Translate this page into:
Attenuating effect of Indian mustard (Brassica juncea) seed and its nano formulation on arsenic induced-oxidative stress and associated genotoxicity in rat
⁎Corresponding authors at: P.O. Box 22452, Riyadh 11495, Saudi Arabia. bvirk@ksu.edu.sa (Promy Virk), mawad@ksu.edu.sa (Manal Awad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objective
Arsenic (As) has been in the forefront of toxicological research due to its continual worldwide human exposure and its adverse effects including genotoxicity. The study assessed the potential ameliorative effect of Indian mustard seeds (Brassica juncea) and their nanoparticles against arsenic toxicity.
Methods
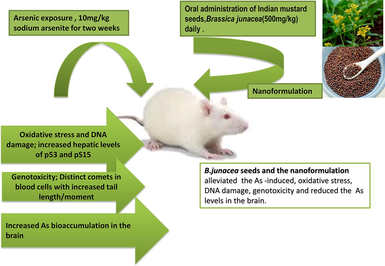
Male Wistar rats were used for the exposure study. Group I was control and group V was exposed only to the nanoparticles. Groups II, III, and IV were exposed to 10 mg/kg sodium arsenite for two weeks. Groups III and IV were treated with mustard seeds and the nanoparticles (500 mg/kg) respectively.
Results
Arsenic intoxication caused a marked increase in the levels of As in the brain. Additionally, oxidative damage was exhibited with significantly enhanced levels of serum 8-OHdG and hepatic p53 and pS15. The pictographs and the analyzed parameters of the COMET assay illustrated DNA damage with significantly increased values of tail moment and length. Treatment with mustard seeds and its nanoparticles markedly reversed the adverse effects of As toxicity, which is attributed to the presence of a gamut of constituent phytocompounds in the mustard seeds.
Conclusion
Taken together the nano formulation was more efficacious and could be introduced as a potential nutraceutical to combat metal toxicity.
Keywords
Arsenic
Indian mustard seed
Brassica juncea
Oxidative stress
Genotoxicity
Nanoformulation
1 Introduction
Arsenic (As) is a ubiquitous naturally occurring toxicant. The International Agency for Research on Cancer (IARC, 2012) has categorized this metalloid as class 1 human carcinogen. The metalloid is a well documented genotoxic agent affecting the skin and internal organs (Mateo et al., 2019). The common routes of exposure to As in humans is via ingestion, drinking As contaminated water (Sheikh et al., 2014) and through wastes of agricultural pesticides and mining activities (Singh et al., 2011). The global estimates show that around 200 million people are exposed to As concentrations more than the permissible level of 10 µg/l set by the World Health Organization (WHO, 2012). Ground water contamination used for irrigation resulting in bioavailability of arsenic to food crops also enhances the uptake along the food chain by humans and livestock. Dietary consumption of contaminated rice primarily contributes towards the chronic exposure of As in humans. Paddy accumulates relatively higher amounts of As and is a staple food for over 3 billion people worldwide, predominantly in Asia, also being used extensively for infant feeding (Mitra et al., 2017). Chemically arsenic occurs in two forms; organic and inorganic with the inorganic forms of trivalent arsenic being more toxic than the organic form (Mateo et al., 2019). Since almost all the vital organs are affected by arsenic intoxication, it causes severe health hazards in the human body with ensued damage or dysfunctioning (Khairul et al., 2017). A recent study reported a correlation between arsenic exposure prevalence of diabetes type 2 which supports the hypothesis that arsenic could be possible diabetogenic risk factor (Grau-Perez et al., 2018). Previous literature reports that generation of reactive oxygen species (ROS) and subsequent oxidative stress is the common denominator in arsenic pathogenesis. Oxidative stress contributes to the carcinogenicity that includes the oxidative DNA damage and chromosomal aberration and its interference with the cellular signaling pathways (Mandal, 2017). In the light of these considerations, developing strategies to efficaciously combat the deleterious effects of arsenic exposure in the human population is imperative. In the recent years, phytocompounds have been extensively used for their potent antioxidant status and have proven to be a promising strategy to combat heavy metal toxicity. The seeds of the Indian mustard (Brassica juncea) have been widely reported for their antioxidant status and antimicrobial activity owing to the presence of sinapic acid in B. juncea. The seeds of this plant are endowed with antioxidants, and phytonutrients, such as alpha-linolenic acid, erucic acid, palmitic acid, tocopherols, tocotrienols, carotene, oryzanol, squalene, and thiamine (Grygier, 2022). A previous study reported that oil free methanol extract of Indian mustard seeds constitutes polyphenols including gallic acid, caffeic acid, quercetin and kaempferol which contribute to their potent free radical scavenging and metal chelating activity (Dua et al., 2014).
