Translate this page into:
Assessment of the physiological markers, oxidative stress and neurotoxic effects of nematode Anisakis sp. on the Luciobarbus callensis (Teleosts, Cyprinids) in northeast of Algeria
⁎Corresponding author. falmekhlafi@ksu.edu.sa (Fahd A. AL-mekhlaf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Our study focused on the effect of parasites on the antioxidant system and their neurotoxic effect in Luciobarbus callensis (Teleosts, Cyprinids). In this context, cellular biomarkers, acetylcholinesterase (AChE), catalase (CAT), molecules H2O2 as well as morphophysiological markers (condition factor (FC), hepatosomatic index (HSI), splenosomatic index (SSI), viscerosomatic index (VSI) were analyzed in fish infested with L3 larvae of Anisakis sp., nematodes of marine organisms, captured in Lake Oubéira (Algeria). The results showed a significant decrease in AChE activities in the heart (68 % p < 0.05) and muscles (81.55 % p < 0.05) in infested fish compared to those recorded in reference fish. In contrast, splenic AChE levels were significantly induced. CAT levels were significantly decreased in the spleen (73 % p < 0.05) and intestine (56 % p < 0.05) of fish parasitized by Anisakis sp. compared to healthy fish. HSI and SSI were significantly higher in healthy fish. On the other hand, Anisakis sp. had no significant effect on FC and VSI at the time of sampling. This study suggests that antioxidant defense disorders (CAT), due to a direct inhibitory action of nematode toxic products on this molecule and an increase in ROS production in these animals, leading to a high pro-oxidant state (H2O2), causing neurological effects (AChE) with enlargement of the spleen and liver of infested barb. In this regard, monitoring the effect of parasitic infections on physiological and biochemical marker profiles can be a crucial means of assessing fish health under natural conditions; to ensure the conservation and sustainability of aquatic biodiversity, including fish farming and artificial production.
Keywords
Anisakidae
Algerian barb
Luciobarbus callensis
Biomarkers
Health condition
Oxidative stress
1 Introduction
Fish are susceptible to parasitic infestations that can harm fish health. Given their importance as indicative tools for ecosystem health (Allan et al., 2020), parasites are still rarely considered biotic stressors in freshwater fish. They can exert several effects on the host, such as toxic, antigenic, and even behavior modification and/or altering organisms' physiology. In the literature, changes in host behavior following a parasite infestation are frequently reported and are often assumed to be adaptive to the host or parasite and rarely cause direct mortality of organisms (Shamsi et al., 2021).
The immune system in fish is quite similar to that of higher vertebrates. Its primary function is to maintain homeostasis and protect the body from parasites or other stressors to minimize the health costs associated with infection (Rohlenová et al., 2011). Parasites affect the physiological homeostasis of their hosts (Sures et al., 2017), by weakening the immune defences and nutrient absorption (Kiron, 2012), thus causing damage at the cellular level and modulating biomarker responses in organisms (Marcogliese and Giamberini, 2013). This survival strategy adopted by the parasite imposes an energy tax that can interfere with the costly demands of the immune response that causes a strategy to reduce energy expenditure by the host to compensate for the metabolic cost of the infestation (Allan et al., 2020).
In fish species, oxidative stress is mainly studied in environmental contamination by pollutants (Yang et al., 2020). However, parasites also cause oxidative stress and a higher level of membrane damage in fish organs (Belló et al., 2000). Understanding the defense mechanisms of fish against parasitic infections leading to physiological alterations (Dautremepuits et al., 2003) prompted biologists to elaborate and develop a wide range of morphological, physiological, and biochemical indices to measure the general state of fish subpopulations and populations (Brown and Murphy, 2004). Condition indices are often used in fisheries to demonstrate the effects of a stressor on an organism and are particularly applicable when investigating integrated health effects.
Fulton condition (FC), and Organosomatic indices (HSI, SSI, VSI) are influenced by environmental factors and density of infection (Ryberg et al., 2020). FC and OI indicate the nutritional status of fish (Aminisarteshnizi, 2021), energy metabolism, nutrient uptake, pathogen recognition, regulation of the intestinal microbiome (Martin et al., 2016), defense mechanisms, flavors, and nutritional qualities of fish fillets (Rasmussen et al., 2000).
Oxidative stress and neurotoxicity are important indicators of the physiological status of the parasite and/or fish behavior (Mehrdana and Buchmann, 2017). Fish have developed enzymatic and non-enzymatic antioxidant defense mechanisms similar to those of mammals to neutralize the impact of Reactive oxygen species (ROS) (Di Giulio and Meyer, 2008). In addition, acetylcholinesterase (AChE) is used to assess the neurotoxic effects of organophosphates and carbamates, insecticides, thus disrupting the transmission of nerve impulses (Adams, 2001). As a result, these disturbances can affect locomotion and balance in exposed organisms (Bretaud et al., 2000).
