7.35 ± 1.10c
IL-6 (ngg−1 tissue)
4.32 ± 1.85 bc
34.39 ± 2.30 a
9.92 ± 2.52b
4.19 ± 1.98c
COX-2 (ngg−1 tissue)
16.12 ± 2.53c
94.99 ± 3.25 a
25.99 ± 2.20b
15.52 ± 2.79c
4 Discussion
Liver is the main detoxifying organ in the body which is involved in metabolism, but becomes susceptible to OS when exposed to toxins. PE-MPs intoxication results in production of ROS in the liver which leads to hepatic damage (Vethaak and Legler, 2021). PE-MPs treatment damages the hepatocytes via disturbing the metabolism, microtubule separation and protein denaturation. PON belongs to a diverse group of phytonutrients (flavonoids) and exhibits versatile biological characteristics such as, anti-inflammatory, antioxidant and immunoregulatory (Ullah et al., 2020). Thus, this research was formulated to access the therapeutic efficacy of PON against PE-MPs-provoked hepatic damages in albino rats by determining the liver function enzymes, biochemical and inflammatory indices.
PE-MPs treatment reduced the expressions of antioxidant genes and Nrf-2, whereas increasing Keap-1 expression. Yamamoto et al. (2018) stated that Nrf-2 performs crucial function in the regulation of OS. While Keap-1 acts as the inhibitor of Nrf-2 and controls its stability (Pintard et al., 2004). Nrf-2 separates from Keap-1 during ROS production through some physical modifications. Following the separation, Nrf-2 is relocated to the nucleus, where it engages with small MAF (musculoaponeurotic fibrosarcoma) proteins. Therefore, Nrf-2 has an indispensable part in monitoring the stimulation of antioxidant genes (Hawkes et al., 2014). Nevertheless, PON administration elevated the expressions of Nrf-2 and antioxidant genes, while lowering Keap-1 expression. Therefore, it is assumed that PON can regulate the expressions of Nrf-2 and Keap-1.
The results of our study indicated that PE-MPs administration decreased the activities of antioxidants i.e., SOD, GSR, GSH, GST, CAT, HO-1 and GPx, while markedly escalating ROS and MDA contents in the liver. These antioxidants are the 1st wall of protection of the cell which protects the cellular proteins, lipids and DNA against OS damage (Bansal et al. 2005). GSR sustains GSH level by converting glutathione disulfide (GSSG) into GSH (Zhu et al., 2012). GST plays a critical role in the detoxification of toxins to safeguard the cells from oxygen free radicals (Hayes et al., 2005). However, the decrease in the activities of antioxidant enzymes leads to excessive ROS generation, which weakens the anti-oxidative capacity of the cell to fight against free radicals and ultimately results in OS. MDA is the byproduct of lipid peroxidation (LP), which is used as a biomarker for the detection of LP and ROS induced cellular damages (He et al., 2012). Nevertheless, the supplementation of PON + PE-MPs augmented the antioxidants activities, while reducing the ROS and MDA concentrations. These attenuative effects of PON in restoring the antioxidants can be due to its free radical scavenging nature.
PE-MPs intoxication dramatically elevated the levels of hepatic markers, including, ALP, ALT and AST. The effect of any toxicant on the function of liver can be analyzed by estimating the level of these function enzymes. These aminotransferases are present in the mitochondria of hepatic cells and their release into the blood indicates hepatic injuries. OS increases the mitochondrial permeability, allowing the leakage of these hepatic markers into blood which leads to hepatotoxicity (Nagai et al., 2016). Additionally, multiple studies proposed that OS is the major reason behind this aberrant increase in hepatic function markers (Knudsen et al., 2016). However, the supplementation of PON with PE-MPs remarkably mitigated PE-MPs prompted hepatotoxicity by substantially lowering the levels of these hepatic enzymes owing to the ROS scavenging and hepatoprotective features of PON.
