Translate this page into:
Association of polymorphisms in inflammatory cytokine genes with the development of head and neck cancer in Pakistani population
⁎Corresponding authors. fiazkhanhu333@gmail.com (Muhammad Fiaz Khan), saminabee@gmail.com (Samina Qamer)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Head and neck cancer (HNC) is the most common type of carcinoma and represents a major health problem in developing countries. HNC is a multifactorial condition associated with a variety of risk factors comprising genetic, environmental and life style. Genetic polymorphisms of interleukin genes might contribute to the development of HNC. Present study was conducted to investigate a possible association of HNC with single nucleotide polymorphisms (SNPs) in interleukin genes (IL-8 rs4073 A/T, IL-4 rs2070874 C/T, IL-10 rs1800896 T/C, IL-10 rs180072 T/G, IL-6 rs1800796 C/G, IL-6 rs1800795 C/G, and IL-1-α rs17561 A/C) and oral hygiene in Pakistani Population. For this purpose, 231 cases and 219 controls were recruited. The SNPs were assessed through QuantStudio real time PCR system. Analysis of the data showed a significant association of IL-10 rs1800896 with HNC while other SNPs were not associated was HNC pathogenicity. Further, no significant association was observed between hygiene associated factors and HNC in the studied population. This study presents useful aspects of HNC in Pakistani cohort.
Keywords
Head and neck cancer
Polymorphism
Interleukin
IL-1α
IL-4
IL-6
IL-8
IL-10
1 Introduction
Head and neck cancer (HNC) comprising the malignancies of oral cavity, pharynx and larynx, is 5th most common cancer type in developing world, and accounts for>650,000 cases and 330,000 deaths per year. Alcohol and tobacco use are the two most important risk factors for HNCs, especially carcinomas of the oral cavity, oropharynx, hypopharynx, and larynx (Gandini et al., 2012). Moreover, some studies suggest the association of HNC with poor oral hygiene (Guha et al., 2007; Gandini et al., 2012; Eliot et al., 2013). Additionally, genetic risk factors of HNC have also been reported extensively (Sturgis and Wei, 2002).
Cytokines are the peptides released in response to inflammation and infection and can function to inhibit carcinogenesis. However, cytokines can cause defects in apoptosis, promote growth and facilitate metastasis. Studies show that disrupted serum level of interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10) and members of interleukin-1 family are associated with oncogenesis via various pathways. Interleukin-1α (IL-1α) (member of IL-1 family), IL-6 and L-10 are involved in differentiation, proliferation, B-cell activation and periodontitis lesion (Yamazaki et al., 1994). IL-4 has an inhibitory effect on inflammation, thrombosis, angiogenesis, growth, or invasion of some types of cancer (Vairaktaris et al., 2007). IL-8 is a pro-inflammatory, which promotes neutrophils chemotaxis and degranulation. The high expression of IL-8 has been reported in endothelial cells, infiltrating neutrophils, and tumor-associated macrophages. IL-8 through its receptor on tumor cells, autocrine pathway, can support tumor migration and invasion, and through its receptors on endothelial cells, paracrine pathway, contributes to angiogenesis (Mojtahedi et al., 2014).
Present study was performed to find out the possible association of inflammation-related genes (IL-1α, IL-4, IL-6, IL-8 and IL-10) SNPs with HNC in Pakistani population. To the best of our knowledge, these genes polymorphisms have not been studied in Pakistani population.
2 Materials and methods
2.1 Study subjects
Present study was approved by the Ethics Committee of the Institute of Radiology and Nuclear Medicine (IRNUM) hospital, Peshawar, Pakistan. Written informed consent was taken from all the subjects. Histopathologically confirmed individuals with HNC including malignant neoplasm of the oral cavity, oropharynx, hypopharynx and larynx were recruited from IRNUM Peshawar, from November 2015 to August 2016. From all the participants including 231 patients and 219 age and sex control individuals, 3–5 ml blood samples were collected in EDTA containing vacutainer tubes.
2.2 Data collection
Data was collected by using a standard questionnaire that included questions regarding demographic variables (age, sex, family income, education level), oral hygiene (self-report of gum bleeding, use of denture, number of missing teeth and periodontal diseases), dental health care (tooth brushing, material used for cleaning teeth, dental floss use, mouthwash) and other risk factors (use of naswar, smoking, paan chewing). To assess the overall oral hygiene, visual inspection of each participant was performed with the help of dentist.