We focused in this study on the genus Barbus presenting the complex polyphyletic group of Cyprinid fish of the old world; this genus has a very wide distribution in Asia, Europe, and Africa. In north-eastern Algeria, the genus Barbus is represented only by the natural populations of Luciobarbus callensis (Valenciennes, 1842); synonym of Barbus callensis, a well-spread and very abundant endemic fish species in Lake Oubéira. Additionally, this species of high heritage value is listed on the IUCN (International Union for Conservation of Nature) Red List as a “minor concern” (Crivelli, 2006). In recent years, according to our observations in the field, this native species has been threatened by a metazoan parasite (Nematode, Anisakidae). In the water bodies of north-eastern Algeria, notably Lake Oubéira, work had mainly focused on eel, parasites of the Algerian barb, and the ecology of parasites of common carp (Brahmia et al., 2016). However, little is known about fish physiology and adaptive responses to oxidative stress; this article is the first to study the possible neurotoxicity and oxidative stress-induced changes caused by larvae (L3) of the genus Anisakis (Nematode, Anisakidae). To this end, several physiological (K, HSI, SSI, VSI) and biochemical (CAT, H202, AChE) responses were evaluated in the Algerian freshwater fish L. callensis from Lake Oubéira. We hypothesized that fish could develop resistance and/or adaptive responses to counteract and avoid the harmful effects of parasites through physiological and biochemical acclimatization. In this regard, monitoring the effect of parasitic infections on physiological and biochemical marker profiles can be a crucial means of assessing fish health under natural conditions; to ensure the conservation and sustainability of aquatic biodiversity, including fish farming and artificial production.
2 Material and methods
2.1 Study area
Oubéira lake is situated in the north-eastern part of Algeria (36°50 N–08°23E) and has a surface area of 2200 ha and a maximum depth of 4 m. It is located approximately 4 km from the shores of the Mediterranean Sea (Fig. 1). This freshwater lake is listed on the Ramsar list and classified as a biosphere reserve by UNESCO in 1990 (Boumezbeur, 2003). The lake contains a diverse ichthyological fauna dominated mainly by the Cyprinidae family, which is represented by a native and abundant L. callensis (Valenciennes, 1842). The second sampling Oued Bounamoussa is a reference site (Fig. 1). The Bounamoussa Oued is the main Oued in the Bounamoussa watershed that drains two major tributaries, the Bouhadjar Ouedon the right bank and the Kebir Oued on the left bank. Freshwater stream located in Cheffia (El Tarf) in the extreme north-east of Algeria, with mountainous relief (36° 36′ 40″ N, 8° 02′ 20″ E).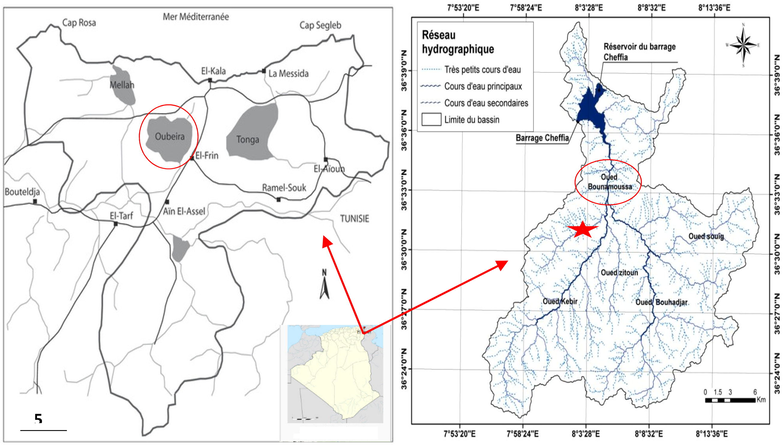
Oubéira Lake and Oued Bounamoussa location map.
2.2 Fish sampling
Professional fishermen sampled fish during the winter (February 2017) using creels. The barb fish sacrificed (CCAC, 2005) were immediately transported to the laboratory (4 °C). Once in the laboratory, fish were weighed, length were measured, and examined for the presence of parasites. External surfaces and all organs were inspected for ectoparasites and endoparasites. The nematodes collected were repeatedly washed in saline solution and preserved in 70 % ethanol. The parasites found in the abdominal cavity, muscle, and intestine were morphologically identified at the genus level by stereomicroscope using the reference guide (Khalil and Polling, 1997). After inspection of many fish, at Lake oubéira, 19 sexually mature fish with an average total length (L) and average total mass (W) of 27.84 ± 0.89 cm and 308.55 ± 28.48 g respectively were separated into two groups. The first group was composed of eight healthy fish that were not infected by the parasite served as control. The second group contained eleven infected fish. In contrast, the second site Oued Bounamoussa being a reference site was composed of 06 healthy fish (23,8 ± 2,52 cm and 173,58 ± 65,85 g). The liver, heart, spleen, intestines, and a part of muscle were weighed to determine indices of fish health status.