Keeping this premise, the current study investigated the putative therapeutic effect of Indian mustard seeds and its nano formulation against As genotoxicity.
2 Materials and methods
2.1 Chemicals and kits
Analytic grade Sodium arsenite (NaAsO2) and methanol were purchased from Sigma Chemicals (St. Louis, Mo., U.S.A.). Indian mustard seeds (Brassica juncea) were procured from a local grocery store in Riyadh City. Commercial ELISA kits for estimation of 8-hydroxy-2-deoxyguanosine and p53 protein were purchased from Abcam (Cambridge, UK). The ‘green’ nanoparticles of B. juncea were synthesized and characterized as reported by Awad et al. (2019).
2.2 Experimental design
Fifty adult male Wistar rats (200 ± 10 g) were procured from the Animal House Facility at King Saud University, Riyadh. The study conformed to the standards within the guidelines of the Institutional Ethics Committee of King Saud University, Riyadh. Rats were acclimated to the laboratory conditions in cages and maintained at 22 ± 2 °C with a 12 h light/dark cycle. Rats were fed commercial chow and given tap water ad libitum. Post acclimation, rats were allocated randomly into five groups of 10 rats each. The rats were given the required doses of arsenic (sodium arsenite) and treated with Indian mustard seed powder and its nanoformulation for a period of two weeks as mentioned below.
Group I - control group was administered normal saline.
Group II- rats were exposed to an oral dose of arsenic (10 mg/kg b.w) (Manna et al., 2007).
Group III- rats were exposed to both an oral dose of arsenic (10 mg/kg b.w) and the B. juncea seed powder (500 mg/kg) (Inyang et al., 2014).
Group IV- rats were exposed to both an oral dose of arsenic (10 mg/kg) and the nanoparticles of B. juncea seed powder (500 mg/kg).
Group V- rats were administered only a daily oral dose of the nanoparticles of the antioxidant B. juncea seed powder (500 mg/kg).
Post exposure period, whole blood samples were collected from rats for the COMET assay. Sera was prepared to assess the endpoints of oxidative stress and DNA damage. Samples of liver and brain were excised out after dissection and stored at −80 °C until further analysis.
2.3 Bioassays
Serum levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) were assessed using a commercial ELISA kit conforming to the manufacturer’s protocol. Hepatic concentrations of p53 protein were also estimated using a commercially available ELISA kits.
2.4 COMET assay
The single-cell gel electrophoresis or Comet assay was used to assess the DNA fragmentation which was quantified and analyzed in individual blood cells following a standard protocol (Ostling and Johanson, 1984; Singh et al., 1994). The slides after being stained in the fluorescent dye were observed under the fluorescence optical microscope (Nikon Eclipse TI-E, Japan) equipped with excitation (465 nm) and barrier (595 nm) filters. Randomly 20 selected cells were scored on each slide using the computer image analysis Comet assay IV Windows’s software with monochrome CCD IEEE1394 FireWire video camera (Perceptive Instruments, Halstead, UK). The evaluating parameters used to measure the extent of DNA damage were the tail intensity (%) and tail moment. The tail intensity assesses the percentage of migrated genomic DNA from the nuclear core to the tail and tail moment is calculated as: tail moment = tail length × tail intensity/100.
2.5 Determination of arsenic concentration in brain
The tissue samples were analyzed with ICP-MS (Inductive Coupled Plasma-Mass Spectrometer, Thermo Fisher Scientific, Instrument) at the College of Science (CLCS) at King Saud University, Riyadh, KSA. The external calibration was carried out by using multi-elements standard of 10 ppm concentration with each sample being run in triplicates. Table 1 highlights the operating conditions of the instrument used for analysis (Cubadda, 2007).
