Translate this page into:
Assessment of green and chemically synthesized copper oxide nanoparticles against hepatocellular carcinoma
⁎Corresponding authors at: Department of Computing, University of Turku, 20500 Turku, Finland (T.N. Gia). tuan.nguyengia@hotmail.com (Tuan Nguyen Gia), hijaz555@gmail.com (Hijaz Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Copper oxide nanoparticle (CuO-NPs) attracts the attentions of human beings due to unique physicochemical and biological properties. The chemical and green synthesis approaches were used to synthesis of CuO-NPs and plant leaves extract Azadirachta indica (A. indica) was preferred in green synthesis approach. The XRD analysis was used to analyze the monoclinic crystal structure and calculate the average crystallite size in the range of 15 ∼ 16 nm. While, the information about different rotational and vibrational modes attached on the spectrum of CuO-NPs was identified by FTIR. After that the UV–VIS analysis provided the information about the absorbance spectrums in the range of 235 and 220 nm. The MTT assay was perfomed to investigate liver carcinoma (HepG2 cells) interaction and absorbance of CuO-NPs towards mentioned cell lines were recorded and loss in HepG2 cells viability. In an overall assessment, anticancer response of comparative study of CuO-NPs towards liver carcinoma treatment contributes significantly after successful demonstration of essential steps of suggested experimental study. Finally, comparative study of experimental and mathematical modeling of anticancer activity towards normal and liver cancer cell lines were conducted and investigated.
Keywords
Chemical synthesis
Green synthesis
Anti-carcinoma activity
Liver carcinoma
Mathematical modeling
1 Introduction
Cancer is an alarming health problem and is becoming the main cause of mortality worldwide. The increasing rate of cancer cases might be due to unhealthy diet, consumption of alcohol and tobacco (Golshah et al., 2019; Vinardell and Mitjans, 2015). Considering the current situation early recognition and appropriate experimental strategy are the basic need of present situation. Many oncologists and researchers work in the field of medical science are trying to resolve the serious health issues. Nowadays, the researchers are played the central role to overcome cancer diagnostic and treatment problems (Shaheen et al., 2017). Continuous efforts have been made to deliver adequate health care benefits at the least possible cost to improve survivability (Sivaraj et al., 2014; Vinardell and Mitjans, 2018; Verma and Kumar, 2019; Rehman et al., 2018a; Ur Rehman et al., 2019).
Previously, available therapies are not suitable for the treatment of carcinoma due to multiple side effects. It was very difficult to differentiate the normal and carcinoma cells. Likewise, the therapies are also damage the healthy cells. These problems were solved to use the transition metals base NPs for the treatment of carcinoma by using different therapies (Sankar et al., 2014; Ramaswamy et al., 2016; Yugandhar et al., 2017; Munir et al., 2019; Naz et al., 2020a,b). Moreover, CuO-NPs are attract the attention of researcher due to incredible properties such as biocompatible, low toxicity, small particle size, high surface area, stability, biodegradability, reasonable bandgap (1.73 eV), small crystallite size and decrease the recombination of charge carriers of CuO-NPs (Ghidan et al., 2016; Kalaiarasi et al., 2018). Presently, CuO-NPs are considered significant due to their excellent catalytic, electrical, textile, intrinsic anticancer activities, gas sensors, biomedicines and environmental remediation (Dey et al., 2019). However, CuO-NPs toxicity influence towards bacterial, algae, fish, rats, human cell lines are remarkable (Naz et al., 2020a,b; Sivaraj et al., 2014; Vasantharaj et al., 2019). It helps to destruction of DNA and slows down the growth rate of cancer bearing cells by releasing copper ions from CuO-NPs induce apoptosis of tumor cells. The CuO-NPs are synthesized by using different methods to attain cost efficiency and reduce time consumption. Among various methods such as sol–gel, microwave, sonochemical, various toxic reducing agents, co-precipitation and extract of plants as reducing agent have been used to synthesize the CuO-NPs (Awwad et al., 2015; Wu et al., 2002; El-Trass et al., 2012; Tadjarodi and Roshani, 2014).