2.3 DNA extraction and genotyping
DNA was isolated from whole blood using phenol chloroform method. Extracted DNA from both cases and controls were used for genotyping. Seven SNPs (IL-8 rs4073 A/T, IL-4 rs2070874 C/T, IL-10 rs1800896 T/C, IL-10 rs180072 T/G, IL-6 rs1800796 C/G, IL-6 rs1800795 C/G, and IL-1-α rs17561 A/C) of five inflammation-related genes were genotyped using Taqman-based allelic discrimination method on a QuantStudio 5 Real Time PCR system (Thermo Fischer). The PCR conditions were as follows: 40 cycles of 60 °C for 30 sec, 95 °C for 5 min, 95 °C for 15 sec, and 60 °C for 60 sec. For each sample reactions were performed in duplicate and included negative control in every reaction.
2.4 Statistical analysis
Patient characteristics (age, gender, socioeconomic status, oral hygiene, alcohol consumption, and use of tobacco or Paan chewing) were evaluated to see if differences existed between cases and controls. These comparisons were examined using medians and interquartile range (25th and 75th percentiles) for age and frequency, percentage, and χ2 or Fisher’s Exact Tests where applicable. Hardy-Weinberg Equilibrium was assessed using χ2 tests. The main focus of the study was to assess the relationship between case-control status and SNPs. This was performed using univariable and multivariable logistic regression to test for any difference in the genotypes for each SNP. In the multivariable model, age and gender were included as adjusters. The relationship between naswar, socioeconomic status variables, and oral hygiene variables were examined to check if one could be used as an adjuster to represent all of them, because of the sample size limit, the number of adjusters and to avoid multicollinearity. This was done using χ2 or Fisher’s exact tests where appropriate.
3 Results
During the study period, 231 HNC cases (147 oral cancers, 29 laryngeal cancers, 24 Hypopharyngeal cancer, 23 Oropharyngeal cancers and 8 Pharyngeal cancers) and 219 controls were recruited.
3.1 Characteristics of the studied subjects
Cases and controls included in the current investigation were considerably different in age. The number of males were higher than females in both cases (55% males and 45% females) and control (72.2% males and 27.9% females) groups. Both cases and control varied in demographic distribution. Resident of rural area found to be high proportion in both cases and control (79.2% cases and 64.8% control) as compared to urban area (20.8% cases and 35.2% control). Highest frequency of cases was found to be illiterate as compared to controls (84.4% cases and 25.1% control) and majority of cases belong to underprivileged socioeconomic families as compared to controls (93.5% cases and 47% control). Also, a significantly high number of cases had poor oral hygiene compared to controls (90.5% cases and 23.7% control). Majority of cases never brush the teeth compared to controls (89.6% cases and 49.3% control). Moreover, the percentag of missing teeth and the use of naswar were significantly greater among cases compared to control. However, very less proportion subjects were found to be smokers. Only 1.7% of cases contrasting to 0.9% controls used to chew paan. Similarly, 1.3% of used to drink alcohol compared to 0.5% of controls (Table 1).
Characteristic
Cases
Controls
N
Median (IQR*) or Frequency (%)
N
Median (IQR*) or Frequency (%)
P-value
Age
231
56.00 (47.00, 62.00)
219
27.00 (22.00, 36.00)
<0.01 *
Gender
231
219
<0.01 *
Female
Male–
104 (45.0)
127 (55.0)–
61 (27.9)
158 (72.2)–
Residence Type
231
–
219
–
<0.01 *
Rural
Urban–
183 (79.2)
48 (20.8)–
142 (64.8)
77 (35.2)–
Literate
231
219
<0.01 *
No
Yes
195 (84.4)
36 (15.6)
55 (25.1)
164 (74.9)–
Family Income
231
–
219
–
<0.01 *
<20,000
20,000–50,000
>50,000–
216 (93.5)
13 (5.6)
2 (0.9)–
103 (47.0)
78 (35.6)
38 (17.4)–
Oral Hygiene
231
219
<0.01 *
Poor
Fair
Good–
209 (90.5)
22 (9.5)
0 (0.00)–
52 (23.7)
93 (42.5)
74 (33.)–
Brush teeth/day
231
–
219
–
<0.01 *
No
Yes
207 (89.6)
24 (10.4)–
108 (49.3)
111 (50.7)
Missing Teeth
231
–
219
–
<0.01 *
None
1–10
>10–
42 (18.2)
93 (40.3)
96 (41.6)–
139 (63.5)
71 (32.4)
9 (4.1)–
Ever Smoke
231
–
219
–
0.58
No
Yes–
194 (84.0)
37 (16.0)–
188 (85.8)
31 (14.1)–
Ever Use Naswar
231
219
–
<0.01 *
No
Yes–
145 (62.8)
86 (37.2)
182 (83.1)
37 (16.9)
Ever Chew Paan
231
–
219
–
0.69
No
Yes–
227 (98.3)
4 (1.7)–
217 (99.1)
2 (0.9)–
Ever Drink Alcohol
231
–
219
–
0.62
No
Yes–
228 (98.7)
3 (1.3)–
218 (99.5)
1 (0.5)–
3.2 Genetic analysis
For genotyping, allele specific PCR was performed. Allele specific PCR revealed that the frequency of IL-8 rs4073 wild homozygous allele AA was 0.13 (15% cases and 11% controls), heterozygous AT alleles were 0.46 (45.1% cases and 48.6% controls), homozygous minor allele was AA 0.4 (39.8% cases and 40.5% control). However, no significant statistical difference was observed for rs4073 between the two groups (Table 2).