Parameters
Value/Condition
RF frequency
40 MHz
RF Power
1548.6 W
Pirani Pressure
1E+2 mbar
Penning Pressure
9.549E-8 mbar
Detector Counting Voltage
1750 V
Detector Analog Voltage
−1825 V
Plasma gas flow[Ar, 99.997]
13.84 L/min
Auxiliary gas flow[Ar, 99.997]
0.8 L/min
Nebulizer gas flow [Ar, 99.997]
0.9 L/min
Sampler and Skimmer cone
Nickel
Mode of operation
Standard mode (STD)
Sample Uptake
30 s
Peristaltic Pump Rate
40 rpm
Nebulizer
Glass concentric type
Spray Chamber
Quartz, Cychronic type
Spray Chamber Temperature
−20 °C
Injector
Quartz, 2.5 mm ID
Torch
Two concentric quartz tubes
Sample tubing
Standard 0.508 mm i.d.
Drain tubing:
Standard 1.29 mm i.d.
Dwell Time
0.01 s
Number of Replicates
3
Rinse Time
30 s
Resolution m/z
238 amu
Isotope ratio precision CeO/Ce
<3 %
Short-term stability
< 3% RSD
2.6 Statistical analysis
The data was analyzed using the SPSS version 22.0 statistical package (SPSS, Chicago, IL, USA). All values are expressed as the mean ± standard deviation (SD). The analysis included one-way analysis of variance (ANOVA) followed by the Tukey's HDS test for post hoc pairwise comparisons with a p-value of ≤0.05 considered statistically significant.
3 Results
3.1 Oxidative DNA damage
The serum 8-OHDG levels in serum were significantly (p ≤ 0.05) increased in the group exposed to As only (67.2688 ± 7.76389 ng/ml), contrary to the control group (5.5875 ± 0.0.71448 ng/ml). Treatment with both mustard seeds (As + Mus) and the nanoparticles (As + NnM) significantly (p ≤ 0.05) lowered the 8-OHDG levels. However, treatment with nanoparticles of mustard seeds (4.9375 ± 0.64096 ng/ml) was significantly (p ≤ 0.05) more efficacious than the mustard seeds (29.664 ± 0.96662 ng/ml). The 8-OHDG levels in serum in the group exposed to nanoparticles only (NnM) was not significantly different from the control group (Fig. 1).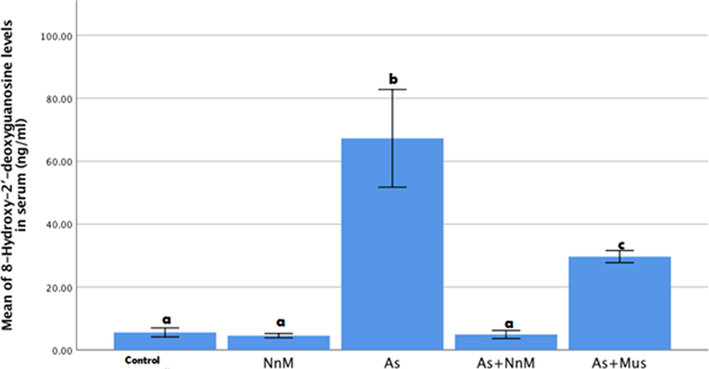
Mean (±SD) serum levels of 8-OHdG (ng/ml)of rats exposed to arsenic (As) and treated with Indian mustard seeds (As + Mus) and nanoparticles of mustard seeds (As + NnM).Different letters indicate significant (p ≤ 0.05) difference between the experimental groups.