2.3 Physiological determinations
The overall health of fish was assessed through direct and indirect indices. In this study, the following four variables were evaluated:
The condition factor (CF) or Fulton’s condition factor is widely used in fisheries and general fish biology studies as an indicator of the general health status of the specimens. Fulton’s index of fish was determined individually using the individual total length (centimeters) and total weight (grams) according to the formula, CF = (W/L3) × 100.
Hepatosomatic index (HSI): Livers of fish were removed, weighed, and HSI was calculated individually using the following formula: HSI = (liver weight/W) × 100.
The HSI permits estimating the energy status of the liver and the process of detoxifying pathogens.
The viscerosomatic index (VSI) was evaluated individually using the individual intestine weight (grams) and total weight (W) that can be expressed by VSI = (viscera weight/W) × 100. The VSI was used to assess fish feeding states at sampling time.
The splenosomatic index (SSI) was determined individually using the individual spleen weight total (grams) and total weight, was defined as SSI = (spleen weight/W) × 100. The SSI is used as an indicator of immunocompetence.
2.4 Biochemical analysis
Catalase, (AChE) activities, hydrogen peroxide levels, and total protein concentration were assessed in the S9 fraction in five vital tissues; the heart, liver, muscle, intestine, and spleen. The tissues were homogenized in PBS (pH 7.4) and centrifuged for 30 min at 9000 g at 4 °C. The supernatant is stored at −80 before biochemical analysis.
Protein estimation: The total protein concentration was estimated by the method of (Bradford, 1976) using bovine serum albumin (BSA) as a standard protein. Various aliquots were created up to 100 µL with distilled water, to which 900 µL of Bradford’s reagent was added, and the color developed was read at 595 nm.
Catalase (CAT) activity (E.C. 1. 11. 1. 6) was evaluated following the method of Aebi et al. (1974), which is based on a spectrophotometric measurement of 10 mM H2O2 breakdown at 240 nm for 3 min. CAT was expressed in µmol/min/mg protein.
Acetylcholinesterase activity (AChE; EC 3.1.1.7) was determined according to the method of (Ellman et al., 1961) using acetylthiocholine (ATCI). The reaction mixture (3.0 ml) in 0.1 M phosphate buffer (pH 7.4) contained 25 ml of 10 % (w/v) tissue homogenate, 0.33 mM DTNB and 0.5 mM ATCI at 37 °C. The addition of the substrate started the reaction, and the change in the absorbance at every 30 s for 5 min was read at 412 nm using a UV–vis spectrophotometer. The activity of AChE was expressed as nmol/min/mg protein.
Hydrogen peroxide (H2O2) level was determined enzymatically using a commercially available kit (Cayman, USA). Briefly, in the presence of peroxidase, H2O2 reacts with 4-amino-antipyrine and phenol to give a red-colored quinine imine which was read at 505 nm and results expressed as mmol H2O2 generated/mg protein.
2.5 Data analyses
All statistical studies were performed with STATISTICA 8.0 software. The results were expressed as mean ± SE. Where appropriate, significant differences between groups were analyzed by one-way analysis of variance (ANOVA) and Tukey HSD test. Asterisks indicated significant differences between parasitized groups and their corresponding controls (p < 0.05).
3 Results
The present study was conducted on a fraction of the population of wild adult fish of Algerian barb (Luciobarbus callensis) sampled in February 2017 from two sites.
3.1 Morphophysiological parameters
All fish sampled were sexually mature. Data on the morphometry and physiology of barb fish have been reported in (Table 1). Under a stereomicroscope (Olympus, SZ), the morphoanatomic characteristics of endoparasites were observed. These observations led to the identification of the L3 larva of the genus Anisakis, nematode belonging to the family Anisakidae. Our study revealed that most fish groups (11 of 19) were infested by a larval of Anisakis sp. with an average parasite intensity of 1.09 (one parasite on average per infested fish). The composition in length and weight of the selected fish samples was almost homogeneous between control fish and fish parasitized by Anisakis sp. (29.01 ± 3.56 cm and 349.60 ± 120.8 g; 26.99 ± 4.0 cm and 278.70 ± 123.32 g respectively). No significant differences (p < 0.05) were found between the two groups. Similarly, the condition factor (CF), whose values ranged from 1.33 ± 0.09 to 1.37 ± 0.18 g/cm3 (parasitized and healthy fish respectively). The viscerosomatic index of nematode infested fish decreased (2.79 ± 0.3 %) but not significantly (p < 0.05) compared with those of the control group (3.08 ± 0.9 %). However, HSI and SSI were significantly increased (p < 0.05) in infested fish compared to healthy specimens. W. weight; L. length; CF. condition factor; SSI. splenosomatic index, HIS hepatosomatic index and VSI. viscerosomatic index.