The previous study was provided the information about the chemically synthesis approach of CuO-NPs to improve the toxicity level for living things. The chemical used during synthesis process irritate skin, nose and eyes. Moreover, the excess used of CuO-NPs cause respiratory system, gastro intestinal tract and skin related diseases. In case of green synthesis approach reduces the toxicity level of CuO-NPs and medicinal green plant was preferred due to cost effective, biocompatible and eco-friendly (Padil and Černík, 2013; Devi and Singh, 2014; Yugandhar et al., 2017). This study focused to synthesis of CuO-NPs via chemical and green synthesis approaches. Azadirachta indica (A. indica) is the common name of Neem plant that is used in green synthesis approach of CuO-NPs. A. indica is a medicinal plant that used to cure the multiple diseases. The multiple characterization techniques such as XRD, FTIR, and UV–VIS were used for material analysis and MTT assay for anti-carcinoma activity. Finally, the prepared CuO-NPs towards mentioned cells were recorded and loss in HepG2 cells viability was assessed by MTT assay.
2 Experimental procedures
2.1 Green synthesis approach
The green synthesis approach was used for the synthesis of CuO-NPs. The fresh leaves Azadirachta indica (Neem) were washed twice and cut into small pieces. After that 20 g leaves were dissolved in 100 mL de-ionized water and boiled at 60 °C for 20 min. Likewise, prepared solution was filtered by using filter paper and 2 g of C4H8CuO5 mixed in 20 mL solution boiled at 80 °C to get the green color mixture. Moreover, the product was dried in an oven and then put into furnace at 400 °C for 2 h. Finally, black color precipitate was grinded in mortar and pestle. The schematic diagram of CuO-NPs for treatment of hepatocellular carcinoma is shown in Fig. 1.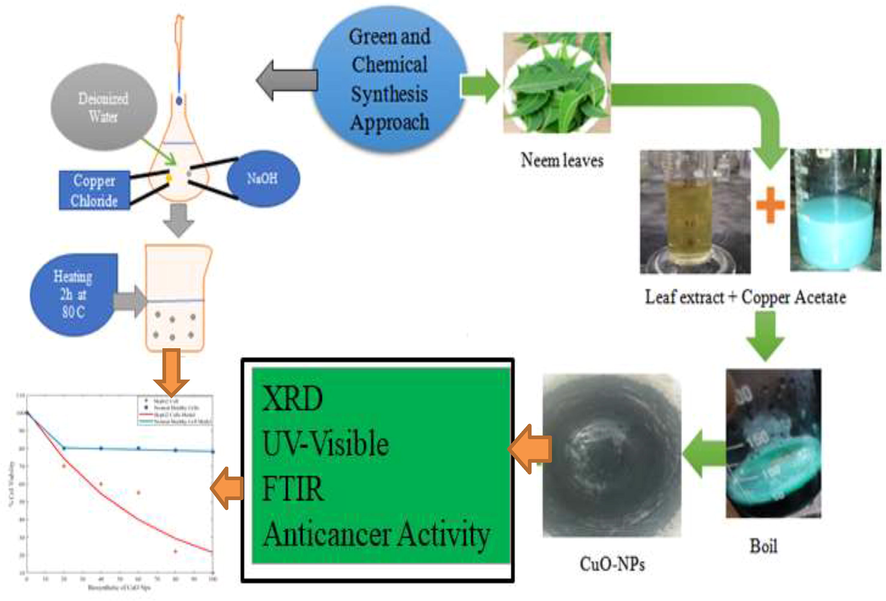
Schematic diagram of CuO-NPs for treatment of hepatocellular carcinoma.
2.2 Chemical synthesis approach
The chemical approach was used for the synthesis of CuO-NPs in which copper chloride (0.06 M) was dissolved in 200 mL de-ionized water and continuous stirred on magnetic stirrer for 30 min. The NaOH (0.15 M) solution was prepared to attain the pH upto 11 of copper chloride solution. The prepared solution was continuously stirred on magnetic stirrer for 3 h to get black color homogeneous mixture. After that, prepared solution was filter by using filter paper with the help of de-ionized water. Finally, the black color product was dried in oven at 80 °C for 2 h. The prepared precipitate of black color powder was grinded in mortar and pestle.
