Association between salivary factors and cariogenic bacteria in type-2 diabetes patients
⁎Corresponding author at: Department of Biochemistry, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. haseeb@ksu.edu.sa (Haseeb A. Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Type-2 diabetes (T2DM) is a global epidemic. Among various complications of T2DM, dental caries is one of its preventable complications. This investigation was aimed to study the association between salivary factors and the growth of cariogenic bacteria in the saliva of T2DM patients. We measured the salivary glucose, saliva flow rate and its buffering capacity in T2DM patients (N = 100). Cariogenic bacteria in saliva were detected by using a Chair-side Test Kit. We also analyzed fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) in all the subjects. A large number of T2DM patients (78%) had high counts (>105 CFU /ml) of streptococcus mutans in their saliva whereas high counts of lactobacilli were observed only in 42% patients. We observed significant associations between streptococcus mutans load and saliva flow rate, saliva buffering capacity and glycemic control however these variable did not show any significant association with lactobacilli. Hypo-salivation, high salivary glucose and poor glycemic control promoted the growth of streptococcus mutans in the saliva of T2DM patients. In conclusion, salivary factors play important roles in controlling the salivary status of cariogenic bacteria. Thus, an adequate oral health and proper glycemic control could help in abolishing the caries risk and its complications.
Keywords
Diabetes
Cariogenic bacteria
Saliva flow rate
Salivary glucose
Streptococcus mutans
Lactobacilli
1 Introduction
Type-2 diabetes mellitus (T2DM) is a global public health issue accounting for about 90–95% of total diabetic cases. According to the International Diabetes Federation (IDF), 415 million persons are affected with diabetes worldwide; 35.4 million of them are in Middle East and North Africa region and 3.4 million in Saudi Arabia. Diabetes mellitus is a metabolic disorder and associated with many complications such as infections, cardiovascular problems, neuropathy, nephropathy, retinopathy, and oral complications (Wilkins, 2005). The important oral and dental complications associated with diabetes include; periodontal diseases, xerostomia, angular cheilitis, oral candidiasis, lichen planus and dental caries (Wilkins, 2005).
In a series of 400 type-2 diabetic patients from India, root caries were prevalent in 42% of patients with significant association between root caries and age, presence of periodontal pockets as well as loss of attachment (>3 mm) (Soni et al., 2014). Another study from India using comparatively small number of patients reported the oral manifestations in the form of periodontal disease (34%), oral candidiasis (24%), tooth loss (24%), oral mucosal ulcers (22%), taste impairment (20%), xerostomia (14%), and dental caries (24%) (Bajaj et al., 2012). In a cross-sectional study comprising 32 patients with controlled T2DM, 31 with poorly controlled T2DM and 37 non-diabetic subjects, the poorly controlled T2DM group exhibited significantly higher mean buffering capacity, plaque index and bleeding on probing than other groups (Kogawa et al., 2016). A Swedish cross-sectional study on 102 diabetic patients and same number of non-diabetic subjects showed significantly higher frequency of xerostomia, initial caries lesions and advanced periodontitis in the diabetic group however diabetes duration or metabolic control was not related to periodontal status (Sandberg et al., 2000).
Dental caries is a chronic infectious disease characterized by destruction of dental hard tissues by lactic acid producing bacteria due to the fermentation of carbohydrates, present in the dietary food particles left adhered in oral cavity (Selwitz et al., 2007). There are two groups of cariogenic bacteria; among them, streptococci mutans are the main initiator of the dental caries whereas lactobacilli are usually more active during the progression of the disease. After adjusting for the diabetic status, the root surface caries showed significant association with high counts of streptococci mutans, lactobacilli and yeasts in saliva, but streptococci mutans in supragingival plaques; while coronal caries showed significant association only with lactobacilli and yeast in saliva of T2DM patients from Thailand (Hintao et al., 2007a). In a stratified cross-sectional study on 105 T2DM patients and 103 non-diabetic subjects, the factors associated with root surface caries included T2DM, a low saliva buffer capacity, more missing teeth, and existing coronal caries; whereas wearing removable dentures, more missing teeth, a high number of lactobacilli, and a low saliva buffer capacity but not T2DM were associated with coronal caries (Hintao et al., 2007b). Almusawi et al (2018) reported high risk of dental caries in Saudi T2DM patients; a large number of these patients had preventable risk factors such as heavy plaque, bacterial load and poor glycemic control.
