Attenuation of oxidative damage-associated hepatotoxicity by piperine in CCl4-induced liver fibrosis
⁎Corresponding authors at: Department of Clinical Pharmacy, College of Pharmacy, King Saud University, P.O. Box-2457, Riyadh 11451, Saudi Arabia (M.U. Rehman). sbilal@skuastkashmir.ac.in (Sheikh Bilal Ahmad), muneebjh@gmail.com (Muneeb U. Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The present research has demonstrated the impact of treatment with piperine on hepatic fibrosis induced by the administration of carbon tetrachloride.
Methods
In order to study the outcome of piperine treatment on hepatic fibrosis caused by carbon tetrachloride administration in male Wistar rats, a total of 24 rats were collected and randomly classified into 4 separate groups each consisting of 6 animals. Group I was assigned as a control group and treated with olive oil for 4 weeks (1 ml/kg, i.p.) with two doses each week. Group II was designated as a toxic group and treated with carbon tetrachloride for 4 weeks (1 ml/kg, 1:1 combination with olive oil, i.p.) with two hepatic fibrosis induction doses each week. Group III was graded as a group I therapy and co-treated with carbon tetrachloride (1 ml/kg, 1:1 combination with olive oil, i.p.) for 4 weeks with two hepatic fibrosis induction doses a week and with piperine (25 mg/kg) for 4 weeks. Group IV was designated as group II therapy and co-treated with carbon tetrachloride (1 ml/kg, 1:1 combination of olive oil, i.p.) for 4 weeks with two hepatic fibrosis induction doses a week and piperine (50 mg/kg) for 4 weeks.
Results
Piperine significantly reduced the carbon tetrachloride augmented activities of liver enzymes, lipid peroxidation, glutathione, hydroxyproline, nitrite, and carbonyl levels. Carbon tetrachloride enhanced Total cholesterol (TC), Triglyceride (TG), Low density lipoprotein (LDL), and Very low density lipoprotein (VLDL) levels, and decreased High density lipoprotein (HDL) level was restored to normal with piperine treatment. Moreover, piperine inhibited the over-expression of pro-inflammatory biomarkers and α-SMA.
Conclusion
The data of the current study demonstrated piperine as a potent hepato-protective agent and recommends the use of piperine for preventing hepatic fibrosis.
Keywords
Fibrosis
Hepatotoxicity
Piperine
Carbon tetrachloride
Proinflammatory markers
1 Introduction
Liver fibrosis is a remedial reaction produced against liver damage caused by its exposure to drugs or toxic substances (Duval et al., 2015). The essential pathological characteristic depicted in hepatic fibrosis is the excess deposition of extracellular matrix (Tacke and Trautwein, 2015). Liver fibrosis can advance to produce more severe conditions such as cirrhosis, portal hypertension, and even hepato-cellular malignancy that resulted in higher rate of disease and death (Branchi et al., 2014).
Carbon tetrachloride (CCl4), a known toxic reagent that induces hepatic fibrosis and hepatic lesion, has been widely employed for hepatic studies (Huang et al., 1998). Liver damage is induced by the development of ROS and trichloromethyl free radicals triggered by liver cytochrome P450 2E1 (CYP2E1). Piperine inhibits NF-κB via activating Nef2, blocking the down-stream inflammatory cytokines or mediators such as IL1β, IL6, TNFα, iNOS, Cox2, NO, PGE2, and MPO, stimulating anti-oxidant reaction machinery including CAT, GSH, GPx, SOD, GR, NQO1, and HO1, ROS scavenging, and reducing lipid per-oxidation (Rehman et al., 2020).
Piper nigrum contains around 2–8% of phytochemical piperine (Iahtisham-Ul et al., 2020). Piperine exhibits a broad array of biologic properties including anti-depressant, anti-anxiety, anti-thyroid, anti-tumor immune-stimulating, anti-inflammatory, and anti-metastatic (Pradeep and Kuttan, 2002). This alkaloid also stimulates the hepato-protection against the toxicity induced by CCl4 and enhances the bioavailability of numerous drugs exhibiting structural and therapeutic diversity. In the current write-up, we will study the effectiveness of piperine for attenuating the oxidative damage associated with hepatotoxicity in CCl4-induced liver fibrosis.