2.3 Material characterizations techniques
The following are the characterization techniques were used to characterize the CuO-NPs powder. The XRD (Cu-Kα radiation 1.5406 Å (D8 Advance, Bruker, X'Pert3 MRD XL) was used to study the crystalline nature of required nanomaterial. To identify the different rotational and vibrational modes were attached on the spectrum of CuO-NPs with the help of FTIR (Spectrum 2, Perkin Elmer). The absorbance bands of CuO-NPs were recorded using UV–VIS (Lambda 25, Perkin Elmer) (Munir et al., 2021).
2.4 Bioassay
2.4.1 HepG2 cell culturing
HepG2 (liver cancer cells) cell lines 1 × 104cells/well and normal human liver cells THLE-2 derived from liver (human) epithelial cells were cultured in 96 well plates. The 25 cm plastic tissue culture flasks and 96 wells plates (Nunc Wiesbaden Germany) individually in DMEM with (10%) fetal bovine serum and Hanks salts. After that 2 mM/L glutamine with some non-essential amino acids were incubated for 24 h. The atmosphere was provided to culture cell lines at 37 °C and it was subcultured three times a week. The 65–75% cell cultured indicate that the confluence was harvested by using trypsin 0.25% (Alam et al., 2012a,b; Alam et al., 2021; Govarthanan et al., 2016).
2.4.2 Cell labeling with CuO-NPs
The stock solution of CuO-NPs via chemical approach as well as green synthesis approach were prepared having compound polarity solution of 100 mg/L by dispersing/considering working solution of CuO-NPs (0, 10, 20, 40, 60, 80 and 100 µg/mL) incorporated into HepG2 cells cultured. Experimental arrangement of 96 wells plate and first ten columns of 96 well plate having confluence of 85% of hepG2 cells confluence were allocated for localized CuO-NPs test and rest of two columns of 96 wells plate were reserved for control/reference HepG2 cells.
2.4.3 MTT assay
The HepG2 cancer cells lines were tested by employing MTT assay (Alam et al., 2015). This assay was used to study the living cells in which active mitochondria. It was responsible to convert the tetrazolium compound into formazan product (insoluble purple colour). Moreover, CuO-NPs were labeled HepG2 cells lines and fixed in MTT for 3 hrs at 37 °C. After that three times washed cell lines and drying at room temperature under the presence of dry air and its color were dissolved in DMSO. Only 15 min of shaking, the plates were assessed using Multiscan MCC/340 microplate reader at filters (540 and 490 nm) respectively. Finally, the photodynamic treatment and cellular viability of cancer cells lines were estimated by applying MTT assay. The percentage feasible cell in each test was calculated by using standard method (Wu et al., 2002; Rehana et al., 2017, Alam et al., 2020).