Fish barbel Site
Length (cm)
Weight (g)
CF %
HSI %
SSI %
VSI %
No-parasitized LO N = 8
29.01 ± 3.56
349.60 ± 120.8
1.37 ± 0.18
0.78 ± 0,12
0,06 ± 0.01
3.08 ± 0.9
Parasitized LO N = 11
26.99 ± 4.05
278.70 ± 123.32
1.33 ± 0.09
1.33 ± 0.12*
0,14 ± 0.02*
2.79 ± 0.3
No-parasitized OB N = 6
23,8 ± 2,52
173,58 ± 65,85
1,22 ± 0,05
1,05 ± 0,12
0,08 ± 0,02
9,10 ± 3,36
3.2 Oxidative stress indices
This study showed that the parasite-induced a non-significant increase in catalase activity in the heart, while in other tissues, it was reduced compared to the control group. About the effect of parasitic infection on hepatic and muscular CAT activities, a non-significant decrease (p < 0.05) was observed between healthy L. callensis and infested with Anisakis sp. (Fig. 2). At the same time, an increase in H2O2 levels was recorded generally in all studied organs of parasitized barbels compared to non-parasitized fish (Fig. 3).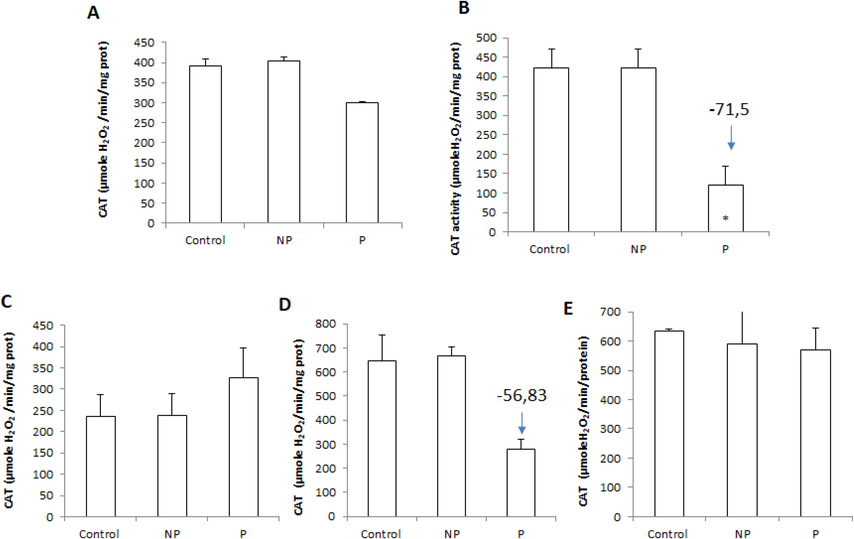
Tissue catalase (CAT) activity: in (a) liver, (b) spleen, (c) heart, (d) intestine, and (e) muscle of freshwater Luciobarbus callensis parasitized (n = 11) and control fish (n = 8). Data are expressed as mean ± S.E. (n = 19). Significant changes vs control are indicated by *P < 0.05.
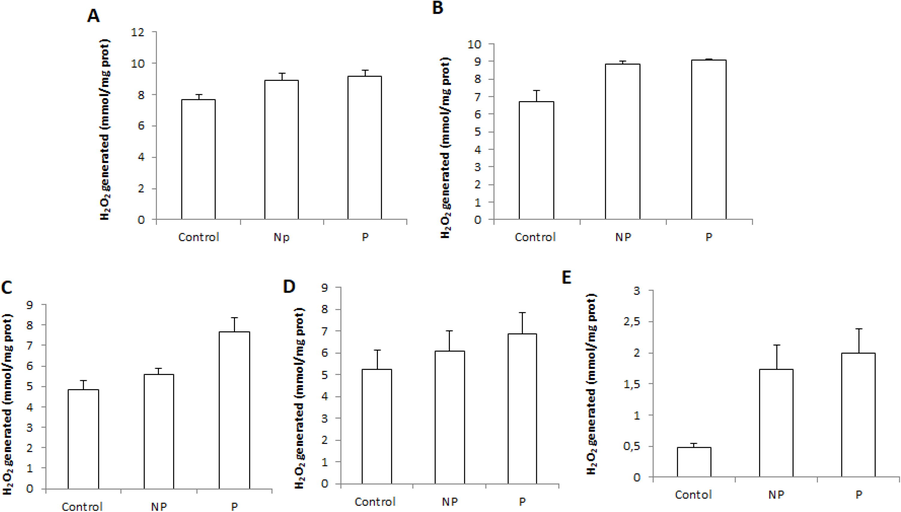
Tissue hydrogen peroxide (H2O2) level: in liver (a), spleen (b), heart (c), intestine(d), and muscle (e) of freshwater Luciobarbus callensis parasitized (n = 11) and control fish (n = 8). Data are expressed as mean ± S.E. (n = 19). Significant changes vs control are indicated by *P < 0.05.