The results of our study demonstrated that PE-MPs administration elevated the expressions of Bax and Caspase-3, besides downregulating Bcl-2 expression. ROS triggered apoptosis and OS are the ultimate causes of hepatotoxicity (Danial and Korsmeyer 2004). The overall process of apoptosis is controlled by the proteins belonging to Caspase and Bcl-2 families. Bax promotes the apoptotic cell death, besides Bcl-2 performs an antagonistic role to Bax and protects the cells from apoptosis. The increase in the expression of Bax along with the reduction in Bcl-2 prompt efflux of cytochrome C from the mitochondrial membrane into the cytosol. The increased contents of cytochrome C content inside the cytosol leads to the activation of Caspase-3. Caspase-3 cleaves the cellular proteins, therefore altering the structural makeup of cells and eventually triggering apoptotic cell death (Végran et al., 2011). However, concurrent treatment of PON + PE-MPs markedly upregulated the expression of Bcl-2, besides reducing the expressions of Caspase-3 and Bax, as a result of anti-apoptotic potential of PON.
PE-MPs treatment noticeably elevated the levels of inflammatory markers. The activation of inflammation modulator, NF-kB, due to any cellular activity such as excessive OS, stimulates the production of IL-6, TNF-α, IL-1β (Nakajima and Kitamura 2013). The generation of these markers leads to acute inflammatory response and several other anomalies linked with abnormal ROS accumulation. COX-2 is another inflammatory marker which is reported to instigate inflammation (Caglayan et al., 2018). Nevertheless, PON supplementation downregulated these levels due to its anti-inflammatory characteristics. This anti-inflammatory potency of PON might be due to the methoxylation of 5′- or 7′ OH groups and non-methoxylation of 3′ OH groups on A and B rings of PON respectively (Yang et al. 2020).
5 Conclusion
In summary, our investigation demonstrated that PE-MPs administration instigated OS and LP in hepatic tissues that potently disturbed the levels of oxidative stress markers, liver function enzymes, inflammatory indices. It also disturbed the expressions of Nrf-2/Keap-1 and apoptotic markers. Nevertheless, PON administration effectively recovered all these impairments due to hepatoprotective, antioxidant, anti-inflammatory as well as anti-apoptotic potential. Thus, it is assumed that PON might be applied as a therapeutic compound in future to treat hepatic damage. Due to its therapeutic potential, PON could be integrated into current treatment protocols, providing a novel approach to treat patients suffering from hepatic damage. However, the current study was performed on model animals, therefore, we recommend clinical trials of this compound to check its efficacy on human beings.
CRediT authorship contribution statement
Naila Ghafoor: Writing – original draft, Methodology, Investigation, Conceptualization. Tooba Mehar: Writing – original draft, Methodology, Investigation, Conceptualization. Moazama Batool: Visualization, Validation, Software, Data curation. Muhammad Zaid Salar: Writing – review & editing, Validation, Methodology, Investigation. Mohammad Z. Ahmed: Writing – review & editing, Resources, Funding acquisition, Data curation. Usman Atique: Writing – review & editing, Formal analysis, Data curation.
Acknowledgement
The authors are thankful to the Researchers Supporting Project number (RSPD2024R728), King Suad University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-Induced Nephrotoxicity in Rats. Pakistan Veterinary Journal. 2023;43(3):623-627.
- [Google Scholar]
- Protective role of vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact.. 2005;156:101-111.
- [Google Scholar]
- Preparation and characterization of low-density polyethylene/thermoplastic starch composites. Adv. Poly. Technol.. 2016;35(1)
- [CrossRef] [Google Scholar]
- Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ. Sci. Pollut. Res.. 2018;25:20968-20984.
- [CrossRef] [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [CrossRef] [Google Scholar]
- Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus) Mar. Pollut. Bull.. 2018;129:231-240.
- [Google Scholar]
- Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol.. 1979;3:451-456.