SNP
Genotype
CasesN (%)
ControlsN (%)
UnivariableP-value
MultivariableP-value
IL8 rs4073 A/T
AA
34 (15.0)
23 (11.0)
0.43
0.68
AT
102 (45.1)
102 (48.6)
.
.
TT
90 (39.8)
85 (40.5)
.
.
IL4 rs2070874 C/T
CC
157 (70.4)
150 (70.8)
0.75
0.19
CT
58 (26.0)
57 (26.9)
.
.
TT
8 (3.6)
5 (2.4)
.
.
IL10 rs1800896 T/C
TT
114 (50.0)
84 (38.9)
0.01
0.01
TC
89 (39.0)
115 (53.2)
.
.
CC
25 (11.0)
17 (7.9)
.
.
IL10 rs180072 T/G
TT
51 (22.7)
38 (17.9)
0.46
0.98
TG
102 (45.3)
104 (49.1)
.
.
GG
72 (32.0)
70 (33.0)
.
.
IL6 rs1800796 C/G
CC
18 (8.0)
15 (7.0)
0.22
0.99
CG
81 (36.0)
95 (44.2)
.
.
GG
126 (56.0)
105 (48.8)
.
.
IL6 rs1800795 C/G
CC
9 (4.0)
7 (3.3)
0.28
0.70
CG
60 (26.7)
72 (33.6)
.
.
GG
156 (69.3)
135 (63.1)
.
.
IL1-α rs17561 A/C
AA
20 (8.9)
14 (6.5)
0.63
0.90
AC
102 (45.5)
99 (46.0)
.
.
CC
102 (45.5)
102 (47.4)
.
.
For IL-4 rs2070874, the frequency of wild type allele CC homozygous was found to be highest (0.7) among both groups (70.4% cases and 70.8% controls) and heterozygous CT alleles frequency was 0.26 (26% cases and 26.9% controls). The homozygous minor allele TT showed frequency of 0.3 (3.6% cases and 2.4% controls) was less frequent. However, the association between case-control was statistically insignificant (Table 2).
For IL-10 rs1800896, the wild type homozygous TT allele frequency was 0.45 (50% cases and 38.9% controls), the heterozygous TC alleles was found with almost similar of 0.46 (39% and 53.2%). While, the minor allele CC frequency was 0.09 (11% and 7.9%). When compared between the two groups, TC genotype was less prevalent in cases as compared to controls, the difference between two groups was statistically significant (P = 0.01) (Table 2).
For IL-10 rs180072, the frequency of homozygous wild type allele TT was 0.2 (22.7% cases and 17.9 controls), heterozygous TG alleles were 0.47 (45.3% cases and 49.1% controls) and homozygous minor allele GG was 0.32 (32% cases and 33% controls) genotype was less frequent (22.7% cases vs 17.9% controls). However, the difference among the two groups was statistically insignificant (Table 2).
For IL-6 rs1800796, the CC wild allele was least with frequency of 0.075 (7% cases and 8% controls), the heterozygous CG alleles have a frequency of 0.4 (36% and 44.2%), while, the homozygous minor allele GG has a frequency of 0.52 (56% cases and 48.8% control). But the association between the two subject groups was statistically insignificant (Table 2).