3.3 Neurotoxic parameters
As expected, the nematode caused a significant inhibition of AChE activity, with the magnitude of this response highly dependent on the target tissue. The hydrolytic activity of this esterase was strongly depressed in the heart and muscle.
3.3.1 Acetylcholinesterase activity
Compared with healthy fish (Fig. 4), AChE activity also decreased in the liver, intestine, muscle, and heart and increased in the spleen. The liver and intestine showed no significant (P < 0.05) decrease in their activity in the parasitized fish when compared to control fish. However, a significant (P < 0.05) decrease in activity was recorded in the heart and muscle. The highest level of decline in AChE activity (81.55 % in muscle and 68.39 % in heart) was observed in parasitized fish. Inversely, the spleen showed a significant (P < 0.05) increase in AChE activity in parasitized fish compared to healthy fish.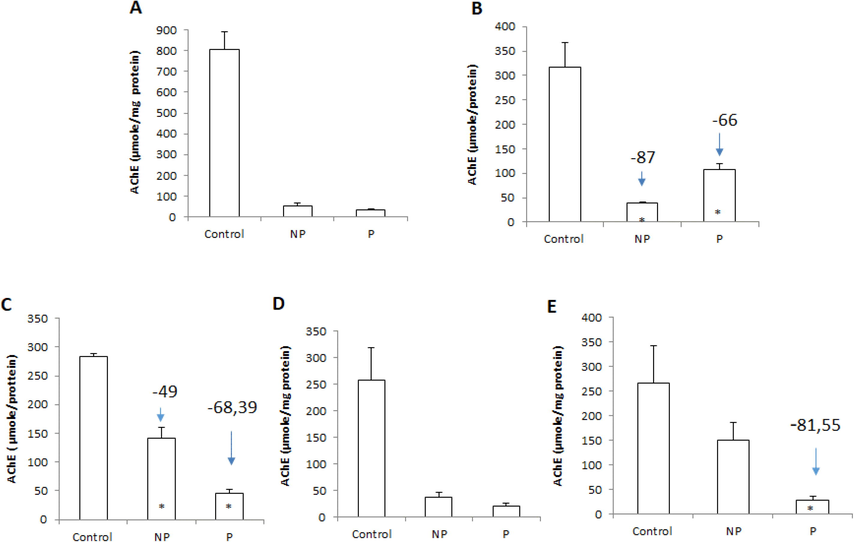
Tissue acetylcholinesterase (AChE) activity: in liver (a), spleen (b), heart (c), intestine (d), and muscle (e) of freshwater Luciobarbus callensis parasitized (n = 11) and control fish (n = 8). Data are expressed as mean ± S.E. (n = 19). Significant changes vs control are indicated by *P < 0.05.
4 Discussion
Evaluating the health status of fish is the missing cornerstone in the assessment and management of ecological risks that could appear as a relevant indicator of the quality of aquatic environments.
Different biomarkers that respond to natural stressors have been recommended to assess the influence of parasites on animal health, such as the morphophysiological indices. We expected parasitized individuals to be leaner than non-parasitized individuals due to the depletion of body reserves when fighting the infestation. Surprisingly, we found no evidence that the harmful effects of parasites were visible from the outside through the loss of condition. A CF value of 1.00 is ideal and indicates that fish have good health conditions. As an unexpected result, the Anisakis sp. had no significant effect on biometric indices at the sampling time. This result was consistent with the conclusions of Skuratovskaya et al. (2015). Indeed, the lack of modulation of CF in the natural environment is a positive element in our study. It is an indicator of long-term response and is relatively insensitive to short-term environmental changes (Adams, S. M. and McLean, R. B., 1985).
The hepatic index is a more sensitive indicator of food intake than the CF. It has been used to estimate fish health or well-being over short periods because it is a sensitive parameter that responds quickly to changing environmental conditions (Van der Oost et al., 2003). As long as fish can feed, this index increases with detoxification systems (Roche et al., 2003). Our results revealed that HSI was significantly increased in parasitized barbels compared to non-parasitized barbels. This increase can be explained by liver enzymes or energy storage induction. This result was supported by Seppänen et al. (2009) and Skuratovskaya et al. (2015). These authors proposed that the increase in HSI in fish may testify to the hypertrophy of the organ due to tissue transformation and the strengthening of the function of metabolites of the detoxification of parasites and the products of peroxidation and oxidation of free radicals. In fact, the nematode induced glucose absorption, increasing energy intake and food and, consequently, an increase in the hepatic index of the host.