- [Google Scholar]
- Attenuative Effects of Ginkgetin Against Polystyrene Microplastics-Induced Renal Toxicity in Rats. Pakistan Veterinary Journal. 2023;43(4):819-823.
- [Google Scholar]
- Regulation of the human thioredoxin gene promoter and its key substrates: a study of functional and putative regulatory elements. Biochim. Biophys. Acta - Gen. Subj.. 2014;1840:303-314.
- [Google Scholar]
- Hayashi, I., Morishita, Y., Imai, K., Nakamura, M., Nakachi, K., Hayashi, T., 2007. High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 631, 55–61. https://doi. org/10.1016/j.mrgentox.2007.04.006.
- Glutathione transferases. Annu. Rev. Pharmacol. Toxicol.. 2005;45:51-88.
- [CrossRef] [Google Scholar]
- In vitro and in vivo antioxidant activity of the ethanolic extract from meconopsis quintuplinervia. J. Ethnopharmacol.. 2012;141:104-110.
- [CrossRef] [Google Scholar]
- Reproductive toxicity of polystyrene microplastics: in vivo experimental study on testicular toxicity in mice. J. Hazard Mater.. 2021;405:124028
- [Google Scholar]
- Toxic effect of polyethylene microplastic on testicles and ameliorative effect of luteolin in adult rats: environmental challenge. J. King Saud Univ. Sci.. 2022;34:102064
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [CrossRef] [Google Scholar]
- Poncirin and its metabolite ponciretin attenuate colitis in mice by inhibiting LPS binding on TLR4 of macrophages and correcting Th17/Treg imbalance. J. Ethnopharmacol.. 2016;189:175-185.
- [Google Scholar]
- Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: a review. J. Hazard. Mater.. 2021;413:125423
- [CrossRef] [Google Scholar]
- Correlation between liver cell necrosis and circulating alanine aminotransferase after ischaemia/reperfusion injuries in the rat liver. Int. J. Exp. Pathol.. 2016;97:133-138.
- [Google Scholar]
- Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun Biochem. Biophys. Res. Commun.. 1976;71:952-958.
- [Google Scholar]
- Plastic debris on pacific islands: ecological and health implications. Sci. Total Environ. 2019:181-187.
- [Google Scholar]
- In vitro and in vivo immunomodulatory effects of RDP1258, a novel synthetic peptide. J. Am. Soc. Nephrol.. 1999;10:1997-2005.
- [Google Scholar]
- Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anti-Cancer Drugs. 2016;27:17-23.
- [Google Scholar]
- Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free Radic. Biol. Med.. 2013;65:162-174.
- [CrossRef] [Google Scholar]
- Reaction of linoleic acid hydroperoxide with thiobarbutiric acid. J. Lipid Res.. 1978;19:1053-1057.
- [CrossRef] [Google Scholar]
- Cullin-based ubiquitin ligases: Cul3–BTB complexes join the family. The Embo. J.. 2004;23:1681-1687.
- [Google Scholar]
- Environmental fate, ecotoxicity biomarkers, and potential health effects of micro-and nano-scale plastic contamination. J. Hazard. Mater.. 2021;403:123910
- [CrossRef] [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- Poncirin attenuates CCL4-induced liver injury through inhibition of oxidative stress and inflammatory cytokines in mice. BMC Complement. Med. Ther.. 2020;20:115.
- [CrossRef] [Google Scholar]
- A short caspase-3 isoform inhibits chemotherapy-induced apoptosis by blocking apoptosome assembly. PLoS One. 2011;6:29058.
- [Google Scholar]
- Microplastics and Human Health. J. Sci.. 2021;371:672-674.
- The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev.. 2018;98:1169-1203.
- [Google Scholar]
- Poncirin suppresses lipopolysaccharide (LPS)-induced microglial inflammation and ameliorates brain ischemic injury in experimental stroke in mice. Ann. Transl. Med.. 2020;8(21)
- [CrossRef] [Google Scholar]
- Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ.. 2020;710:136279
- [Google Scholar]