3.2 Biomarkers of genotoxicity
There was a significant (p ≤ 0.05) increase observed in the hepatic levels of p53 protein on exposure to As (3.2625 ± 0.20073 µg/ml) contrary to the control (0.4957 ± 0.02089 µg/ml). A significant (p ≤ 0.05) reduction was observed in the levels in both the treated groups, with mustard seeds and its nanoparticles (As + Mus; 0.7274 ± 0.14021 µg/ml and As + NnM; 0.6099 ± 0.15738 µg/ml). However, there was no significant difference observed between the treated groups (Fig. 2).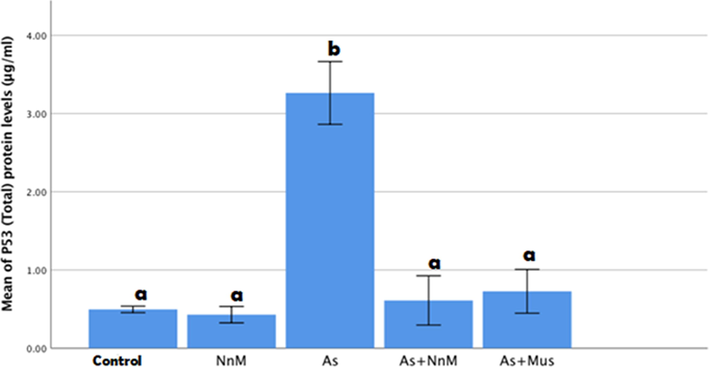
Mean (±SD) hepatic levels of p53 protein (μg/ml)of rats exposed to arsenic (As) and treated with Indian mustard seeds (As + Mus) and nanoparticles of mustard seeds (As + NnM). A significant (p ≤ 0.05) difference is observed in values with different letters.
An estimation of the levels of pS15 protein showed that exposure to arsenic exhibited a significant (p ≤ 0.05) increase in the levels of the protein (3.1505 ± 0.25128 µg/ml) contrary to control (1.6202 ± 0.04479 µg/ml). The levels of pS15 protein were significantly (p ≤ 0.05) reduced in both treated groups, (As + Mus and As + NnM). Nevertheless, significantly (p ≤ 0.05) lower levels were observed in the group treated with nanoparticles (1.4844 ± 0.12606 µg/ml) in comparison to the group treated with mustard seeds (2.4862 ± 0.15876 µg/ml). The levels of pS15in the As + NnM group was comparable to the control group (Fig. 3).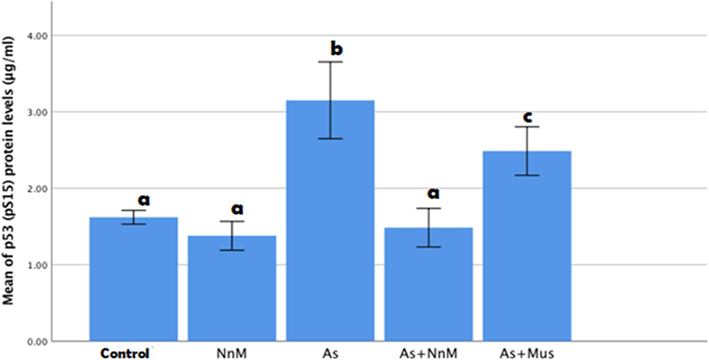
Mean (±SD) hepatic levels of p53 (pS15) protein (μg/ml) of rats exposed to arsenic (As) and treated with Indian mustard seeds (As + Mus) and nanoparticles of mustard seeds (As + NnM). A significant (p ≤ 0.05) difference is observed in values with different letters.
3.2.1 Comet assay
Distinct comets were observed in the group exposed to arsenic in comparison to the control group. The DNA disintegration was evaluated based on parameters of the comets; tail length and tail moment (Fig. 4). A significant (p ≤ 0.05) increase was observed in the tail length (170.1467 ± 11.3580) post exposure to As for two weeks when compared to the control group (94.9333 ± 0.4133) (Fig. 6). Additionally, tail