Translate this page into:
Association of NAD + ADP-ribosyltransferase 1 gene polymorphism with the development of neonatal diabetes mellitus
⁎Corresponding author at: Department of Pediatrics, China-Japan Union Hospital of Jilin University, No.126, Xiantai Street, Changchun, Jilin 130033, China. ShaneBeardiky@yahoo.com (Xu Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Regulation of glucose metabolism by NAD-dependent ADP-ribosyltransferase is well investigated in different populations. Genetic variations NAD + ADP-ribosyltransferase has been shown to alter the activity of the enzyme and associated with susceptibility to diabetes. However, reports on neonatal diabetes are minimal. We, therefore, in the present report aimed to investigate the involvement of NAD + ADP-ribosyltransferase variants as a possible risk factor for predisposition to neonatal diabetes mellitus in Chinese patients. Neonatal subjects diagnosed with diabetes mellitus and healthy infants from a similar geographical area were enrolled at China-Japan Union Hospital of Jilin University, China. The involvement of NAD + ADP-ribosyltransferase with neonatal diabetes mellitus was investigated in DNA samples of all participants by PCR methods. One hundred Twenty neonatal diabetes mellitus cases and a matched number of healthy neonates were enrolled in the present study. GG and G SNPs of NAD + ADP-ribosyltransferase were mainly involved in developing neonatal diabetes mellitus and the individuals with GA SNPs protect responsible for protecting from neonatal diabetes mellitus. Thus, NAD + ADP-ribosyltransferase involved in neonatal diabetes mellitus and risk of developing is approx. 2 times higher compared to healthy subjects. Our finding showed that polymorphism in NAD + ADP-ribosyltransferase gene is associated with the predisposition to neonatal diabetes mellitus in Chinese. The present study also suggested that NAD + ADP-ribosyltransferase is a promising target for the treatment of neonatal diabetes mellitus and an effective care of patients. Our study results encourage conducting further investigation in the multi-centric clinical genetic study including larger samples to assesses the role of NAD + ADP-ribosyltransferase polymorphism in neonatal diabetes mellitus.
Keywords
Diabetes mellitus
Type-2
Genetic variations
Polymorphism
1 Introduction
Type 2 diabetes mellitus (T2DM) is mainly characterized by the individuals inability to respond or produce insulin. The resistance of insulin was also reported and is highly associated with dyslipidemia and hypertension. Dyslipidemia is one of the risk factors of this metabolic syndrome (Ansarimoghaddam et al., 2018) and also associated with the possible development of cardiovascular diseases (Martins et al., 2018; Zafar et al., 2019). In recent years the prevalence of resistance to insulin and the metabolic syndrome is increasing, mainly among younger populations in developing countries (Johnson, 2006; Ansarimoghaddam et al., 2018; Ali et al., 2012). Lifestyle changes in unhealthy manner also lead to both metabolic syndrome and insulin resistance. Generally, genetic factors in the individuals are useful to determine metabolic syndrome and insulin resistance. It was reported that the majority of loci related to T2DM is initially associated with the production of insulin and the function of β‐cell and fewer factors involving insulin resistance. Plasma membrane glycoprotein 1 (PC‐1) is mainly located on the chromosome 6 and specifically encodes for certain proteins which determine insulin sensitivity. However, the role of PC‐1 is not clearly determined; it involved in inhibiting the function of insulin receptor and disrupts signaling (Pappalardo et al., 2017; Johnson, 2006).
Diagnosis of diabetes in “early-onset” stage can happen within first months or days of life with presentation of hyperglycemia. However in some cases there are complications in neuron. Also, the time of diagnosis is very important, and problem persists to differentiate autoimmune type 1 diabetes and a monogenic cause. Many findings show that these early age diabetic cases are of monogenic origin and report for autoimmune markers of destruction of β-cell markers are poorly understood (Flanagan et al., 2006; Flanagan et al., 2007; Shield et al., 1997; Edghill et al., 2006). Although neonatal diabetes mellitus (NDM) is frequently considered a rare disease, however this disease affects a newborn for every 400,000 newborns. However this frequency was very high in European countries. Based on the condition, NDM is grouped as permanent NDM and transient NDM. In the case of transient NDM, patients required insulin treatment initially (12 weeks of age) and prolonged application is required in the case of transient NDM. Autoimmune diabetes is rare before 6 months of age, NDM is the result of impaired function of B-cell, decreased B-cell mass and abnormal pancreatic islet development (Polak and Cave, 2007; Grulich-Henn et al., 2010; Aguilar-Bryan and Bryan, 2008)
T2DM is the most lethal disease that contributes to the significant reason for morbidity, mortality, which leads to an essential factor of the economic burden of the country (Li et al., 2014). In children, the prevalence of neonatal T2DM is close to 10% and increased mortality rate was reported in various developing countries, including China. More than 1.4 million deaths per year are attributable to T2DM cases among the age of less than five years (Weng et al., 2016). However, previous epidemiological analysis revealed that among neonates prevalence of T2DM is higher than estimated. T2DM is a progressive disorder due to the diminished insulin secretion and resistance to insulin, which leads to dysfunction of glycemic control and increased risk of diabetic induced complications (Pratley et al., 2014).