In this cross-sectional study, we investigated the role of salivary factors including saliva flow rate, saliva buffering capacity and salivary glucose on the growth of cariogenic bacteria (streptococcus mutans and lactobacilli) in the saliva of T2DM patients. We also evaluated the association between glycemic control and cariogenic bacterial load in the saliva of T2DM patients from Saud Arabia.
2 Materials and methods
This cross-sectional study was conducted on T2DM patients enrolled at the outpatient department of Sheikh Abdul Malik bin Ibrahim Al Sheikh Diabetic Center at King Salman Hospital in Riyadh, Saudi Arabia. The inclusion criteria were Saudi type-2 diabetic patients aged ≥30 years. The exclusion criteria were type-1 diabetes, pregnancy, edentulous patients, smokers, patients with oral injuries or severe gingivitis, patients taking antibiotics or antibacterial mouth rinses, and xerostomia or dry mouth due to medications for certain diseases. The study protocol was approved by Institutional Review Board and all the participants signed an informed consent. The research has been conducted in full accordance with the World Medical Association Declaration of Helsinki. Patients were interviewed by one investigator and one dental hygienist to collect socio-demographic data. Prior to saliva and blood collection, all the patients were informed about the collection procedures and precautions.
Saliva samples were collected in the morning time from the fasted patients attending to their routine examination. They had been advised not to brush their teeth for at least one hour before collecting the samples. The subjects were asked to rinse their mouth with distilled water and wait for at least 5 min to avoid any dilution of samples. Then they were suggested to sit in an upright position and tilt their heads slightly forward. Paraffin pellet stimulated whole saliva was collected for 5 min in sterile sample collection tubes by spitting method and stored at −20 °C until analyzed. Blood samples were collected from the patients just before collecting of the saliva samples. The data of fasting blood glucose (FBG) and glycosylated hemoglobin (HbA1c) were obtained from patients' records. The concentration of salivary glucose was estimated by glucose oxidase method using a commercially available colorimetric assay kit (United Diagnostics Corporation, Riyadh, KSA).
Saliva flow rate was calculated by weighing the tube before and after saliva collection and graded into three categories, normal flow rate (>1 g/min), moderately low flow rate (1–0.7 g/min) and very low flow rate (≤0.7 g/min). Determination of salivary buffering capacity and cariogenic bacteria (streptococcus mutans and lactobacilli) load in saliva were performed by using a Chair-side Test Kit (CRT Bacteria, Ivoclar Vivadent, Liechtenstein) according to manufacturer's instructions. The semi-quantitative analysis of saliva buffering capacity was performed by comparing the color of the test field with the color of the sample; blue, green and yellow colors indicated high, moderate and low buffering capacity, respectively. Quantification of the bacterial load was based on the colony forming units per milliliter of saliva (CFU/ml) as low (<105 CFU/ml) or high (≥105 CFU/ml).
Data were analyzed by Statistical Package for Social Sciences (SPSS) software version 21. Shapiro-Wilk test was performed to determine the normality of the variables. Chi-square test was used to test the association between salivary parameters (saliva flow rate and saliva buffering capacity) and cariogenic bacteria. The frequencies of high and low counts of bacteria were compared by Fisher’s exact test using the CalcFisher software (Khan, 2003). Pearson test was used for correlation analysis. P values <0.05 were considered as statistically significant.