The ISS (or spleen weight) is a good indicator of the state of activity of the immune system and the onset and intensity of infection and disease (Dekić et al., 2016). In fish, few studies have shown the effect of metazoan parasites on the spleen, scavenging lymphoid organs, which, at its level, immune cells develop and store themselves. On the other hand, many pathogenic effects have focused on bacteria, viruses, or protozoa responses. A common feature of responses to these agents was splenomegaly. However, an increase and decrease in the ISS appear to be possible outcomes of toxic exposure. In addition, SSI showed a significant increase similar to HIS. This result is consistent with data from several researchers (Seppänen et al., 2009) who reported that the increase in spleen mass may lead to the rise in ISS, which may be explained by splenic immune cell infiltration attributed to the proliferation of lymphocytes, thrombocytes, and macrophages, and dilatation of blood vessels following hyper-synthesis of erythrocyte by the spleen in response to the hematophagous activity of worms. In contrast, (Skuratovskaya et al., 2015) found a significant decrease in ISS in the Black Sea whiting with medium and high intensity of infection with myxosporeans and nematodes. However, the increase in ISS and HSI in parasitized fish species may indicate a relationship between parasites and biomarkers of oxidative stress and neurotoxicity, as endoparasites are intimately linked to the physiology of their host mainly oxidative processes.
The interactions between nematodes and fish have rarely been studied in natural environments because these interactions are quite complex and promote constant changes in the delicate balance between pro-oxidant and antioxidant molecules since the host and parasite can produce both. Generally, oxidative stress comes from inflammatory processes initiated in the host in response to parasitic infection and the parasite's direct production of reactive species (Percário et al., 2012). The reaction in the host is characterized by an increase in oxygen consumption in leukocytes (Skuratovskaya et al., 2015), causing oxidative stress that can be detected and measured by giving a quantitative indication of the health status of fish.
This result indicated that parasitism did not lead to oxidative stress in the muscle and liver of fish. In contrast, significant differences (p < 0.05) were observed between activities measured in the spleen and intestines of L. callensis infested and non-infested fish. Our results are supported by other researchers who suggested that the inhibition of CAT activity is likely due to increased ROS generation of host macrophages and high content of parasite metabolites, which affect fish metabolism stimulating oxidative stress and thus modulating the antioxidant status of the host (Tkachenko et al., 2014).
In the context of neurotoxicity, the activity of AChE is more convincing and clearer. Monitoring AChE activity in fish has become a technique commonly applied to diagnose environmental exposure to cholinergic poisons (Podolska and Napierska, 2006). AChE is found in nerve tissue and some non-neural cells such as erythrocytes (Massoulié et al., 1993) and is most often assessed in the brain and muscles of fish in the presence of pesticides. We found it very interesting to evaluate the effect of Anisakis sp. (Nematode, Anisakidae) larvae (L3) on AChE activity in vital tissues of the L. callensis: the heart, spleen, intestine, liver, and muscles. AChE is involved in the transmission of nerve impulses; it is not surprising that this enzyme was localized mainly in these two tissues (heart and muscle). Our results are consistent with the studies of Podolska and Napierska (2006), which suggest that parasites are generally considered stressors for their host by inhibiting AChE that blocks the transmission of nerve impulses to various types of cholinergic synapses. What is surprising is that the response pattern observed in this study was similar in terms of sensitivity of AChE activity to those described in many aquatic organisms after exposure to pesticides (organophosphates and carbamates (Al-Ghais, 2013).
In fact, AChE activity in hosts and parasites was inversely related (Pritchard, 1993). Indeed, the secretory form plays an important role in host-parasite interactions (Poisot et al., 2009) because parasites that establish long-term infections are relatively non-pathogenic and secrete large amounts of AChE, while parasites that show acute infections secrete relatively small amounts of AChE (Pritchard, 1993). In addition, AChE can also modulate intestinal peristalsis and modify the permeability of the host's intestinal cells to ensure the parasite's diet and, therefore its survival; by destroying host ACh, thereby providing the parasite with biochemical retention by affecting glycogenesis in the host and acting as an anticoagulant by inactivating platelet-activating factor (Lee, 1996). The celluxlar exudates that initially accompany the visceral reaction consist mainly of fibroblasts; this is found in our study. In addition, the AChE produced by some gastrointestinal nematodes (hookworm) may also reduce inflammation and local ulceration by hydrolyzing the ACh that stimulates this gastric acid secretion (Lee, 1996). ACh is an anti-inflammatory molecule with an important role in host immunomodulation (Jaguezeski et al., 2018). Moreover, inflammation is a source of oxygen radicals produced directly by activated phagocytic cells. Interestingly, one of the essential functions of AChE secreted by nematodes is the induction of an immune response in their hosts and modulation of that response interfering with cholinergic stimulation of receptors on leukocyte membranes (You et al., 2018).