NAD dependent adenosine diphosphate-ribosyltransferases is an important enzyme responsible for PARylation of ADP-ribose on important protein motif via ester linkage (Ren et al., 2018). NAD + ADP-ribosyltransferase induces various cellular activities such as structure of chromatin, repair of DNA deformatives, apoptosis, necrosis and differentiations of cells (Cohen and Chang, 2018). In humans, a total of seventeen types of NAD + ADP-ribosyltransferase have been reported and out of them expression of NAD + ADP-ribosyltransferase has been reported in nucleus and believe to regulated majority of NAD + ADP-ribosyltransferase actions (Zhu et al., 2013). It was reported that damage in the DNA trigger the NAD + ADP-ribosyltransferase and elicit the DNA repair process (Hur et al., 2006; Hassa and Hottiger, 2008; Corcoran et al., 2016; Smulson et al., 2000). Importantly, NAD + ADP-ribosyltransferase facilitate maintenance of genomic integrity by PARylation of histones proteins and other important enzymes molecules. It also helps in proper functioning of DNA repair system, protein–protein interaction and regulation of gene expression for optimal cellular homeostasis (Yu et al., 2002; Phulwani and Kielian, 2008; Chiarugi and Moskowitz, 2003). However, during the genotoxic and cellular and anxiety, delay stimulation of NAD + ADP-ribosyltransferase lead to a decline in NAD+ and ATP function and causes the discharge of mitochondrial pro-apoptotic protein, AIF. Dysregulation of cellular homeostasis by induction of stress mostly mediated through NAD + ADP-ribosyltransferase lead to necrosis of cells by induction of downstream signaling (Du et al., 2003).
The functional role of NAD + ADP-ribosyltransferase in the neonatal type 2 diabetes mellitus has not been explored in Chinese subjects. We, therefore, designed the present investigation to check the involvement of NAD + ADP-ribosyltransferase in neonatal T2DM in Chinese individuals. We hypothesized the possible relationship between NAD + ADP-ribosyltransferase with neonatal T2DM and aimed to investigate the involvement of NAD + ADP-ribosyltransferase a crucial risk factors for susceptibility to neonatal T2DM among Chinese individuals for effective patient care.
2 Materials and methods
2.1 Diagnosis and analysis
Neonates having T2DM diagnosed by expert clinician as per world heath organization criteria reporting to China-Japan Union Hospital of Jilin University, China. Healthy neonates were enrolled as controls from similar geographical areas. The association of NAD + ADP-ribosyltransferase was investigated by genotyping of all enrolled subjects by polymerase chain reaction (PCR). In the present investigation, the patients of both gender-confirming the diagnosis of neonatal type 2 diabetes mellitus or neonatal subject without T2DM were used. This experiment was performed at China in a single centre. The Institutional Human Ethical Committee was approved the study protocol and written consent was obtained from parents of each enrolled subject. Parents were earlier explained about the analytical procedure, and possible benefits of society were elegantly described. All enrolled patients and healthy controls were undergone routine laboratory investigations. About 2.5 ml of blood samples of all subjects were collected with an anticoagulant and genomic DNA was further isolated. The isolated genomic DNA were stored at −80 °C till genotyping procedure. Genotyping of NAD + ADP-ribosyltransferase’s variants were accessed by PCR followed by restriction fragment length polymorphism (RFLP) as described earlier (Yadollahi-Farsani et al., 1999). For genotyping of NAD + ADP-ribosyltransferase polymorphism the 25 μl reaction mixture was prepared with a set pf primers, 50 mM KCl, 2 μl of genomic DNA, 0.5 mM dNTP, 10 mM Tris–HCl (200 pm), one unit of the Taq polymerase enzyme with MgCl2. The temperature cycle was optimized. The amplicon was further digested with 5 U of FastDigest DdeI at 37 degree for one hour and differential banding patter was evaluated in the UV transilluminator. Similarly, other nuclear factor genes were amplified and genotyped with similar concentration of reaction mixtures.