moment was also significantly (p ≤ 0.05) elevated (57.1300 ± 10.11894) contrary to the control (16.5433 ± 0.29242) (Fig. 6). Treatment with both mustard seed extract and its nanoparticles exhibited a significant (p ≥ 0.05) decrease in the tail length and tail moment. However, the treatment with the nanoparticles (tail length: 64.4533 ± 6.30159; tail moment: 3.4161 ± 0.34056) was significantly (p ≤ 0.05) more marked than the bulk mustard seed extract (tail length: 127.4133 ± 8.19874; tail moment: 32.1599 ± 5.78339) (Fig. 5).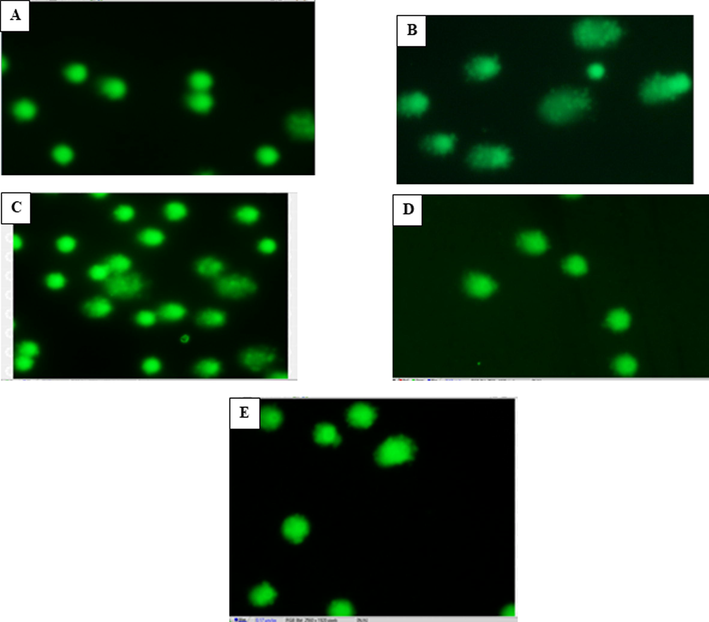
Microphotographs of Comet assay showing effect of Indian mustard seeds and the nanoparticles of mustard seeds on arsenic induced DNA damage in whole blood samples. (A) Micrograph showing fluorescent stained supercoiled DNA intact within the nuclear membrane of control cells. (B) Micrograph showing DNA migration out of the cell as long comet tails (arrows) in cells exposed to arsenic (As) contrary to the time-matched controls. (C) Micrograph of cells exposed to arsenic and treated with mustard seeds (As + Mus), showing mild degree of degeneration of DNA contrary to the As-exposed group. (D) Micrograph of cells exposed to arsenic and treated with nanoparticles of mustard seeds (As + NnM), showing fewer comets with reduced DNA migration compared with the As-exposed group. (E) Representative micrograph of fluorescent DNA stain of positive control cells mustard nanoparticles, showing undamaged and supercoiled DNA remaining within the nuclear cell membrane.
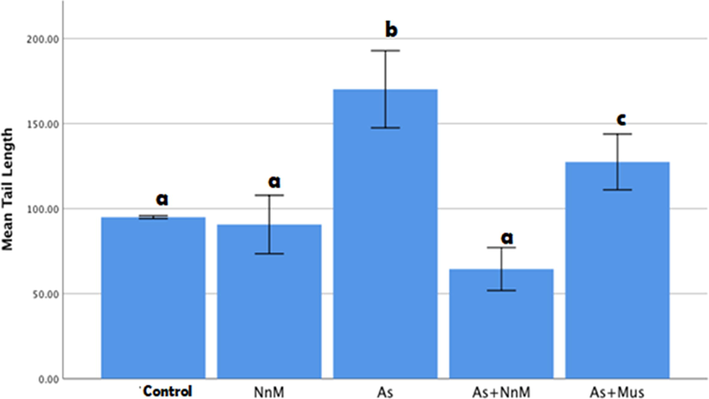
Mean (±SD) tail length measured to assess DNA damage in blood cells of rats exposed to arsenic (As) and treated with Indian mustard seeds (As + Mus) and nanoparticles of mustard seeds (As + NnM). A significant (p ≤ 0.05) difference is observed in values with different letters.
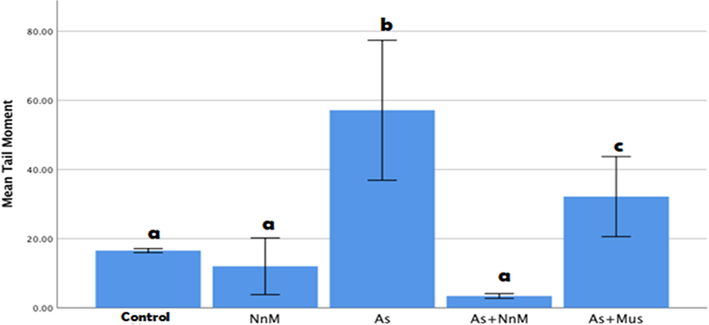
Mean (±SD) tail moment measured to assess DNA damage in blood cells of rats exposed to arsenic (As) and treated with Indian mustard seeds (As + Mus) and nanoparticles of mustard seeds (As + NnM). A significant (p ≤ 0.05) difference is observed in values with different letters.