2 Materials and methods
2.1 Chemicals
CCl4, bovine serum albumin (BSA), Piperine, reduced glutathione (GSH), 4-hydroxy-L-proline, hexadecyltrimethylammonium, 5,5-dithio-bis (2-nitrobenzoic acid) (Ellman’s reagent), and tetramethylbenzidine. N-butanol, NADPH, EDTA, thiobarbituric acid (TBA), Tris Hcl, DNPH, CDNB, chloramine-T, trichloroacetic acid (TCA), NaOH, dL-Aspartate, FAD, PCA, Tween 20, DCPIP, α-ketoglutaric acid, NaCl, and sodium pyruvate were procured from Sigma-Aldrich (St. Louis, MO, USA). Piperine had a purity grade of 97%.
2.2 Animals
Male Wistar rats aged from 7 to 9 weeks with 180–220 g of body weight were employed in the current study. The rats were acquired from the Central Animal House Facility and housed at the standard temperature (25 ± 1 °C) and light/dark period (12/12 h) in the animal care facility. Normal food pellets and tap water were fed to these rats. Rat acclimatization was done for 7 days before treatment. All the tests have been carried out according to guidelines. The project has been accepted by the Institutional Board of Approval (No. RAKMHSU-REC-7-2017-F-P).
2.3 Treatment protocol and induction of hepatic fibrosis
In order to research the outcome of treatment with piperine (Sigma-Aldrich) for hepatic fibrosis caused by CCl4 administration in male Wistar rats, a total of 24 Wistar rats were collected and randomly classified into 4 separate groups each containing 6 animals. Group I was assigned as a control group and given olive oil for 4 weeks (1 ml/kg, i.p.) with two doses each week. Group II was designated as a toxic group and treated with CCl4 for 4 weeks (1 ml/kg, 1:1 combination with olive oil, i.p.) with two hepatic fibrosis induction doses each week. Group III was graded as the group I therapy and co-treated with CCl4 (1 ml/kg, 1:1 combination with olive oil, i.p.) for 4 weeks with two hepatic fibrosis induction doses a week and with piperine (25 mg/kg) for 4 weeks. Group IV was designated as group II therapy and co-treated with CCl4 (1 ml/kg, 1:1 combination with olive oil, i.p.) for 4 weeks with two doses each week for hepatic fibrosis induction, and piperine (50 mg/kg) for 4 weeks with two doses per week for hepatic fibrosis induction. 12 h after the last CCl4 injection, all the rats were euthanized under light ether anesthesia.
2.4 Determination of the liver enzymes functional tests
The hepatoprotective property of piperine was investigated by estimating total protein, albumin, bilirubin, ALP, ALT, and AST level in serum by diagnostics kits commercially available.
2.5 Assessment of catalase activity (CAT)
The enzymatic activity of the catalase was determined with the procedure described by Claiborne (1984).
2.6 Assessment of superoxide dismutase activity (SOD)
Super-oxide dismutase (SOD) activity was determined with the protocol described by Marklund and Marklund (1974).
2.7 Assessment of reduced glutathione (GSH)
The procedure described by Jollow et al. (1974) was used for estimating reduced glutathione levels.
2.8 Assessment of glutathione reductase activity (GR)
The activity of Glutathione reductase was determined with the protocol described by Carlberg and Mannervik (1975).
2.9 Assessment of glutathione peroxidase activity (GPx)
Glutathione peroxidase activity was measured by the modus operandi of Mohandas et al., 1984.
2.10 Assessment of protein carbonyl
The assessment of protein carbonyl was done by following the modified version of the protocol of Levine et al. (1990) modified by Floor and Wetzel (1998).
2.11 Assessment of hepatic 4-hydroxyproline
The colorimetric quantification of hepatic 4-hydroxyproline was done by the chloramine-T technique of Lee et al. (2005) after minor modifications.
2.12 Assessment of malondialdehyde (MDA)
Lipid peroxidation (LPO) was performed with the protocol described by Wright et al. (1981).
2.13 Estimation of TNF-α, IL-6, TGF-β, and NFκ-B
TNF-α (Tumor necrosis factor α), IL-6 (Interleukin-6), TGF-β (Transforming growth factor β), and NFκ-B were determined by ELISA technique-based (enzyme-linked Immunosorbent assay) rat TNF-α, IL-6, TGF-β, and NFκ-B kits (Bioscience, Inc., San Diego., USA). Each of the pro-inflammatory cytokine was measured from colonic tissue samples with the help of ELISA. Sample preparation was done in phosphate-buffered saline (PBS) containing protease inhibitor cocktail and each sample was analyzed with the help of Elisa Plate Reader (Multiskan EX, Thermo) by following the manufacturer’s instructions.
2.14 Assay for hepatic Immunohistochemistry
The assessment of α-smooth muscle actin was done by following the modified procedure of Hosoya et al. (2006).