3 Results
A total of 100 type-2 diabetes patients (43 male and 57 female) consented to participate in this study. The mean age of the patients was 54.66 (SD ± 8.97) years and 80% were married. The duration of diabetes mellitus ranged from 1 week to 35 years with a median of 10 years. Most of the patients (77%) belonged to the low educational level (23% illiterate and 54% had secondary education or lower) while 23% had higher education. Almost half of the study participants were housewives (51%) and 22% were retired. The major comorbidities were hypertension (58%) and neuropathy (55%) whereas 13% patients had physical inability. Most of the patients either received oral medications to treat diabetes (45%) or took both oral medications and insulin injections (43%) whereas 12% received insulin injections only.
The saliva flow rate ranged from 0.17 to 3.10 g/min with a median of 0.82 g/min. More than one third of the patients (37%) suffered from xerostomia (Fig. 1). About two third of the patients (62%) had medium saliva buffering capacity (Fig. 2). The fasting blood glucose (FBG) ranged from 4.7 mmol/L to 25.0 mmol/L with a median of 8.95 mmol/L. The level of salivary glucose ranged between 0.12 mg/dL and 22.77 mg/dL with a median value of 0.74 mg/dL. The average concentration of HbA1c in the sera of patients was 8.89 ± 1.68%. A significantly large number of diabetic patients (78%) had high counts (>105 CFU /ml) of streptococcus mutans in their saliva as compared to patients with the high counts of lactobacilli (42%) (Fig. 3).
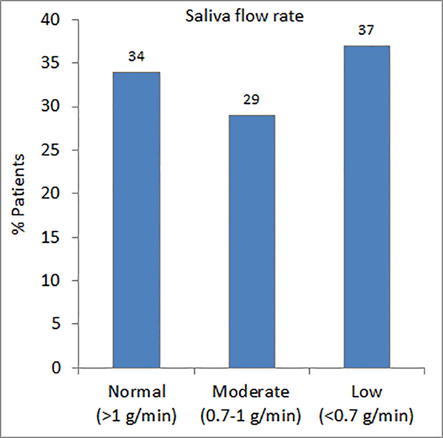
- Distribution of patients according to their saliva flow rate.
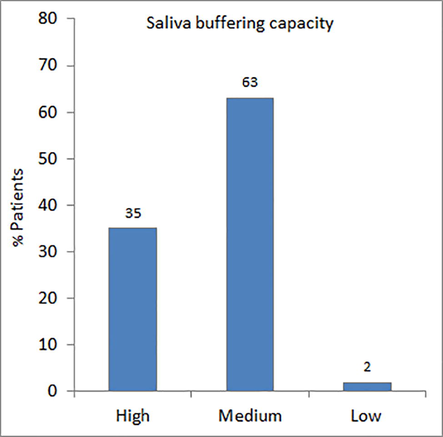
- Distribution of patients according to saliva buffering capacity.
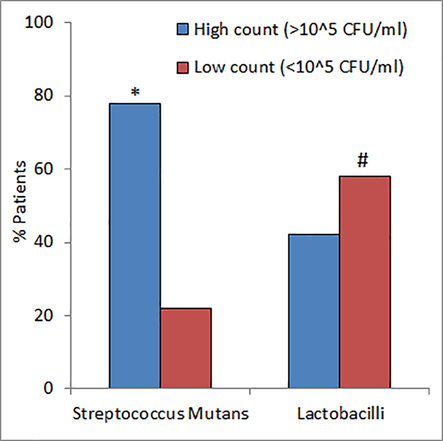
- Prevalence of cariogenic bacteria in diabetic patients. *P < 0.001 versus high counts of Lactobacilli and #P < 0.001 versus low count of S. Mutans using Fisher’s exact test.