2.2 Role of NAD + ADP-ribosyltransferase
As the present investigation was aimed to perform an initial study to decipher possible role of NAD + ADP-ribosyltransferase in Chinese neonatal T2DM patients, no power analysis was performed to determine appropriate sample size of the present study. We planned to enroll One hundred and Thirty subjects from each group. Before performing routine statistical analysis all data were subjected to normality test by KM or SW test. Data, those follow Gaussian distribution or inverted bell-shaped were compared using an unpaired t-test analysis. On the other hand, if results not following Gaussian distribution or inverted bell-shaped will be tested using an appropriate statistical test based on the number of sub-groups. Mean ± Standard deviation was considered for all experiments and the results were compared using non-parametric/parametric statistical analysis depend on their type of distribution. Categorical variables of results expressed as percentage and number format in every category were finally compared with other by Fisher exact test or Chi-square test using 2 × 2 contingency table. All statistical investigation were carried out by using Graph Pad Prism (version 6.2) and the P value <0.05 was considered as statistically significant.
3 Results
Two hundred and forty Chinese subjects were enrolled in the present investigation comprising of equal number of T2Dm cases and healthy controls. After the completion of experiment, both neonatal T2DM subjects and healthy control groups were analyzed using appropriate statistical analysis. An average age of neonatal type 2 diabetes mellitus patients was 3.1 (0.6) weeks and the age of healthy subjects was 3.5 (0.3) weeks. Gender dispersal was highly comparable among the subjects of neonatal T2DM subjects and healthy control groups (Table 1). Values are expressed as Mean (SD) for Age and BMI, and absolute values are presented for gender. N indicates the total number of individuals.
Variables
Neonatal type 2 diabetes mellitus Subjects
N = 120Healthy Subjects
N = 120
Age (in weeks), Mean (SD)
3.1 (0.6)
3.5 (0.3)
Gender
Male
85
100
Female
35
20
Bodyweight, kg
6.16
(1.47)5.7
(1.26)
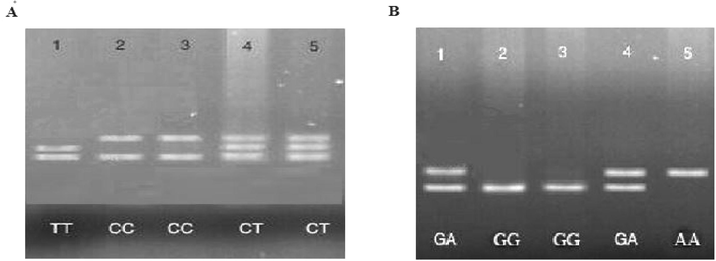
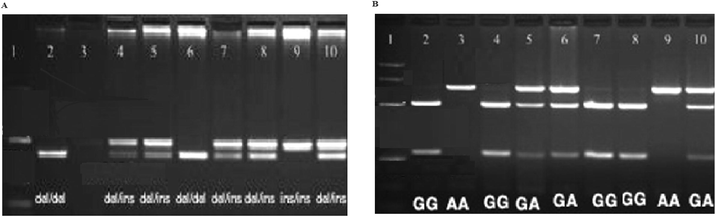
We observed that the subject with GA gene was susceptible to developing neonatal T2DM; the risk of neonatal T2DM was significantly higher in cases as compared to patients without no GG gene (p < 0.001). The present finding revealed that the presence of A allele in the individuals are highly susceptible to type 2 diabetes mellitus. The risk of neonatal T2DM was more than 1.7 fold in this group of patients than individuals without any G allele group (p < 0.001). In our study, we found the role of genotypes such as GA and GG among patients from neonatal T2DM group (Figs. 1 and 2; Table 2). We noticed the positive relationship of NAD + ADP-ribosyltransferase polymorphisms with neonatal T2DM when the analyzed association between neonatal T2DM and NAD + ADP-ribosyltransferase polymorphisms among subjects with neonatal T2DM. No significant association of NAD + ADP-ribosyltransferase polymorphism was observed in neonatal T2DM patients when compared with healthy subjects. We also found that the subjects with C allele were more susceptible to neonatal T2DM; the risk of neonatal T2DM was about 1.5 times higher among individuals having C allele than the subjects without any C allele. Values are expressed as absolute numbers. N = Total number of patients in each group.