3 Results and discussion
3.1 XRD measurement
The XRD analysis was used to identify the crystal structure and calculate the crystallite size of CuO-NPs obtained through green and chemical synthesis approach. XRD pattern of CuO-NPs as shown in Fig. 2(a) green synthesis and Fig. 2(b) Chemical synthesis approach represented the monoclinic phase and matched well with (JCPDS-80-1916) card (Awwad, et al., 2015). In both cases, the same pattern was observed and no extra peak appeared in case of green synthesis method. The green synthesis peaks indicate slight fluctuation as compare to chemical approach. The different diffracted peaks indicate the 2θ values at 32.3, 35.7, 38.4, 48.9, 53.1, 58.5 corresponding to following miller indices such as (
1 0), (0 0 2), (1 1 1), (
02), (0 2 0) and (2 0 2) (Awwad et al., 2015; Zhao et al., 2013). Furthermore, the crystallite size of these NPs was calculated by using the scherrer equation. The average crystallite size (CuO-NPs) for chemical and green synthesis approach in between (15 ∼ 16 nm) as expressed in Table 1.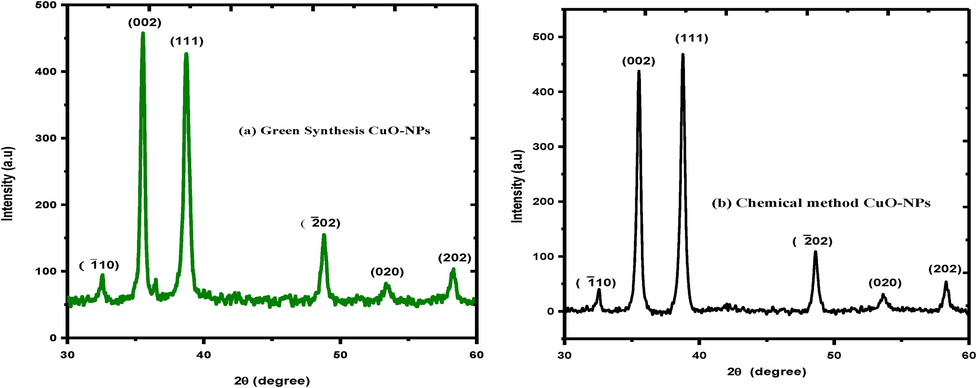
XRD spectrum of CuO-NPs (a) green synthesis (b) chemical approach.
CuO-NPs
Peak (0 0 2)
Peak (1 1 1)
Average size (nm)
Green synthesis
18
14
16
Chemical approach
17
13
15
3.2 FTIR measurement
The FTIR analysis was used identify the different rotational and vibrational modes attached on the spectrum of CuO-NPs. Fig. 3 exhibited FTIR spectrum of CuO-NPs (a) green synthesis approach (b) chemical synthesis approach. Fig. 3(a) indicates the peak of CuO-NPs is shifted, when the reaction between plants leaves extract and copper nitrate take place. The FTIR spectrum of CuO-NPs represented that the broader absorption band at 3742 cm−1 corresponds to the hydroxyl (OH) functional group. The few other absorption peaks were observed at different wavenumber 2360 cm−1 IR band at 1537 cm−1 can be assigned to alkenes group (C=C). Moreover, the peaks at 1535 cm−1 indicate stretching vibration of C–O group due to the presence of alcohols (C–O) at 1537, while smaller peaks at 780 and 768 cm−1 and 465 cm−1 were assigned vibration of C–H group. Fig. 3(b) chemical approach range from 450 to 4500 cm−1. At 3500–3700 cm−1 indicate the OH starching vibrational mode, while the band 1647–1689 represents 1st amide and 1500–1530 shows 2nd amide region. Finally, the bands 500–600 cm−1 indicated that metal oxide (CuO-NPs) due to the vibration in monoclinic phase (Sharma et al., 2015).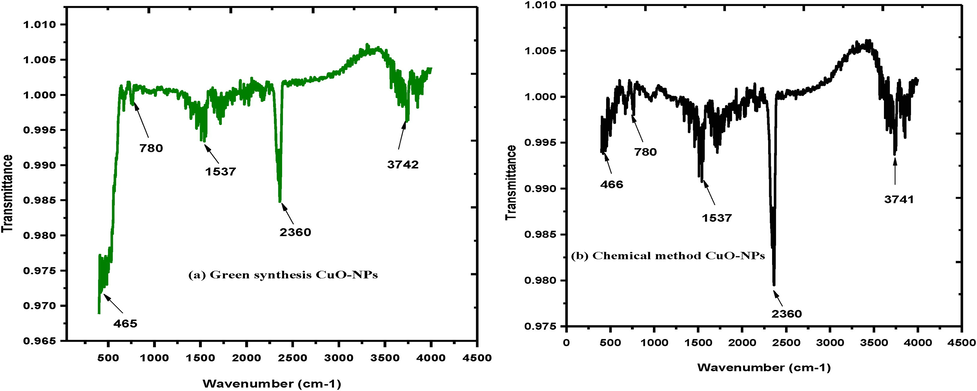
FTIR spectrum of CuO-NPs (a) green synthesis approach (b) chemical synthesis approach.