Nonparametric statistics for categorical variables showed significant associations between streptococcus mutans load and saliva flow rate (χ2 = 14.71, P = 0.001), saliva buffering capacity (χ2 = 10.63, P = 0.002) and glycemic control (χ2 = 5.76, P = 0.049) however these variable did not show any significant association with lactobacilli (Table 1). Parametric correlations for continuous variables showed significant correlations between streptococcus mutans load and saliva flow rate (R = -0.322, P = 0.003) as well as salivary glucose (R = 0.287, P = 0.008) however lactobacilli bacteria were not correlated with these variables (Table 2). Both FBG and HbA1c were neither correlated with streptococcus mutans nor lactobacilli. There were significant correlations between lactobacilli load and patient age as well as duration of diabetes (Table 2).
| Variable | Streptococcus Mutans | Lactobacilli | |||||||
|---|---|---|---|---|---|---|---|---|---|
| High | Low | χ2 | P | High | Low | χ2 | P | ||
| Saliva flow rate | Normal | 19 | 15 | 14.71 | 0.001* | 16 | 18 | 0.69 | 0.711 |
| Moderate | 24 | 4 | 11 | 17 | |||||
| Low | 34 | 3 | 14 | 23 | |||||
| Saliva buffering capacity | High | 21 | 14 | 10.63 | 0.002* | 14 | 21 | 0.37 | 1.00 |
| Medium | 55 | 7 | 26 | 36 | |||||
| Low | 2 | 0 | 1 | 1 | |||||
| Glycemic control | ≤7% | 12 | 3 | 5.76 | 0.049* | 7 | 8 | 2.89 | 0.249 |
| >7–8% | 9 | 8 | 4 | 13 | |||||
| >8% | 50 | 11 | 28 | 33 | |||||
| Variable | Streptococcus Mutans | Lactobacilli |
|---|---|---|
| Age | R = −0.086, P = 0.435 | R = 0.290, P = 0.007* |
| Duration of diabetes | R = 0.106, P = 0.338 | R = 0.367, P = 0.001* |
| Saliva flow rate | R = −0.322, P = 0.003* | R = 0.174, P = 0.113 |
| Salivary glucose | R = 0.287, P = 0.008* | R = 0.094, P = 0.394 |
| FBG | R = 0.175, P = 0.112 | R = −0.043, P = 0.699 |
| HbA1c | R = −0.025, P = 0.821 | R = 0.111, P = 0.314 |
4 Discussion
Our results showed that a large number of T2DM patients suffered from xerostomia (dry mouth) (Fig. 1) and impaired saliva buffering capacity (Fig. 2). Khovidhunkit et al. (2009) reported significantly higher prevalence of xerostomia (62% versus 36%) and hyposalivation (46% versus 28%) in T2DM patients as compared to non-diabetic controls. Sandberg et al. (2000) also observed that T2DM patients suffered from xerostomia to a significantly higher degree than non-diabetic controls. The salivary flow rate was reported to be lower in the T2DM patients, regardless of whether they were well or poorly metabolically controlled, compared with healthy individuals (Bernardi et al., 2007). Puttaswamy et al. (2017) observed low salivary flow rate and buffering capacity in T2DM (N = 60) as compared to non-diabetic controls (N = 40). The salivary buffering capacity was found to be comparable among the three groups including controls with normal blood glucose levels, diabetic patients with FBG ≤ 200 mg/dL and with FBG > 200 mg/dL; however the salivary pH levels were significantly lower in diabetic patients with FBG > 200 mg/dL (Elkafri et al., 2014). In a case-control study comprising 30 diabetic patients and 60 healthy subjects, the salivary pH was significantly higher in diabetic groups whereas the salivary acid buffering capacity did not differ significantly between the two groups (Wang et al., 2013). In type-1 diabetic patients, the mean values for salivary buffering capacities and salivary pH were significantly lower than controls (Aren et al., 2003). However, if the patients’ type-1 diabetes is well controlled, their salivary and caries data does not differ from that of healthy controls (Swanljung et al., 1992).