For IL-6 rs1800795, the homozygous wild allele CC was found less frequent with 0.035 frequency (4% and 3.3%), heterozygous CG was 0.3 (26.7% cases and 33.6% controls) and GG homozygous allele was 0.66 (69.3% cases and 63.1% control). However, no significant association between rs1800795 and disease was found (Table 2).
For IL-1α rs17561, the wild type allele AA frequency was 0.077 (8.9% cases and 6.5% controls), the heterozygous AC was 0.455 (45.5% cases and 46% controls) and CC homozygous allele was 0.46 (45.5% vs 47.4%). Statistical analysis did not revealed any significant association of rs17561 and HNC in the studied population (Table 2).
Findings of present study show that frequencies of IL8 rs4073, IL4 rs2070874, IL10 rs1800896, IL-10 rs180072, IL-6 rs1800796, IL-6 rs1800795 and IL-1α rs17561 genotypes were in line with the Hardy-Weinberg Equilibrium in controls (P < 0.05) except IL-10 rs1800896 T/C (Table 3).
SNP
Homozygous Major Allele
HeterozygousAlleles
Homozygous Minor Allele
Controls N
P value
IL8 rs4073 A/T
23
102
85
210
0.35
IL4 rs2070874 C/T
150
57
5
212
0.88
IL10 rs1800896 T/C
84
115
17
216
0.01
IL10 rs180072 T/G
38
104
70
212
0.95
IL6 rs1800796 C/G
15
95
105
215
0.29
IL6 rs1800795 C/G
7
72
135
214
0.48
IL1-α rs17561 A/C
14
99
102
215
0.12
In the present study logistic regression analysis (Univariate model) shows that when the IL10 rs1800896 T/C TT homozygous genotype was used as reference the CC genotype was not associated with a significantly increased risk for oral cancer. While TC genotype was associated with a significantly increased risk for oral cancer (Fig. 1). However, multivariate model revealed that when the IL10 rs1800896 T/C TT homozygous genotype was used as reference the TC genotype and CC genotype were not associated with a significantly increased risk for oral cancer (Fig. 2).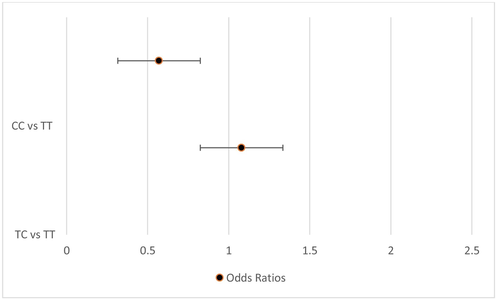
Logistic regression analysis of association among significant SNP (IL10 rs1800896 T/C) and risk of oral cancer (univariate model).
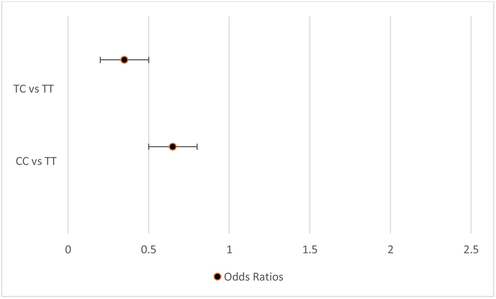
Logistic regression analysis of association among significant SNP (IL10 rs1800896 T/C) and risk of oral cancer (multivariate model).
4 Discussion
HNC is becoming a serious health threat worldwide and accounts for>550,000 cases and 380,000 deaths per year (Fitzmaurice et al., 2017). In Pakistan, HNC is most prevailing (32.6%) in male and second most prevalent (15.1%) malignancy after breast cancer (38.2%) in female (Hanif et al., 2009). It has been revealed that use of tobacco, alcohol intake, infection with human papillomavirus (HPV) and Epstein-Barr virus (EBV), and exposure to environment are the main etiologic factors contributing to HNC. In relation to oral health, the polymicrobial supragingival plaque has a pertinent mutagenic association with saliva, it may be contemplated as a potential independent factor, and individual oral hygiene may be a co-factor in the progression of oral cancer by excessive secretion of inflammatory mediators like cytokines (Gaudet et al., 2010). In addition to exogenous risk factors, development of cancer is also dependent on individual genetic makeup such variations in genes related to growth, development, differentiation, immune system, apoptotic pathways, alcohol metabolism, DNA repair, and activation of proto oncogenes (Lin and Karin, 2007; Ma et al., 2011; Bediaga et al., 2015). Single nucleotide polymorphisms (SNPs) are the most common genetic variation in humans, which may affect gene expression, protein function, and disease predisposition (Nachman, 2001; Hsu et al., 2014).