2.15 Histopathological analysis of hepatic tissue
The wet hepatic tissues are fixed and dehydrated in a 10% neutral buffered solution. Following the prescribed histopathological investigation procedure, liver tissue samples were treated after fixation. For examination with hematoxylin and eosin (H&E) dye, a 5 μm part of the liver was cut and dyed. In all groups, the pathological features of liver tissues were observed under the fluorescent microscope for histopathological improvement (Olympus Life Science, Europa GMBH, Wendenstrasse 14–18, 20,097 Hamburg, Germany).
2.16 Assessment of lipid profile
Serum samples were collected and analyzed for lipid profile. Triglyceride (TG), HDL-cholesterol, and Total cholesterol (TC) levels were estimated by kit (Genzyme Diagnostics). However, the estimation of LDL-cholesterol was done as the remaining difference of TC and HDL-cholesterol.
2.17 Statistical analysis
The individual group data were presented as mean ± SEM. Mean ± SEM was viewed as the individual community results. As statistical methods to evaluate disparities between groups, ANOVA (analysis of variance) and Tukey-Kramer multiple comparison tests were used. At p < 0.05, all the comparisons were considered to be statistically important.
3 Results
3.1 Effect of CCl4 and piperine on the functions of liver enzymes
The CCl4 treated Group II showed a remarkably elevated (***p < 0.001) level of ALT, AST, ALP, and Bilirubin whereas total protein and albumin declined in contrast with the control group. This depicts that CCl4 can induce hepatotoxicity by enhancing the functions of liver enzymes. However, piperine treatment significantly decreases the functions of liver enzymes both at low-dose (25 mg/kg) in group III (##p < 0.01) and at high-dose (50 mg/kg) in group IV (###p < 0.001) as evident from Table 1.
| Treatment Groups | ALT (IU/L) | AST (IU/L) | Albumin (g/dl) | Bilirubin (mg/dl) | Total Protein (g/dl) | ALP (IU/L) |
|---|---|---|---|---|---|---|
| Group I | 42.76
|
76.75
|
6.32
|
0.23
|
6.16
|
25.13
|
| Group II | 167.1 ± 28.83*** | 174.3 ± 16.7*** | 3.01 ± 0.27** | 0.99 ± 0.17*** | 4.91 ± 0.29 ns | 44.32 ± 4.87** |
| Group III | 123.1 ± 14.03* | 110.9 ± 9.14# | 4.93 ± 0.19# | 0.69 ± 0.10# | 5.32 ± 0.32 ns | 32.72 ± 4.14# |
| Group IV | 70.93 ± 7.86## | 95.1 ± 9.31## | 5.92 ± 0.61## | 0.32 ± 0.10### | 6.26 ± 0.33# | 29.12 ± 2.32## |
Each value represents the mean of 6 rats ± SE.
3.2 Effect of piperine on glutathione and other antioxidant parameters
The effect of piperine treatment on CCl4-induced reduction in anti-oxidant enzyme activities was investigated and the outcome is depicted in Table 2. The antioxidant enzymatic activities showed a considerable disparity (***p < 0.001) between groups I and II. With the piperine treatment, antioxidant enzymatic activities were re-established to normal.
| Group I | Group II | Group III | Group IV | |
|---|---|---|---|---|
| Reduced glutathione (GSH; nmol mg protein) | 369.24 ± 23.5 | 145.31 ± 13.7*** | 281.53 ± 16.4# | 299.53 ± 14.9## |
| Oxidized glutathione (GSSG; nmol mg protein) | 38.63 ± 9.31 | 91.63 ± 8.32*** | 71.34 ± 9.75# | 48.22 ± 7.23### |
| GSH/GSSG Ratio | 9.551 ± 1.52 | 1.580 ± 0.19*** | 3.946 ± 0.73# | 6.211 ± 0.86### |
| GPx (nmol/min/mg protein) | 219.57 ± 24.5 | 97.32 ± 9.15*** | 146.73 ± 11.2# | 165.12 ± 14.7## |
| GR (nmol min/min/mg protein) | 233.75 ± 21.7 | 101.43 ± 12.3*** | 174.72 ± 18.1## | 214.22 ± 21.8### |
| SOD (units/min/mg protein) | 14.531 ± 1.63 | 6.533 ± 0.91*** | 8.559 ± 0.79# | 11.158 ± 1.77### |
| Catalase (nmol H2O2 consumed/min/mg protein) | 8.54 ± 0.97 | 4.11 ± 0.19*** | 6.21 ± 0.54# | 7.95 ± 0.65## |
Each value represents the mean of 6 rats ± SE.