In fish, the spleen, a secondary lymphatic organ, and erythrocyte reservoir, plays a potential role in the immune response against parasitic infection through the degradation of the antigen and the production of antibodies. In this sense, the ISS clarifies the information given by other biomarkers such as AChE. A significant increase in splenic activity of AChE was recorded in parasitized fish compared to non-parasitized fish. This result raises the possibility of a role for AChE other than in synaptic transmission but also as a potential immunological target (You et al., 2018). This suggests that the changes in the SSI which occurred after handling stress were probably attributed to the AChE secretion by the nematode. Indeed, AChE secreted by the filarial nematode where the suppressive effect is due to the degradation of ACh, a neurotransmitter, was responsible for releasing lysosomal enzymes and phagocytosis in the host (Torrealba et al., 2018). The changes also observed by (Scharsack et al., 2007) on the leukocyte responses of three-spined sticklebacks, during a late infection with the cestode Schistocephalus solidus led to neuronal changes, inducing behavioral changes in this fish.
5 Conclusion
Our results indicate that the presence of the Anisakis sp. nematode showed a host immune response against infection, manifested by enlargement of the spleen and liver of infested barbels. It was also associated with a significant increase in somatic indices (HSI, SSI) and no significant effect on the biometric index (FC, VSI). In addition, antioxidant defense disorders (CAT) suggest oxidative stress due to a direct inhibitory action of nematode toxic products on this molecule and an increase in ROS production in these animals, leading to a high pro-oxidant state (H2O2), causing neurological effects (AChE) observed in this study., antioxidant defense disorders (CAT), suggest oxidative stress due to a direct inhibitory action of nematode toxic products on this molecule and an increase in ROS production in these animals, leading to a high pro-oxidant state (H2O2), causing neurological effects (AChE) observed in this study.
This study also highlights the importance of considering the effects of pathogens as stressors in the natural environment. In addition, further investigations are still needed to assess the cost of stress responses in the context of multiple exposures, and in several wild fish populations. Indeed, this work should be completed by monitoring other biomarkers of oxidizing stress (lipid peroxidation rate, superoxide dismutase activity, glutathione peroxidase, GST, etc.) and Lysozyme activity. It would also be important to supplement this work by studying the variation of catalase and AChE activities in beards according to season, sex, and water quality being important factors in the variation of biomarkers.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research, king Saud University for funding through Vice Deanship of Scientific Research Chairs; Research Chair of Bioproducts Research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biomarker/bioindicator response profiles of organisms can help differentiate between sources of anthropogenic stressors in aquatic ecosystems. Biomarkers. 2001;6(1):33-44.
- [Google Scholar]
- Estimation of largemouth bass, Micropterus salmoides Lacepede, growth using the liver somatic index and physiological variables. J. of Fish Biol.. 1985;26(2):111-126.
- [Google Scholar]
- Heterogeneity of erythrocyte catalase II: isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur. J. Biochem.. 1974;48(1):137-145.
- [Google Scholar]
- Parasite infection directly impacts escape response and stress levels in fish. J. Exp. Biol.. 2020;223(16):jeb230904.
- [Google Scholar]
- Length-Weight Relationship and Fulton’s Condition Factor of Macrobrachium nipponense (De Haan, 1849) in Siah Darvishan River, Iran. Egypt. J. Aquat. Biol. Fisheries. 2021;25(2):551-560.
- [Google Scholar]
- Lipid peroxidation induced by Clinostomum detruncatum in muscle of the freshwater fish Rhamdia quelen. Diseases of aquatic organisms. 2000;42(3):233-236.
- [Google Scholar]
- Boumezbeur, A., 2003. Réserve intégrale du lac oubeira, Wilaya El Tarf. Fiche descriptive sur les zones humides Ramsar. Ministère de l’agriculture et du développement rural 7. https://rsis.ramsar.org/fr/ris/280.(Acecced in 1/7/2022).
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72(1–2):248-254.
- [Google Scholar]
- Environmental parameters and parasitism in common carp (Cyprinuscarpio Linnaeus, 1758) caught from Oubeira Lake (North-East of Algeria) Res. J. Fisheries Hydrobiol.. 2016;11(4):27-36.
- [Google Scholar]
- Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus) Ecotoxicol. Environ. Saf.. 2000;47(2):117-124.
- [Google Scholar]
- Seasonal dynamics of direct and indirect condition indices in relation to energy allocation in largemouth bass Micropterus salmoides (Lacepede) Ecol. Freshw. Fish. 2004;13(1):23-36.
- [Google Scholar]
- CCAC, 2005. Canadian Council on Animal Care Guidelines on: The Care and Use of Fish in Research, Teaching and Testing. https://norecopa.no/3r-guide/ccac-guidelines-on-the-care-and-use-of-fish-in-research-teaching-and-testing (accessed 1/7/2022).
- Crivelli, A.J., 2006. IUCN Red List of Threatened Species: Luciobarbus setivimensis.
- Stimulation of antioxidant enzymes levels in carp (Cyprinus carpio L.) infected by Ptychobothrium sp. (Cestoda) Fish Shellfish Immunol.. 2003;15(5):467-471.
- [Google Scholar]
- Condition factor and organosomatic indices of rainbow trout (Onchorhynchus mykiss, Wal.) from different brood stock. Biotechnol. Anim. Husbandry. 2016;32(2):229-237.