This study was conducted to find out the association of oral cancer with oral hygiene, use of tobacco and SNPs in interleukin genes. Oral squamous cell carcinoma (OSCC) was found to the most frequent (63.6%) followed by tumors of larynx, hypopharynx, and oropharynx, which is different from previous studies, where cancer of buccal mucosa (32%) and cancer of tongue (50%) were reported the most frequent (Tahir et al., 2013; Gul et al., 2017). The highest frequency of oral cancer patients was observed in the age group of >40–60 consistent to previous studies (Divaris et al., 2010). The proportion of affected males was higher than female which is consistent to previous report (Teofil et al., 2007). In Pakistan, one reason for increased incidence may be the use of tobacco in males. Data indicated that oral cancer are more common in individuals with poor oral health, low socioeconomic background and deprived education, consistent with previous reports across the globe (Zheng et al., 1990; Marshall et al., 1992; Talamini et al., 2000). Also, a significant association of missing teeth and risk of oral cancer was observed, which is consistent with previous studies (Marshall et al., 1992; Balaram et al., 2002; Lissowska et al., 2003).
Smoking is always supposed to be a vital risk factor in the progression of different malignancies particularly oral carcinoma (Tai et al., 2010; Wyss et al., 2013). Though, in this study there was an insignificant association with smoking. However, a significant association between naswar use and oral cancer was found (Table 1). Use of naswar is common practice in Pakistan, particularly in Khyber Pakhtunkhwa (KP) province, and it is particularly limited to population low socioeconomic strata (Imam et al., 2007). The association of alcohol and Paan is not clear, due to their rare use.
In present study, association of rs4073, rs2070874, rs1800896, rs180072, rs1800796, rs1800795 and rs17561 polymorphisms was studied with risk of HNC in Pakistani population. Among the studied SNPs, of rs4073, rs2070874, rs180072, rs1800796, rs1800795 and rs17561 didn’t showed association with the oral carcinoma in the studied population, which is in contrast to previous reports. The different results can be explained by difference in genetic backgrounds, ethnicities, geographical area, environmental factors, and also a correlation of the SNP with different risk factors.
However, only rs1800896 was found associated with the disease, consistent with Vairaktaris et al. (2008) study of European population. The SNP rs1800896 is located upstream of IL-10 gene, which is known to be involved in angiogenesis, inflammation, autoimmune diseases and many types of malignancies (including oral carcinoma). IL-10 is produced by lymphoid cells, monocytes and macrophages, a multifunctional immunosuppressant that not only interfere with the function of T-cells but also associated with the cessation of inflammatory responses (Rojas et al., 2017). Moreover, it regulates growth and differentiation of
B-cells, NK, cytotoxic and T helper cells, mast cells, granulocytes, dendritic cells, keratinocytes and endothelial cells (Iyer and Cheng, 2012).
Increased levels of IL-10 have been reported in cases with solid tumors, including oral squamous cell carcinoma (SCC), indicating that this pleiotropic cytokine may have a vital role in carcinoma (10–12). IL-10 has been observed to suppressing the immune and inflammatory responses and ultimately promote tumor proliferation (Ma et al., 2011; Hsu et al., 2014; Mojtahedi et al., 2014). Variations in promoters region of IL-10, including rs1800896, upregulate IL-10, which consequence in tumor proliferation by suppressing the immune and inflammatory responses.
One of the main limitation of the study is that sample size was not large enough. Further investigations with large sample size are required to disentangle and explain the observed link between polymorphism in IL10 rs1800896 and HNC.
5 Conclusion
In conclusion, this is the first attempt to study the association of interleukins with HNC in addition to oral-hygiene. The study shows that rs1800896 is a risk factor of HNC in Pakistani population.
Acknowledgments
The authors wish to thanks Higher Education Commission of Pakistan and Northwestern University, Chicago, USA for supporting this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oral cancer in southern India: The influence of smoking, drinking, paan-chewing and oral hygiene. Int. J. Cancer. 2002;98(3):440-445.