3.3 Effect of CCl4 and piperine on lipid profile
The lipid profile was significantly enhanced (***p < 0.001) except HDL that showed a reduction with CCl4 treatment in comparison to control as illustrated in Table 3. The treatment with piperine at both doses (25 mg/kg & 50 mg/kg) decreases the lipid profile except HDL that showed an increasing trend.
| Treatment Groups | TC (IU/L) |
HDL (IU/L) |
LDL (g/dl) |
TG (mg/dl) |
VLDL (g/dl) |
|---|---|---|---|---|---|
| Group I | 82.80 ± 14.7 | 29.43 ± 0.92 | 41.70 ± 3.45 | 89.10 ± 9.43 | 17.53 ± 3.32 |
| Group II | 162.10 ± 21.3*** | 17.23 ± 0.73*** | 98.32 ± 7.31*** | 163.3 ± 15.1*** | 39.22 ± 3.32*** |
| Group III | 123.14 ± 14.9## | 21.28 ± 0.69# | 67.33 ± 4.32## | 101.4 ± 8.17## | 26.04 ± 2.05## |
| Group IV | 101.92 ± 9.41# | 51.21 ± 0.41## | 53.12 ± 4.03## | 123.6 ± 9.31## | 23.05 ± 2.19## |
Each value represents the mean of 6 rats ± SE.
3.4 Effect of piperine serum inflammatory cytokines levels
In CCl4 treated group II the pro-inflammatory cytokine levels of TNFα, TGFβ, NF-κB, and IL6 depicted a considerable elevation (***p < 0.001) in contrast to the untreated group I. Nevertheless, piperine treatment in groups III and IV significantly reduced these cytokine levels near to normal at both dosages. A non-significant difference in these cytokines was observed between groups I and IV (Table 4).
| Group-I | Group-II | Group-III | Group-IV | |
|---|---|---|---|---|
| TGF-β (pg/ml) | 876.64 ± 71.8 | 2121.63 ± 193.9*** | 1667.72 ± 107.8# | 1004.32 ± 115.3### |
| TNF-α (pg/ml) | 518.31 ± 78.63 | 1425.32 ± 153.5*** | 1123.73 ± 125.4# | 732.64 ± 91.94## |
| NFκ-B (pg/ml) | 872.65 ± 84.20 | 2002.7 ± 186.1*** | 1401.43 ± 132.7## | 994.15 ± 88.135## |
| IL-6 (pg/ml) | 761.83 ± 43.62 | 1537.62 ± 143.8*** | 1046.71 ± 92.47## | 875.64 ± 78.70### |
Each value represents the mean of 6 rats ± SE.
3.5 Effect of piperine on CCl4-induced MDA levels
The levels of oxidative stress biomarker MDA increased with the elevated oxidative stress. With the administration of CCl4, MDA levels of group II depicted a sharp elevation than group I (***p < 0.001). The piperine treatment evidently decreased the aberrantly increased MDA levels of group III (#p < 0.5), and IV (##p < 0.01) (Fig. 1).
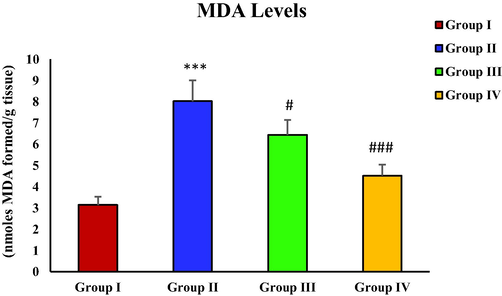
- Effect of piperine treatment on MDA levels in CCl4-induced hepatic fibrosis.
3.6 Effect of piperine on CCl4-induced hydroxyproline levels
With the administration of CCl4 the concentration of hydroxyproline was remarkably raised in the toxic group (group II) in contrast with group I (control) (***p < 0.001). On the other hand, a considerable reduction in hydroxyproline levels was seen subsequent to piperine treatment in group III (#p < 0.05), and IV (##p < 0.01). Moreover, an immeasurable difference was observed in hydroxyproline levels between groups I and IV (Fig. 2).
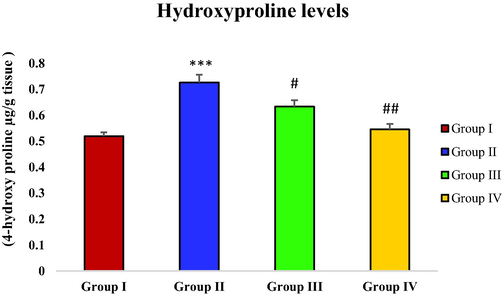
- Effect of piperine treatment on hydroxyproline levels in CCl4-induced hepatic fibrosis.