3.3 UV–visible spectroscopy measurement
Here, UV–VIS analysis was used to study the absorbance spectra of CuO-NPs Fig. 4(a) green synthesis Fig. 4(b) chemical synthesis approach. Fig. 4 shows that the absorbance band at 235 nm for chemical synthesis and 220 nm for green synthesis approach CuO-NPs respectively. Furthermore, UV–VIS spectroscopy of CuO-NPs shows blue shift as compare to CuO bulk close agreement with previous publishes data (Zain et al., 2007).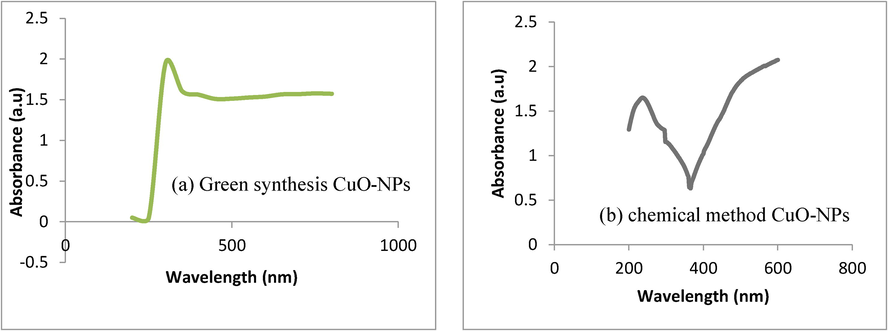
UV–VIS spectra of CuO-NPs (a) green synthesis approach (b) chemical synthesis approach.
3.4 Antioxidant and tumoricidal activities of green synthesis as well as chemically synthesized CuO-NPs (experimental as well as mathematical modulated comparison)
In this section the cell viability loss (%) due to biosynthetic procedure of CuO-NPs were demonstrated which depicts that beyond a certain concentration of CuO-NPs (20 µg/mL) the cell viability loss in HepG2 cell were significant but superficial effects of green synthesized CuO-NPs towards health liver cells were counted/recorded which identify the satisfactory and great development in field of biomedical science. In addition, experimental and mathematical modeling results reflect very good agreement in overall cell viability loss trend seen in Fig. 5 compare with our previous published data (Alam et al., 2015). Similar pattern of results was recorded, which of course depicts the non-invasiveness of photodynamic therapy.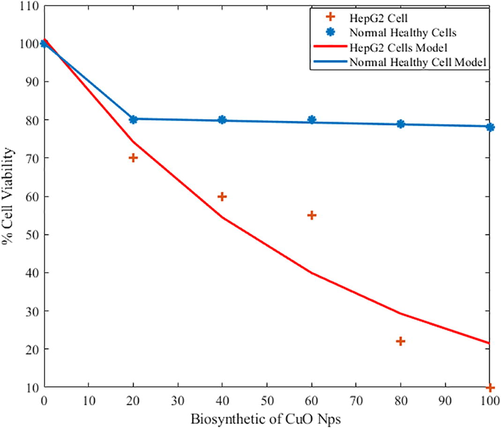
Comparative study of tumoricidal effect (loss in cell Viability %) of biosynthetic of CuO-NPs (microgram/mL) in healthy liver and HepG2 cells (modeling and Experimental Data).
The chemically synthesized CuO-NPs, very toxic effects towards normal healthy liver were investigated along with HepG2 cells (liver cancer cells). As compared to green synthesis procedure of CuO-NPs grown material, chemical synthesized materials are not feasible for PDT as cell viability loss of experimental and modeling data were shown in Fig. 3.5.