- [Google Scholar]
- Polymorphisms in alcohol and tobacco metabolism genes in head and neck cancer in the B asque C ountry. J. Oral Pathol. Med.. 2015;44(10):769-775.
- [Google Scholar]
- Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 2010;21(4):567-575.
- [Google Scholar]
- Periodontal disease and mouthwash use are risk factors for head and neck squamous cell carcinoma. Cancer Causes Control. 2013;24(7):1315-1322.
- [Google Scholar]
- Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol.. 2017;3(4):524-548.
- [Google Scholar]
- Mouthwash and oral cancer risk quantitative meta-analysis of epidemiologic studies. Ann. Agric. Environ. Med.. 2012;19(2)
- [Google Scholar]
- Body mass index and risk of head and neck cancer in a pooled analysis of case–control studies in the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Int. J. Epidemiol.. 2010;39(4):1091-1102.
- [Google Scholar]
- Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am. J. Epidemiol.. 2007;166(10):1159-1173.
- [Google Scholar]
- Epidemiology and pathological trends in oral squamous cell carcinoma in a local tertiary care hospital. Int. J. Community Med. Public Health. 2017;4(12):4440-4444.
- [Google Scholar]
- Institution-based cancer incidence in a local population in Pakistan: nine year data analysis. Asian Pac. J. Cancer Prev.. 2009;10(2):227-230.
- [Google Scholar]
- Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell. 2014;26(4):534-548.
- [Google Scholar]
- Use of smokeless tobacco among groups of Pakistani medical students–a cross sectional study. BMC Public Health. 2007;7(1):1-6.
- [Google Scholar]
- Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev.™ Immunol.. 2012;32(1)
- [Google Scholar]
- A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig.. 2007;117(5):1175-1183.
- [Google Scholar]
- Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur. J. Cancer Prev.. 2003 Feb 1;12(1):25-33.
- [Google Scholar]
- Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin. Cancer Res.. 2011;17(10):3478-3489.
- [Google Scholar]
- Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Eur. J. Cancer B Oral Oncol.. 1992;28(1):9-15.
- [Google Scholar]
- Serum levels of interleukin-7 and interleukin-8 in head and neck squamous cell carcinoma. Indian J. Cancer. 2014;51(3):227.
- [Google Scholar]
- Single nucleotide polymorphisms and recombination rate in humans. Trends Genet.. 2001;17(9):481-485.
- [Google Scholar]
- Rojas, J.M., Avia, M., Martín, V., Sevilla, N., 2017. IL-10: a multifunctional cytokine in viral infections. J. Immunol. Res. 2017.
- Genetic susceptibility–molecular epidemiology of head and neck cancer. Curr. Opin. Oncol.. 2002;14(3):310-317.
- [Google Scholar]
- The role of mast cells and angiogenesis in well-differentiated oral squamous cell carcinoma. J. Cancer Res. Ther.. 2013;9(3):387.
- [Google Scholar]
- Genetic polymorphisms in cytochrome P450 genes are associated with an increased risk of squamous cell carcinoma of the larynx and hypopharynx in a Chinese population. Cancer Genet. Cytogenet.. 2010;196(1):76-82.
- [Google Scholar]
- Oral hygiene, dentition, sexual habits and risk of oral cancer. Br. J. Cancer. 2000;83(9):1238-1242.
- [Google Scholar]
- Teofil, L.U.N.G., TĂŞCĂU, O.C., ALMĂŞAN, H.A. and MUREŞAN, O., 2007. Head and neck cancer, epidemiology and histological aspects–Part 1: A decade's results 1993–2002. J. Cranio-Maxillofacial Surg. 35(2), 120–125.
- Strong association of interleukin-4 (− 590 C/T) polymorphism with increased risk for oral squamous cell carcinoma in Europeans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol.. 2007;104(6):796-802.
- [Google Scholar]
- The interleukin-10 (-1082A/G) polymorphism is strongly associated with increased risk for oral squamous cell carcinoma. Anticancer Res.. 2008;28(1A):309-314.
- [Google Scholar]
- Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol.. 2013;178(5):679-690.
- [Google Scholar]
- IL-4-and IL-6-producing cells in human periodontal disease tissue. J. Oral Pathol. Med.. 1994;23(8):347-353.
- [Google Scholar]
- Dentition, oral hygiene, and risk of oral cancer: a case-control study in Beijing, People's Republic of China. Cancer Causes Control. 1990;1(3):235-241.
- [Google Scholar]