3.7 Effect of piperine on CCl4-induced nitrite level
With CCl4 administration in the toxic group (group II), a considerable rise in nitrite level was observed in contrast with the control group (***p < 0.001). The piperine treatment with 25 mg/kg dosage depicted statistically no remarkable decrease in nitrite level of group III. However, the considerable decline in nitrite level was noticed in group IV (###p < 0.001) after treatment with piperine at the dosage of 50 mg/kg (Fig. 3).
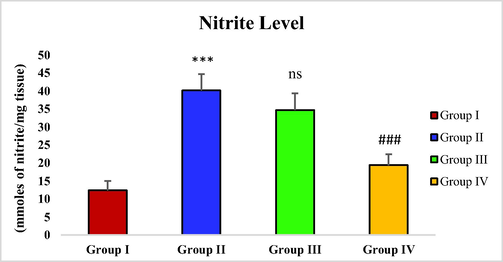
- Effect of piperine treatment on nitrite levels in CCl4-induced hepatic fibrosis.
3.8 Effect of piperine on CCl4 induced protein carbonyl level
Protein Carbonyl concentration was found considerably elevated (***p < 0.001) in group II treated with CCl4 in contrast with group I. However, a significant decline in protein Carbonyl level was observed with piperine administration in groups III and IV (#p < 0.05) (Fig. 4).
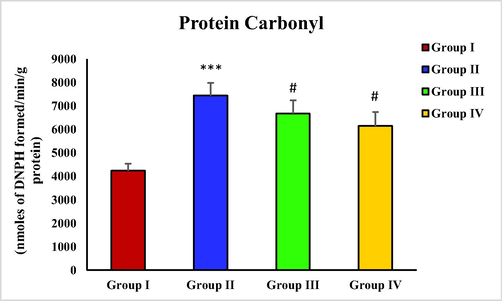
- Effect of piperine treatment on protein Carbonyl level in CCl4 induced hepatic fibrosis.
3.9 Effect of piperine on CCl4 α-SMA expression
In the process of staining of α-smooth muscle actin (α-SMA) immunohistochemically, insignificant staining was observed in group I, taken as control while group II intoxicated with CCl4 showed a marked α-SMA expression as evident in the form of the strongly brown-stained portion. The treatment with piperine at 25 mg/kg dose remarkably reduced the expression of α-SMA as evident in the form of reduced brown-stained portion when compared with group II. However, the piperine treatment at 50 mg/kg dose in group IV completely reversed the CCl4 induced expression of α-SMA and returned the hepatic tissue to a normalized state similar to group I (Fig. 5).
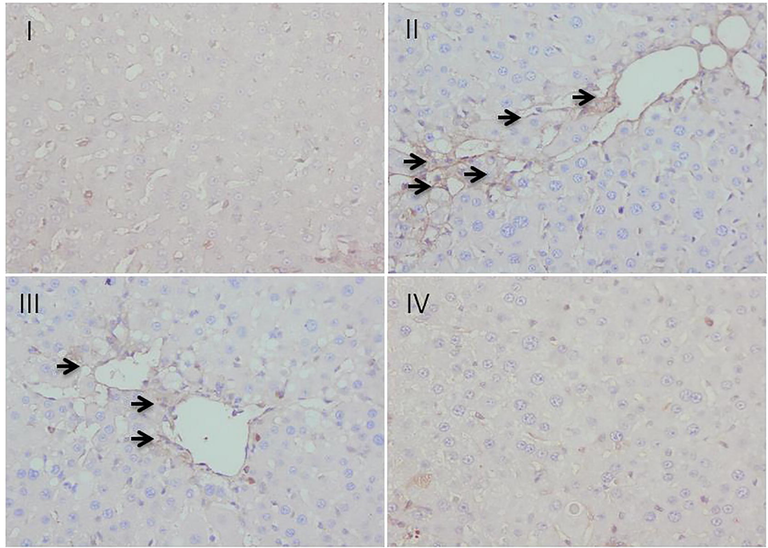
- Effect of piperine treatment on CCl4-induced α-SMA expression.