- [Google Scholar]
- Reactive oxygen species and oxidative stress. In: Di Giulio R., Hinton D., eds. The Toxicology of Fishes. CRC Press; 2008. p. :273-324.
- [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7(2):88-95.
- [Google Scholar]
- Changes on the activity of cholinesterase's in an immunomodulatory response of cattle infected by Listeria monocytogenes. Microb. Pathog.. 2018;114:36-40.
- [Google Scholar]
- Khalil, L.F., Polling, L., 1997. Check list of the helminth parasites of African freshwater fishes. Check list of the helminth parasites of African freshwater fishes (Ed. 2).
- Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol.. 2012;173(1–2):111-133.
- [Google Scholar]
- Why do some nematode parasites of the alimentary tract secrete acetylcholinesterase? Int. J. Parasitol.. 1996;26(5):499-508.
- [Google Scholar]
- Parasites and ecotoxicology: fish and amphibians. In: Férard J.-F., Blaise C., eds. Encyclopedia of Aquatic Ecotoxicology. Dordrecht: Springer Netherlands; 2013. p. :815-826.
- [Google Scholar]
- Transcriptomic responses in the fish intestine. Dev. Comp. Immunol.. 2016;64:103-117.
- [Google Scholar]
- Molecular and cellular biology of cholinesterases. Prog. Neurobiol.. 1993;41(1):31-91.
- [Google Scholar]
- Excretory/secretory products of anisakid nematodes: biological and pathological roles. Acta Vet. Scand.. 2017;59(1):1-12.
- [Google Scholar]
- Acetylcholinesterase activity in hosts (herring Clupea harengus) and parasites (Anisakis simplex larvae) from the southern Baltic. ICES J. Mar. Sci.. 2006;63(1):161-168.
- [Google Scholar]
- Interactions between immunocompetence, somatic condition and parasitism in the chub Leuciscus cephalus in early spring. J. Fish Biol.. 2009;75(7):1667-1682.
- [Google Scholar]
- Why do some parasitic nematodes secrete acetylcholinesterase (AChE)? Int. J. Parasitol.. 1993;23(5):549-550.
- [Google Scholar]
- Manipulation of end-product quality of rainbow trout with finishing diets. Aquac. Nutr.. 2000;6(1):17-24.
- [Google Scholar]
- Caractéristiques écophysiologiques d'une population d'anguilles de Camargue exposée à une pollution clandestine par des polluants organiques persistants. Revue d'Ecologie, Terre et Vie. 2003;58(1):103-126.
- [Google Scholar]
- Are fish immune systems really affected by parasites? An immunoecological study of common carp (Cyprinus carpio) Parasites Vectors. 2011;4(1):1-18.
- [Google Scholar]
- Physiological condition of Eastern Baltic cod, Gadus morhua, infected with the parasitic nematode Contracaecum osculatum. Conserv. Physiol.. 2020;8(1):coaa093.
- [Google Scholar]
- Scharsack, J.P., Koch, K., Hammerschmidt, K., 2007. Who is in control of the stickleback immune system: interactions between Schistocephalus solidus and its specific vertebrate host. Proc. R. Soc. B 274 (1629) 3151–3158.
- Metabolic depression and spleen and liver enlargement in juvenile Arctic charr Salvelinus alpinus exposed to chronic parasite infection. J. Fish Biol.. 2009;74(3):553-561.
- [Google Scholar]
- Do parasites influence behavioural traits of wild and hatchery-reared Murray cod, Maccullochella peelii? Parasitol. Res.. 2021;120(2):515-523.
- [Google Scholar]
- Response of the antioxidant system of Black Sea whiting Merlangius merlangus euxinus (Nordmann, 1840) to parasitic nematode Hysterothylacium aduncum (Rudolphi, 1802) infection. Bull. Eur. Assoc. Fish Pathol.. 2015;35(5):170-176.
- [Google Scholar]
- Parasite responses to pollution: what we know and where we go in ‘Environmental Parasitology’. Parasites Vectors. 2017;10(1):1-19.
- [Google Scholar]
- Tissue-specific responses of oxidative stress biomarkers and antioxidant defenses in rainbow trout Oncorhynchus mykiss during a vaccination against furunculosis. Fish Physiol. Biochem.. 2014;40(4):1289-1300.
- [Google Scholar]
- Functional evidence for the inflammatory reflex in teleosts: A novel α7 nicotinic acetylcholine receptor modulates the macrophage response to dsRNA. Dev. Comp. Immunol.. 2018;84:279-291.
- [Google Scholar]
- Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharmacol.. 2003;13(2):57-149.
- [Google Scholar]
- Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2020;234:108758
- [Google Scholar]
- Suppression of Schistosoma japonicum acetylcholinesterase affects parasite growth and development. Int. J. Mol. Sci.. 2018;19(8):2426.
- [Google Scholar]