Majority of the T2DM patients (78%) had high counts of streptococcus mutans in their saliva whereas high counts of lactobacilli were observed in only 42% of patients (Fig. 3). A recent study has shown high prevalence of oral bacterial in subgingival pockets of T2DM patients as compared to normal subjects (Al-Obaidaa et al., 2020). Association between diabetes and the frequency of cariogenic bacteria in saliva is not clear as some studies reported significant difference for the main cariogenic bacteria between diabetic and non-diabetic subjects (Lai et al., 2017) whereas other studies did not find any such difference (Hintao et al., 2007a,b; Rezazadeh et al., 2016). Increased counts of streptococcus mutans, lactobacilli and yeasts in saliva were associated with root surface caries in T2DM patients whereas coronal caries was only associated with lactobacilli and yeasts in saliva (Hintao et al., 2007a,b). Among T2DM patients from Thailand, the incidence of active dental caries was greater than non-diabetics, and the numbers of total streptococci and lactobacilli were significantly higher in supragingival plaque from diabetic patients than normal subjects. Diabetes patients with active caries showed significantly higher degree of lactobacillus counts in the saliva and supragingival plaque as compared to those diabetic patients who did not have active caries (Kampoo et al., 2014). Goodson et al. (2017) have suggested that hyperglycemia due to obesity or diabetes results in high salivary glucose and subsequent acidification of the oral environment, leading to a generalized perturbation in the oral microbiome as well as an increased risk of dental erosion, dental caries, and gingivitis.
We observed significant correlations between streptococcus mutans counts and salivary factors including saliva flow rate, saliva buffering capacity, salivary glucose in T2DM patients (Tables 1 and 2). Patient age and duration of diabetes were significantly associated with the high counts of lactobacilli (Table 2). Bernardi et al (2007) observed that salivary glucose concentrations were significantly higher in diabetic patients than controls, irrespective of the status of glycemic control in T2DM patients. In a case-control study, among the salivary factors studied, salivary glucose significantly influenced the periodontal status in T2DM (Puttaswamy et al., 2017). Hypo-salivation reduced the buffering capacity of saliva and promoted the growth of cariogenic bacteria such as streptococci mutans and lactobacillus (Khovidhunkit et al., 2009; Karjalainen et al., 1996). After categorizing the glycemic control into three categories, we observed significant association between HbA1c levels and high counts of streptococci mutans but not lactobacilli (Table 1). Syrjälä et al. (2003) have observed that high levels of salivary streptococci mutans and lactobacilli are significantly associated with the risk of dental caries. Moreover, the occurrence of dental caries was highly associated with elevated counts of streptococci mutans and lactobacilli among the subjects with HbA1c ≥ 8.5 compared to those with HbA1c < 8.5, suggesting that poor glycemic control enhances the risk of dental caries.
In conclusion, salivary factors such as saliva flow rate, saliva buffering capacity and salivary glucose play important roles in controlling the salivary status of cariogenic bacteria. Hypo-salivation, high salivary glucose and poor glycemic control promoted the growth of streptococcus mutans in the saliva of T2DM patients. In view of high prevalence of uncontrolled T2DM in Saudi Arabia (Khan et al., 2007, 2014) as well as oral bacterial load (Almusawi et al., 2018; Khan, 2012), the findings of this study have direct implications about designing strategies for minimizing the risk factors for oral complications. Thus, routine monitoring of T2DM patients for oral health as well as glycemic control is important to prevent the growth of cariogenic bacteria and the resulting caries development in risky individuals.
Acknowledgments
We are thankful to the clinical and nursing staff of Sheikh Abdul Malik bin Ibrahim Al Sheikh Diabetic Center for their support and assistance. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the Research Group No. RG-009.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Potential risk factors for dental caries in Type-2 diabetes patients. Int. J. Dent. Hyg.. 2018;16:467-475.
- [Google Scholar]
- Molecular identification and diversity analysis of dental bacteria in diabetic and non-diabetic females from Saudi Arabia. Saudi J. Biol. Sci.. 2020;27:358-362.