3.10 Histopathological investigation
The histopathological investigation displayed supportive shreds of evidence for the liver enzymes and oxidative markers. Histopathological findings showed a normal structure with separate cells, precise central vein, and sinusoidal spaces for the animals' regular group. Group II, on the other hand, confirmed necroinflammation, ballooning degeneration, steatosis, and loss of cellular bourgeons. Piperine treatment at a dosage of 25 mg/kg (group III) and 50 mg/kg (group IV) displayed a relatively normal pattern with a moderate degree of necrosis and infiltration of inflammatory cells compared to group II (Fig. 6).
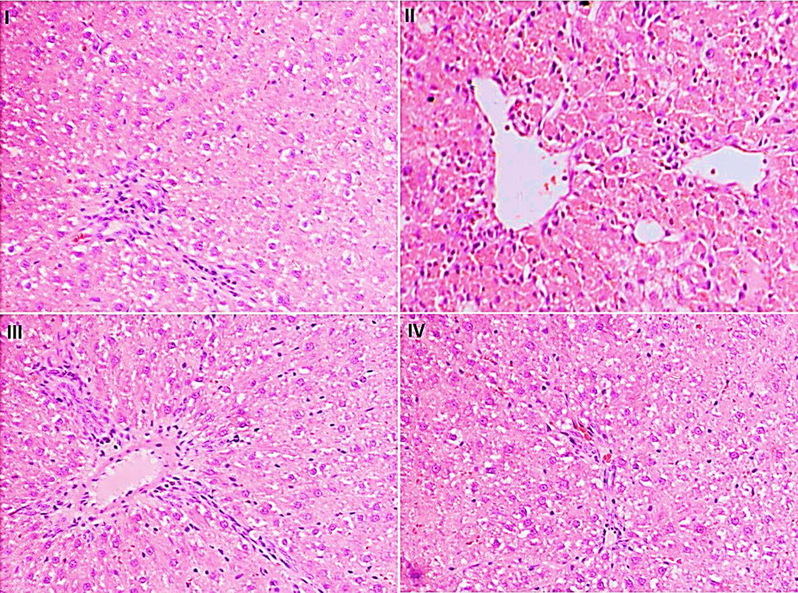
- Microphotograph of hematoxylin and eosin (H & E)-stained sections of liver.
4 Discussion
Hepatic fibrosis develops as a reversible hepatopathy condition during the progressive course of chronic hepatic impairment to irreversible hepatic cirrhosis (Hafez et al., 2017). CCl4 is the toxic reagent most frequently utilized to establish animal models with hepatic fibrosis and hepatic cirrhosis (Scholten et al., 2015). The cytochrome P450 enzyme causes CCl4 biotransformation into harmful metabolic product trichloromethyl free radicals leading to CCl4 toxicity (Anusha et al., 2011).
In this study, the hepato-protective and anti-fibrotic potential of piperine was demonstrated in the Wistar rat model to ameliorate the hepatic fibrosis induced with CCl4 by investigating different parameters. The increased levels of hepatic enzymes in serum have been accepted as non-invasive substitute markers to detect hepatocellular damage (Hann et al., 2012). Our findings showed the evident development of hepatic injury in CCl4 treatment Wistar rats that was supported by significantly increased (***p < 0.001) levels of AST, ALP, ALT, and bilirubin, presenting CCl4 as hepato-necrotic and inflammatory agent (Bahcecioglu et al., 2008). The treatment with piperine significantly retrieved the augmented activities of liver enzymes (Table 1), indicating the hepato-protective potential of piperine that perhaps averts the CCl4-induced loss of functional integrity of membranes of hepatocytes and leaking of sensitive enzymes. Our finding concords with the previous study that demonstrated the hepato-defensive action of piperine against CCl4-induced hepatotoxicity (Desai et al., 2008).
The SOD activity serves as a sensitive indicator in hepato-renal injury since it reduces the toxic effects via scavenging the superoxide anions to produce hydrogen peroxide. In hepatic cells, glutathione and catalase form the critical systems for the detoxification of peroxides (Solanki and Jain, 2011). The cellular GSH, as well as the anti-oxidant enzymes including GPx and SOD are the critical representative elements of endogenous antioxidant systems that mainly participate in the scavenging of free radicals. In this study, GPx, GR, SOD, CAT, and GSH content was drastically depleted in rats treated with CCl4. However, a remarkable restoration in GPx, SOD, GR, CAT, and GSH content was detected with the piperine treatment (Table 2), indicating the piperine's role in promoting the effective and quick consumption of ROS produced by CCl4 induced P450 bio-activation. The present data concords with the study of (Abdel-Daim et al., 2019), where piperine treatment in microcystin-induced neurotoxicity in mice has been found to increase GPx, CAT, SOD, and GSH content in the brain tissue. In this study, CCl4 treated rats showed significantly enhanced lipid profile (TC, TG, LDL, and VLDL), but HDL level was decreased. With the piperine treatment, the lipid profile was retrieved (Table 3). A previous study conducted on cholesterol-rich diet-fed rats reported that methyl piperine remarkably prevented increasing serum TC and TC/HDL ratio (Tu et al., 2014).