The cytotoxic effect of green and chemical synthesis approach of CuO-NPs was tested against normal healthy liver and HepG2 cell lines. Moreover, the Fig. 6 indicated that % cell viability by applying CuO-NPs of both mathematical modeling and experimental analysis. The analysis represented that CuO-NPs provided the significant toxicity effect toward liver carcinoma. The low density lipoproteins (LDL) in liver cancer cells provided the toxicity and the LDL have excellent ability to attract the antioxidant agents. The CuO-NPs occupied the different type of antioxidant due to this ability; it was used for the treatment of liver carcinoma. Few other types of chemical reaction take place inside the cells by applying the nanoparticles and these nanoparticle create the oxidative stress due to loss of membrane was used to kill the carcinoma cells. The significance of this experiment is that first time reported the comparison of chemical and green synthesis approach of CuO-NPs. The cell viability loss 85% of liver carcinoma call and approximately 15% loss of healthy cells were calculated. But in case of green synthesis approach only target the carcinoma cells and these nanoparticles have no any side effect on the healthy cells. Similar pattern of study were conducted in the previous published data (Alam et al., 2012a,b; AlSalhi et al., 2020).
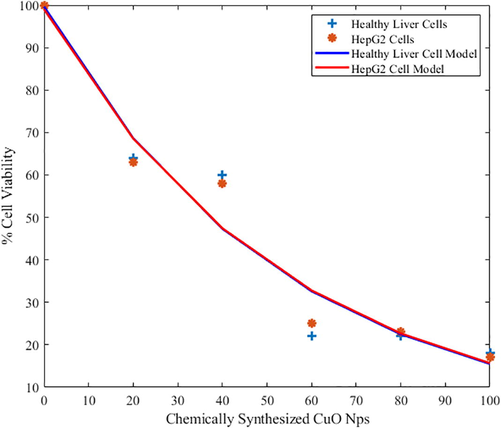
Comparative study of tumoricidal effect (loss in cell Viability %) of chemically synthesized CuO-NPs (microgram/mL) in healthy liver and HepG2 cells (modeling and experimental data).
Eq. (1) shows the proposed mathematical model. This type of model is known as bi-exponential model due to the presence of two weighted summation of exponential functions. This model is used to find fitness of experimental data collected from 4 different experiments. These experiments were conducted using biosynthetic CuO-NPs for healthy and HepG2 cells. Similarly, another experimental setup is established for chemically synthesized CuO-NPs for healthy and HepG2 cells. The data is experimentally collected from above said procedure and used to extract different parameters appeared in Eq. (1). The parameters are extracted using method of least square errors and listed in Table 2. Figure of merits for the goodness of fit are also evaluated such as SSE, R-square, Adjusted R- Square (A R-square) and RMSE and listed in the Table 2. A Mat lab was used to written the extract these model parameters along with goodness of fit (Jain et al., 2007). Moreover, ROS confirms the DNA damage via ROS detection assay and try pan blue staining assay. Results depicted that CuO nanoparticle responsible for DNA damage which leads to oxidative stress lesions causes’ cell viability loss.
Biosynthetic
A
B
C
D
HepG2
101
−0.0155
0
0
Healthy
19.24
−0.9901
10.76
−0.0003083
SSE
R-Square
A R-square
RMSE
HepG2
462.2
0.9138
0.8922
10.75
Healthy
0.2728
0.9992
0.9981
0.3693
Chemically Synthesized
A
B
C
D
HepG2
99.64
−0.01862
0
0
Healthy
−192.3
−0.02001
291.3
−0.01945
SSE
R-Square
A R-square
RMSE
HepG2
301.8
0.9436
0.9296
8.686
Healthy
206.2
0.9599
0.8998
10.15
It can be observed that in biosynthetic of CuO-NPs, the healthy cells have stable behaviour for % cell viability and did not dropped below 80% for increasing biosynthetic of CuO-NPs (Islas et al., 2020). Likewise; HepG2 cells have dropped % cell viability below 15%. This particular behaviour shows that the normal healthy cell will remain unaffected while the HepG2 cells are affected drastically. On the other side, chemically synthesized CuO-NPs have shown the drastic drop of % cell viability for both normal health cells and for HepG2 cells. Mathematical functions in terms of bi-exponential model are presented for any future comparison.