3.3 Bioaccumulation of arsenic in brain
Exposure to As significantly (p ≤ 0.05) increased the arsenic concentration (117.6488 ± 18.2854 ppb) in the brain in comparison to the control (2.8115 ± 0.91007 ppb). Treatment with mustard seeds (As + Mus; 66.1218 ± 2.73890 ppb) and nanoparticles (As + NnM; 56.5976 ± 2.89225 ppb) significantly reduced the arsenic concentration in the brain. However, there were no significant between the two treatments (Fig. 7).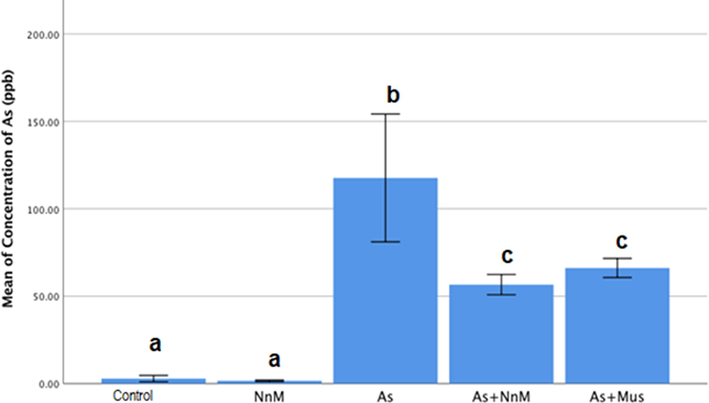
Mean (±SD) concentration (ppb) of arsenic in brain tissue of rats exposed to arsenic (As) and treated with Indian mustard seeds (As + Mus) and nanoparticles of mustard seeds (As + NnM). A significant (p ≤ 0.05) difference is observed in values with different letters.
4 Discussion
The generation of reactive oxygen species (ROS) has been incriminated in the arsenic induced toxicity (Akram et al., 2016). Genotoxicity due to arsenic is not directly communicated to DNA, however, it exhibits its effect on DNA indirectly via oxidative damage triggered by the ROS or by the dysregulation of DNA repair enzymes (Kitchin and Ahmad, 2003). In the present study, the serum levels of 8-OHdG were significantly elevated corresponding to the As-induced oxidative DNA damage. The free radicals generated by heavy metals such as As, target both the purines and pyrimidines, with guanine being most vulnerable to oxidation (Delaney et al., 2012). The addition of hydroxyl radical to the eighth carbon of the molecule leads to the formation of a modified product 8-hydroxydeoxyguanosine (8-OH) which has been recognized as a biomarker of oxidative damage (Valavanidis et al., 2009). Previous studies have reported a positively significant correlation between arsenic exposure through contaminated water or coal burning with elevated urinary 8-OHdG levels (Yamauchi, 2004; Li et al., 2008).
Further, As induced genotoxicity was assessed by comet assay in whole blood samples. The comet tail length and tail moment are the most frequent parameters evaluated to illustrate DNA damage in this technique. A significant increase in both tail moment and tail length was observed on As exposure in the present study. This is in line with a previous study that reported marked increase in comet tail length and tail moment in ovarian cells of rat exposed to As in comparison to control (Arslan-acaroz et al., 2018). Additionally, Flora et al. (2012) also reported that the COMET assay performed in whole blood exhibited a remarkable increase in tail length of rats exposed to As. Further, Akram et al. (2016) reported a marked relationship between the As accumulation and DNA fragmentation.