The levels of proinflammatory cytokines including TNFα, TGFβ, NF-κB, and IL6 were drastically raised in group II treated with CCl4 in contrast with the untreated group (group I). However, the piperine treatment considerably reduced these cytokines to almost average levels (Table 4), depicting its role as an anti-inflammatory agent. Our results concord with the established anti-inflammatory property of piperine (Aswar et al., 2015). Guo et al. demonstrated a considerable decrease in 2,4,6 trinitrobenzene sulfonic acid (TNBS) up-regulated proinflammatory cytokines TNFα, IL6, ILβ1, and NF-κB after piperine treatment in TNBS induced colitis rats as compared to controls (Guo et al., 2020). Furthermore, piperine has been reported to alleviate microcystin-LR-induced hepato-toxicity and neurotoxicity via significantly reducing serum biochemical parameters, proinflammatory cytokines (TNFα, IL6, and ILβ1), and improving oxidative/anti-oxidative state (Abdel-Daim et al., 2019). Choi et al., 2019 also reported a decrease in TNFα, TGFβ, IL1β, and IL6 mRNA levels after piperine treatment during chronic pancreatitis.
MDA levels in CCl4 treated group were sharply elevated. However, the groups (group III and IV) treated with piperine showed a decreasing trend in MDA levels (Fig. 1). A similar result has been observed with piperine treatment in the case of microcystin-induced elevation in MDA levels in nervous tissue (Mohammed et al., 2019). Hydroxyproline levels were drastically increased in the toxic group with CCl4 administration in contrast with the control group. However, the hydroxyproline level was significantly reduced in piperine treated groups (Fig. 2). The results of the current study correspond to the survey conducted by Pradeep and Kuttan (2002) who reported that piperine treatment considerably attenuated the elevated lung collagen hydroxyproline in mice with metastasized lungs. In CCl4 toxic group, the nitrite level was remarkably increased in contrast with the control group. The piperine treatment only at a higher dose (50 mg/kg) significantly restored the nitrite level (Fig. 3). In a previous study, piperine treatment of lipopolysaccharide and concanavalin-A-treated balb/c mice significantly decreased the nitrite level (Pradeep and Kuttan, 2003). In this study, a significant rise in protein carbonyl level was reported in the group treated with CCl4 in contrast with the control group. However, the piperine treatment considerably decreased the protein carbonyl level in treatment groups (Fig. 4). Our data is endorsed by the finding of Dhivya et al. (2017), they also reported a considerable decreased content of protein carbonyl in piperine pre-treated rats followed by isoproterenol treatment compared to isoproterenol intoxicated group in case of isoproterenol-induced myocardial ischemia. A markedly increased expression of α-SMA was observed in the group intoxicated with CCl4. However, with the piperine treatment, α-SMA expression was remarkably reduced at 25 mg/kg and completely reversed at 50 mg/kg in treatment groups (Fig. 5). The current data is endorsed by the observations of Choi et al. (2019), who found that treatment with piperine during chronic pancreatitis decreased the expression of various fibrotic mediators, including α-SMA in pancreatic stellate cells and pancreas. Thus, in this study, we demonstrated piperine as a potential hepato-defensive agent against CCl4-induced toxicity via regulating different biochemical parameters. These findings recommended that treatment with a higher piperine dosage (50 mg/kg) enhanced the histo-architecture and well-defined the standard arrangements for hepatic architecture.
5 Conclusions
This study demonstrated the hepatoprotective potential of piperine against CCl4-induced hepatic fibrosis. Piperine exerts an anti-fibrotic effect via inhibiting the excessive expression of hepatic markers, proinflammatory cytokines, α-SMA, preventing hepatic cell apoptosis, quenching free radicals, and enhancing antioxidant agents via modulating NF-κB signaling. Thus, our study recommends the use of piperine as an effective treatment for preventing hepatic fibrosis.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through Research Group Number (RG-1441-396).Authors are also grateful to the Faculty of Veterinary Science and Animal Husbandry, SKUAST-Kashmir, Shuhama, J&K, India for the technical support provided.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Piperine Enhances the Antioxidant and Anti-Inflammatory Activities of Thymoquinone against Microcystin-LR-Induced Hepatotoxicity and Neurotoxicity in Mice. Oxid Med Cell Longev.. 2019;2019:1309175. Published 2019 Apr 16
- [CrossRef] [Google Scholar]
- Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J. Pharmacol.. 2011;43(5):563-567.