Neonatal T2DM patients
N = 120Healthy Subjects
N = 120OR (95% CI)
p-valueGenotype – G1672A
Existence – GG
42
41
2.63 (2.26–5.14)
0.067
Existence – GA
44
43
3.18 (2.32–4.92)
0.014
Existence – AA
46
35
3.53 (2.34–5.28)
0.054Allele – G1672A
Allele G
223
213
2.42 (1.34 –3.77)
< 0.0001
Allele A
24
34
Genotype – C410T
Existence – CC
47
44
3.08
Existence – CT
63
76
3.21 (2.42–5.45)
0.014
Existence – TT
95
87
3.23 (3.22–5.42)
0.072Allele – C410T
Allele C
72
73
3.13 (2.33–4.68)
0.082
Allele T
36
42
In general, G SNPs and GG of NAD + ADP-ribosyltransferase were highly responsible for neonatal T2DM development, and the individuals with GA SNPs protect from neonatal type 2 diabetes mellitus. Thus, NAD + ADP-ribosyltransferase involved in neonatal T2DM and risk of forming type 2 diabetes mellitus is about 2 times higher than healthy individuals.
4 Discussion
Determination of specific target for neonatal T2DM treatment is very important for effective management, and this assists in altering the risk factor associated with neonatal T2DM with improving patient care. This is the first report to explore the association of polymorphisms in the development of neonatal T2DM in Chinese individuals for effective patient care. A total of 120 Chinese subjects with neonatal T2DM and 130 healthy Chinese individuals were completed the study. In our study, G SNPs and GG of NAD + ADP-ribosyltransferase were found responsible for developing neonatal T2DM. However, GA SNPs in the individuals protect from the development of neonatal T2DM. Thus, NAD + ADP-ribosyltransferase involved in neonatal T2DM and risk of developing is approx. 2 times higher than healthy individuals. We observed that the individuals with GG genotype were highly susceptible to develop neonatal T2DM; the risk of neonatal T2DM was about more than 1 times higher than individuals without GG DNA genotype. We found that the subject with GG gene was at susceptible of developing neonatal T2DM; the risk of neonatal type 2 diabetes mellitus was 1.5 higher in neonatal T2DM patients as compared to subjects who had no GG gene (p < 0.001). We found that the subjects with the G allele are also highly susceptible to a greater risk of neonatal T2DM; the risk of neonatal T2DM was 1.3 times more in these individuals than subject with no G allele (p < 0.001). We also notice the involvement of another genotype such as AA and GA among neonatal T2DM subjects. In our study we found the positive relationship of NAD + ADP-ribosyltransferase polymorphisms with neonatal T2DM when analyzed association between neonatal T2DM and NAD + ADP-ribosyltransferase polymorphisms among subjects with neonatal T2DM. There was no evidence of polymorphism of NAD + ADP-ribosyltransferase when compared to the results with the patients of neonatal T2DM with healthy subjects. The subjects with C allele were more susceptible to neonatal T2DM; the risk of neonatal T2DM was 1.5 times higher among individuals having C allele than individuals with no C allele (Sharma et al., 2011). Our finding showed that polymorphism of NAD + ADP-ribosyltransferase is mainly involved in the development of neonatal T2DM in Chinese population.
5 Conclusion
To conclude, our study results showed that the polymorphism of NAD + ADP-ribosyltransferase is involved in the development of neonatal T2DM in China. Our result suggested that NAD + ADP-ribosyltransferase is an important target for the treatment of neonatal diabetes mellitus; the treatment targeting NAD + ADP-ribosyltransferase is highly effective. Our findings encourage conducting large multi-centric, multi-country, a clinical genetic analysis that assesses the role of NAD + ADP-ribosyltransferase polymorphism in neonatal diabetes mellitus.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Retrospective analysis of metabolic syndrome: prevalence and distribution in executive population in urban Pakistan. Int. J. Family Med.. 2012;649383
- [CrossRef] [Google Scholar]
- Prevalence of metabolic syndrome in Middle-East countries: meta-analysis of cross-sectional studies. Diabet. Metabol. Syn: Clin. Res. Rev.. 2018;12(2):195-201.