The tumour suppressor protein, p53 plays a pivotal role in response to toxins that cause DNA damage and is at the node of the cellular DNA damage response pathways (Loughery et al., 2014). In the present study, As exposure for two weeks resulted in a marked induction of hepatic levels of p53 protein and pS15 protein, a form of p53 phosphorylated at serine 15. It is noteworthy that the role of As exposure on p53 expression is conflicting and varies with arsenic species, concentration, exposure time, and cell types. It has been reported that p53 is activated by high doses and suppressed by low levels of As. Indeed, the activation at high concentrations of As (>20 µmol/L) is mediated through DNA damage (Huang et al., 2008). Menendez et al. (2001) reported As induced p53 expression a human lyphoblastoid cell line, via an ataxia telangiectasia mutated (ATM) (a member of PI3-kinase-related protein kinase)-dependent pathway, which phosphorylates p53 at serine 15 (Menendez et al., 2001). Further, Vogt and Rossman (2001) in a cell culture of W138 normal human lung fibroblasts, treatment with 0.1 µM arsenite for 14 days exhibited a 3-fold enhancement in the cellular levels of p53 protein without any concomitant increase in p21 protein, a major downstream protein of the cell-cycle arrest that often mirrors an increase in the p53 expression. One of the first events that occur as a part of the p53 modification post metabolic stress or DNA damage is the phosphorylation of p53 at Ser15 (Loughery et al., 2014). Under normal stable conditions transactivation of p53 is suppressed as it is associated with its inhibitor MDM2. During genotoxic assaults, such as As toxicity, phosphorylation at serine 15 modulates the p53 leading to a conformational change in the protein. Subsequently, the inhibitor MDM2 is unable to bind p53 in the latter new conformation thereby relieving the transcriptional inhibition which eventually results in elevated levels of p53 (Hu et al., 2020). Furthermore, the findings of the present study, showed a significantly enhanced bioaccumulation of As in the brain in all the groups exposed to As. Similar increased concentrations of arsenic were observed in the brain tissue of bats foraging on waste water treatment plant effluent contrary to bats in the reference site. This explains the previous observations that As has the ability to cross the blood brain barrier and could be potentially cause neurological impairment (Hill et al., 2018). There have been reports from several epidemiological studies in the past decade which provide substantial evidence that supports a strong correlation between As exposure and neurological dysfunctioning leading to cognitive impairment in children and adults (Tyler and Allan, 2014). Marked neurodegenerative symptoms similar to that of Alzheimer’s Disease have also been reported on chronic exposure to As (Andrade et al., 2015) which is best explained as a result of As- induced oxidative damage in brain of rats (Noman et al., 2015; Arslan-Acaroz et al., 2018).
A recent review by Hu et al. (2020) shows a comprehensive account of studies that reported the ameliorative effect of several natural antioxidants against As toxicity including genotoxicity. In the present study, the aqueous extract of B. juncea seed and its nanoformulation exhibited marked potency in reversing the modulatory effects of As exposure on the endpoints evaluated, namely accumulation of arsenic in brain, serum levels of 8-OHdG, p53, pS15 and DNA damage as assessed by the COMET assay. This is plausibly attributed to the anti-genotoxic and antioxidant status of the mustard seeds (Parikh et al., 2015). Taken together, a broad assessment of the comparative efficacy of the mustard seed extract and its nanoparticles illustrated that the ameliorative effect of the nanoparticles was more pronounced.
Owing to its potent antioxidant potential the use of Indian mustard seed (B. juncea) (Sahu et al., 2020), could be a promising therapeutic nutritional intervention in combating arsenic-induced oxidative stress.Further, the bioavailability of the seeds could be enhanced with the nanosization which could prove to be an effective potential nutraceutical.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for funding this work through research group no (RG-1441-311).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Assessment of arsenic induced DNA fragmentation by using comet assay. J. Carcinogen. Mutagene.. 2016;7:258.
- [CrossRef] [Google Scholar]
- Lead, arsenic, and manganese metal mixture exposures: focus on biomarkers of effect. Biol. Trace Elem. Res.. 2015;166(1):13-23.
- [CrossRef] [Google Scholar]
- In vivo assessment of polydatin, a natural polyphenol compound, on arsenic-induced free radical overproduction, gene expression, and genotoxicity. Environ. Sci. Pollut Res.. 2018;25:2614-2622.
- [Google Scholar]
- Chemical and biological consequences of oxidatively damaged guanine in DNA. Free Radic. Res.. 2012;46:420-441.
- [CrossRef] [Google Scholar]
- Antioxidants from defatted Indian Mustard (Brassica Juncea) protect biomolecules against in vitro oxidation. Physiol. Mol. Biol. Plants. 2014;20(4):539-543.
- [CrossRef] [Google Scholar]
- A possible mechanism for combined arsenic and fluoride induced cellular and DNA damage in mice. Metallomics. 2012;4(1):78-90.
- [Google Scholar]
- Arsenic exposure, diabetes-related genes and diabetes prevalence in a general population from Spain. Environ. Pollut.. 2018;235:948-955.