- [Google Scholar]
- Antiallergic effect of piperine on ovalbumin-induced allergic rhinitis in mice. Pharm. Biol.. 2015;53(9):1358-1366.
- [Google Scholar]
- Hepatoprotective effect of infliximab, an anti-TNF-alpha agent, on carbon tetrachloride-induced hepatic fibrosis. Inflammation. 2008;31(4):215-221.
- [Google Scholar]
- Non-invasive assessment of liver fibrosis in chronic hepatitis B. World J. Gastroenterol.. 2014;20(40):14568-14580.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250(14):5475-5480.
- [Google Scholar]
- Piperine ameliorates the severity of fibrosis via inhibition of TGF-β/SMAD signaling in a mouse model of chronic pancreatitis. Mol. Med. Rep. 2019:3709-3718.
- [Google Scholar]
- Catalase activity. In: Greenwald R.A., ed. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press Inc.; 1984. p. :283-284.
- [Google Scholar]
- Potentiating effect of piperine on hepatoprotective effect of Boerhaavia diffusa to combat oxidative stress. Int. J. Pharmacol.. 2008;4:393-397.
- [Google Scholar]
- Piperine modulates isoproterenol induced myocardial ischemia through antioxidant and anti-dyslipidemic effect in male Wistar rats. Biomed. Pharmacother.. 2017;87:705-713.
- [Google Scholar]
- Liver fibrosis and mechanisms of the protective action of medicinal plants targeting inflammation and the immune response. Int. J. Inflam.. 2015;2015:1-14.
- [Google Scholar]
- Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem.. 1998;70(1):268-275.
- [Google Scholar]
- Piperine, a functional food alkaloid, exhibits inhibitory potential against TNBS-induced colitis via the inhibition of IκB-α/NF-κB and induces tight junction protein (claudin-1, occludin, and ZO-1) signaling pathway in experimental mice. Hum. Exp. Toxicol.. 2020;39(4):477-491.
- [Google Scholar]
- Effect of ginseng extract on the TGF-β1 signaling pathway in CCl4-induced liver fibrosis in rats. BMC Complement. Altern. Med.. 2017;17(1)
- [CrossRef] [Google Scholar]
- Comprehensive analysis of common serum liver enzymes as prospective predictors of hepatocellular carcinoma in HBV patients. PLoS ONE. 2012;7(10):e47687.
- [CrossRef] [Google Scholar]
- Immunohistochemical localization of alpha-Smooth muscle actin during rat molar tooth development. J. Histochem. Cytochem.. 2006;54(12):1371-1378.
- [Google Scholar]
- Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J. Gastroenterol.. 1998;4(3):206-209.
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151-169.
- [Google Scholar]
- Hydroxyproline content of needle biopsies as an objective measure of liver fibrosis: Emphasis on sampling variability. J. Gastroenterol. Hepatol.. 2005;20(7):1109-1114.
- [Google Scholar]
- Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol.. 1990;186:464-478.
- [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47(3):469-474.
- [Google Scholar]
- Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem. Pharmacol.. 1984;33(11):1801-1807.
- [Google Scholar]
- Effect of piperine on the inhibition of lung metastasis induced B16F–10 melanoma cells in mice. Clin. Exp. Metast.. 2002;19(8):703-708.
- [Google Scholar]
- Effect of piperine on the inhibition of nitric oxide (NO) and TNF-alpha production. Immunopharmacol. Immunotoxicol.. 2003;25(3):337-346.
- [Google Scholar]
- Piperine regulates Nrf-2/Keap-1 signalling and exhibits anticancer effect in experimental colon carcinogenesis in wistar rats. Biology. 2020;9(9):302.
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of Clitoria terenatea and Vigno mungo against acetaminophen and carbon tetrachloride-induced hepatotoxicity in rats. J. Pharmacol. Toxicol.. 2011;6:30-48.
- [Google Scholar]
- Tu Y, Sun D, Zeng X, et al 2014. Piperine potentiates the hypocholesterolemic effect of curcumin in rats fed on a high fat diet. Exp. Ther. Med. 8(1):260–266.
- Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch. Biochem. Biophys.. 1981;206(2):296-304.
- [Google Scholar]