- [Google Scholar]
- Poly(ADP-ribose) polymerase-1 activity promotes NF-kappaB-driven transcription and microglial activation: implication for neurodegenerative disorders. J. Neurochem.. 2003;85(2):306-317.
- [Google Scholar]
- Insights into the biogenesis, function, and regulation of ADP-ribosylation. Nat. Chem. Biol.. 2018;14(3):236-243.
- [Google Scholar]
- Molecular pathways: targeting DNA repair pathway defects enriched in metastasis. Clin. Cancer Res.. 2016;22(13):3132-3137.
- [Google Scholar]
- Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J. Biol. Chem.. 2003;278(20):18426-18433.
- [Google Scholar]
- HLA genotyping supports a nonautoimmune etiology in patients diagnosed with diabetes under the age of 6 months. Diabetes. 2006;55:1895-1898.
- [Google Scholar]
- Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49:1190-1197.
- [Google Scholar]
- Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930-1937.
- [Google Scholar]
- Entities and frequency of neonatal diabetes: data from the diabetes documentation and quality management system (DPA) Diabet. Med.. 2010;27(6):709-712.
- [Google Scholar]
- The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front. Biosci.. 2008;13:3046-3082.
- [Google Scholar]
- Poly(ADP-ribose) polymerase (PARP) polymorphisms associated with nephritis and arthritis in systemic lupus erythematosus. Rheumatology (Oxford). 2006;45(6):711-717.
- [Google Scholar]
- Johnson, K.A., 2006. Transglutaminase Mediation of Pathologic Chondrogenic Differentiation in Cartilage and Aortic Smooth Muscle (Doctoral dissertation. UC San: Diego).
- Efficacy and safety comparison of add-on therapy with liraglutide, saxagliptin and vildagliptin, all in combination with current conventional oral hypoglycemic agents therapy in poorly controlled Chinese type 2 diabetes. Exp. Clin. Endocrinol. Diabetes. 2014;122(8):469-476.
- [Google Scholar]
- Metabolic syndrome and male fertility. World J. Men's Health. 2018;37(2):113.
- [CrossRef] [Google Scholar]
- Gly972Arg of IRS-1 and Lys121Gln of PC-1 polymorphisms act in opposite way in polycystic ovary syndrome. J. Endocrinol. Invest.. 2017;40(4):367-376.
- [Google Scholar]
- Poly (ADP-ribose) polymerases (PARPs) 1–3 regulate astrocyte activation. J. Neurochem.. 2008;106(2):578-590.
- [Google Scholar]
- Neonatal diabetes mellitus: a disease linked to multiple mechanism. Orphanet. J. Rare Dis.. 2007;2:12.
- [Google Scholar]
- Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diab. Endocrinol.. 2014;2(4):289-297.
- [Google Scholar]
- Involvement of poly(ADP-ribose) polymerase-1 in Chinese patients with glioma: a potential target for effective patient care. Int. J. Biol. Markers. 2018;33(1):68-72.
- [Google Scholar]
- Type 2 diabetes (T2D) associated polymorphisms regulate expression of adjacent transcripts in transformed lymphocytes, adipose, and muscle from Caucasian and African-American subjects. J. Clin. Endocrinol. Metab.. 2011;96(2):394-403.
- [Google Scholar]
- Aetiopathology and genetic basis of neonatal diabetes. Arch. Dis. Child Fetal Neonatal Ed.. 1997;76:39-42.
- [Google Scholar]
- Roles of poly(ADP-ribosyl)ation and PARP in apoptosis, DNA repair, genomic stability and functions of p53 and E2F–1. Adv. Enzyme Regul.. 2000;40:183-215.
- [Google Scholar]
- Standards of care for type 2 diabetes in China. Diabetes Metab. Res. Rev.. 2016;32(5):442-458.
- [Google Scholar]
- Polymorphic forms of human ADP-ribosyltransferase-1 differences in their catalytic activities revealed by labeling of membrane-associated substrates. Eur. J. Biochem.. 1999;262(2):342-348.
- [Google Scholar]
- Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259-263.
- [Google Scholar]
- Serum profile of cytokines and their genetic variants in metabolic syndrome and healthy subjects: A comparative study. Biosci. Report. 2019;39(2) pii: BSR20181202.10.1042/BSR20181202
- [Google Scholar]
- Comparative efficacy of glimepiride and metformin in monotherapy of type 2 diabetes mellitus: meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr.. 2013;5(1):70.
- [Google Scholar]