- [Google Scholar]
- Periodontal health, salivary status, and metabolic control in children with type 1 diabetes mellitus. J. Periodontol.. 2003;74:1789-1795.
- [Google Scholar]
- Oral manifestations in type-2 diabetes and related complications. Indian J. Endocrinol. Metab.. 2012;16:777-779.
- [Google Scholar]
- Study of the buffering capacity, pH and salivary flow rate in type 2 well-controlled and poorly controlled diabetic patients. Oral Health Prev. Dent.. 2007;5:73-78.
- [Google Scholar]
- Relationship between blood glucose levels and salivary pH and buffering capacity in type II diabetes patients. East Mediterr. Health J.. 2014;20:139-145.
- [Google Scholar]
- The salivary microbiome is altered in the presence of a high salivary glucose concentration. PLoS One. 2017;12:e0170437
- [Google Scholar]
- The microbiological profiles of saliva, supragingival and subgingival plaque and dental caries in adults with and without type 2 diabetes mellitus. Oral Microbiol. Immunol.. 2007;22:175-181.
- [Google Scholar]
- Root surface and coronal caries in adults with type 2 diabetes mellitus. Commun. Dent. Oral Epidemiol.. 2007;35:302-309.
- [Google Scholar]
- Oral bacterial communities in individuals with type 2 diabetes who live in southern Thailand. Appl. Environ. Microbiol.. 2014;80:662-671.
- [Google Scholar]
- Salivary factors in children and adolescents with insulin-dependent diabetes mellitus. Pediatr. Dent.. 1996;18:306-311.
- [Google Scholar]
- Molecular identification and phylogeny of commonly occurring periodontal bacteria using 16S rRNA gene sequences. J. Pure Appl. Microbio.. 2012;6:517-523.
- [Google Scholar]
- Evaluation of HbA1c criteria for diagnosis of diabetes mellitus: a retrospective study of 12785 type 2 Saudi male patients. Endocr. Res.. 2014;39:61-65.
- [Google Scholar]
- Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin. Exp. Med.. 2007;7:24-29.
- [Google Scholar]
- Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: a preliminary study. J. Med. Assoc. Thai.. 2009;92:1220-1228.
- [Google Scholar]
- Impact of glycemic control on oral health status in type 2 diabetes individuals and its association with salivary and plasma levels of chromogranin A. Arch. Oral Biol.. 2016;62:10-19.
- [Google Scholar]
- Evaluation of the difference in caries experience in diabetic and non-diabetic children-A case control study. PLoS One. 2017;12:e0188451
- [Google Scholar]
- Correlation between salivary glucose and blood glucose and the implications of salivary factors on the oral health status in type 2 diabetes mellitus patients. J. Int. Soc. Prev. Commun. Dent.. 2017;7:28-33.
- [Google Scholar]
- Comparison of oral Lactobacillus and Streptococcus mutans between diabetic dialysis patients with non-diabetic dialysis patients and healthy people. J.. Renal. Inj. Prev.. 2016;5:148-152.
- [Google Scholar]
- Type 2 diabetes and oral health: a comparison between diabetic and non-diabetic subjects. Diabetes Res. Clin. Pract.. 2000;50:27-34.
- [Google Scholar]
- Caries and saliva in 12–18-year-old diabetics and controls. Scand. J. Dent. Res.. 1992;100:310-313.
- [Google Scholar]
- Metabolic control as a modifier of the association between salivary factors and dental caries among diabetic patients. Caries Res.. 2003;37:142-147.
- [Google Scholar]
- Root caries among type 2 diabetes mellitus patients visiting a hospital. Spec Care Dentist.. 2014;34:273-277.
- [Google Scholar]
- The salivary factors related to caries and periodontal disease in children and adolescents with diabetes mellitus. Zhonghua Kou Qiang Yi Xue Za Zhi.. 2013;48:545-549.
- [Google Scholar]
- Clinical Practice of the Dental Hygienist (Ninth edition). Lippincott Williams & Wilkins; 2005.