- [Google Scholar]
- The brains of bats foraging at wastewater treatment works accumulate arsenic, and have low non-enzymatic antioxidant capacities. Neurotoxicology. 2018;69:232-241.
- [CrossRef] [Google Scholar]
- The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules. 2020;10(240)
- [CrossRef] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Arsenic, Metals, Fibres and Dusts. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100C.) ARSENIC AND ARSENIC COMPOUNDS. Available from: https://www.ncbi.nlm.nih.gov/books/NBK304380/.
- Inyang, I. J., Eyo, A. O., Olajide, T. M., & Essien, A., (2014). Effects of ethanolic extract of Brassica juncea (Mustard Seed) on the brain and kidney tissues of albino wistar rats, 04(22), 75-82.
- Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget. 2017;8(14):23905-23926.
- [CrossRef] [Google Scholar]
- Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol. Lett.. 2003;137(1-2):3-13.
- [Google Scholar]
- Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res.. 2014;42(12):7666-7680.
- [Google Scholar]
- Molecular insight of arsenic-induced carcinogenesis and its prevention. Naunyn-Schmiedeberg's Arch. Pharmacol.. 2017;390:443-455.
- [CrossRef] [Google Scholar]
- Protection of arsenic-induced hepatic disorder by arjunolic acid. Basic Clin. Pharmacol. Toxicol.. 2007;101(5):333-338.
- [Google Scholar]
- Arsenic phytoremediation: finally a feasible approach in the near future. In: Saldarriaga-Noreña H., Alfonso Murillo-Tovar M., Farooq R., Dongre R., Riaz S., eds. Environmental Chemistry and Recent Pollution Control Approaches. IntechOpen; 2019.
- [Google Scholar]
- status confers sensitivity to arsenic cytotoxic effects. Mutagenesis. 2001.ATM;16:443-448.
- [Google Scholar]
- Arsenic accumulation in rice and probable mitigation approaches: A review. Agronomy. 2017;7(4):67.
- [CrossRef] [Google Scholar]
- Arsenic-induced histological alterations in various organs of mice. J. Cytol. Histol.. 2015;6:323.
- [CrossRef] [Google Scholar]
- Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun.. 1984;123(1):291-298.
- [Google Scholar]
- Phytoextract of Indian mustard seeds acts by suppressing the generation of ROS against acetaminophen-induced hepatotoxicity in HepG2 cells. Pharm. Biol.. 2015;53(7):975-984.
- [CrossRef] [Google Scholar]
- Sahu, M., Devi, S., Mishra, P., Gupta, E., 2020. Mustard Is a Miracle Seed to Human Health. 10.4018/978-1-7998-2524-1.ch012. In: Ethnopharmacological Investigation of Indian Spices (pp.154-162): IGI.
- Protective effects of Moringa oleifera Lam. leaves against arsenic-induced toxicity in mice. Asian Pac. J. Trop. Biomed.. 2014;4:S353-S358.
- [Google Scholar]
- Mechanisms Pertaining to Arsenic Toxicity. Toxicol. Int.. 2011;18(2):87-93.
- [CrossRef] [Google Scholar]
- Modifications of alkaline microgel electrophoresis for sensitive detection of DNA damage. Int. J. Radial Biol.. 1994;66(1):23-28.
- [Google Scholar]
- The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: A review. Curr. Environ. Health Rep.. 2014;1(2):132-147.
- [Google Scholar]
- Valavanidis, A., Vlachogianni, T., Fiotakis, C. (2009). hydroxy-2’-deoxyguanosine (8-ohdg): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 27, 120–139.
- Effects of arsenite on p53, p21 and cyclin D expression in normal human fibroblasts—a possible mechanism for arsenite's comutagenicity. Mutat. Res.. 2001;478(1–2):159-168.
- [Google Scholar]
- World Health Organization.2012. Arsenic, fact sheet No 372 [Internet]. Geneva: Available from: http://www.who.int/mediacentre/factsheets/fs372/en/ [cited 2014 May 16].
- Evaluation of DNA damage in patients with arsenic poisoning: Urinary 8-hydroxydeoxyguanine. Toxicol. Appl. Pharmacol.. 2004;198(3):291-296.
- [CrossRef] [Google Scholar]