4 Conclusion
The chemical and green synthesis approach was used to synthesize the CuO-NPs. In case of green synthesis approach the plant leaves extract of Azadirachta indica (A. indica) was used to prepare the CuO-NPs. The XRD depicted monoclinic phase and the crystallite size in the range 15 to 16 nm. After that the FTIR spectrum was used to identify the presence of different rotational and vibrational modes attached on the spectra of CuO-NPs. The UV–VIS analysis represented the absorbance peaks at 220 and 235 nm. In vitro MTT assay towards HepG2 was carried out for the determination of anti-carcinoma activity comprises of chemical and green synthesized of CuO-NPs. By using green synthesis approach, the development of CuO-NPs shows significant toxicity towards liver carcinoma and it proved bio-safe towards healthy liver tissue island when assessed MTT assay. But opposite relation of chemically grown CuO-NPs were recorded. We conclude that CuO-NPs synthesized using Azadirachta indica (A. indica) leaves extract may be implemented as chemotherapeutic agents for anticancer activity which significantly play a vital role for cancer treatment purpose in near future.
Acknowledgement
Researchers Supporting Project number (RSP-2021/397), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Spectroscopic features of PHOTOGEM® in human Rhabdomyosarcoma (RD) cellular model. J. King Saud Univ.-Sci.. 2020;32(7):3131-3137.
- [Google Scholar]
- Synthesis of NiO nanoparticles and their evaluation for photodynamic therapy against HeLa cancer cells. J. King Saud Univ.-Sci.. 2020;32(2):1395-1402.
- [Google Scholar]
- Antibacterial activity of synthesized copper oxide nanoparticles using Malva sylvestris leaf extract. SMU Med. J.. 2015;2(1):91-101.
- [Google Scholar]
- Synthesis of copper oxide nanoparticles by a novel method and its application in the degradation of methyl orange. Adv. Electron. Electr. Eng.. 2014;4(1):83-88.
- [Google Scholar]
- Azadirachta indica leaves mediated green synthesized copper oxide nanoparticles induce apoptosis through activation of TNF-α and caspases signaling pathway against cancer cells. J. Saudi Chem. Soc.. 2019;23(2):222-238.
- [Google Scholar]
- CuO nanoparticles: synthesis, characterization, optical properties and interaction with amino acids. Appl. Surf. Sci.. 2012;258(7):2997-3001.
- [Google Scholar]
- Sensitivity of A-549 human lung cancer cells to nanoporous zinc oxide conjugated with Photofrin. Lasers Med. Sci.. 2012;27(3):607-614.
- [Google Scholar]
- Anticancer effects of nanometallic oxides and their ligands with photosensitizers in osteosarcoma cells. J. Optoelectron. Adv. Mater.. 2015;17:1808-1815.
- [Google Scholar]
- The photodynamic effect of ZnO nanorods and their ligands with different photosensitizers. Rev. Nanosci. Nanotechnol.. 2012;1(1):40-51.
- [Google Scholar]
- Green synthesis of copper oxide nanoparticles using Punica granatum peels extract: Effect on green peach Aphid. Environ. Nanotechnol. Monit. Manage.. 2016;6:95-98.
- [Google Scholar]
- Applying the Taguchi method to the optimization of anticancer activity of bacterial alginate-CuO bionanocomposite. Open Access Macedonian J. Med. Sci.. 2019;7(1):1-5.
- [Google Scholar]
- Govarthanan, M., Cho, M., Park, J. H., Jang, J. S., Yi, Y. J., Kamala-Kannan, S., & Oh, B. T. (2016). Cottonseed oilcake extract mediated green synthesis of silver nanoparticles and its antibacterial and cytotoxic activity. J. Nanomater., 2016.
- Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics. 2007;2(3):107-118.
- [Google Scholar]
- Copper oxide nanoparticles induce anticancer activity in A549 lung cancer cells by inhibition of histone deacetylase. Biotechnol. Lett.. 2018;40(2):249-256.
- [Google Scholar]
- Treatment of breast cancer with capped magnetic-NPs induced hyperthermia therapy. J. Mol. Struct.. 2019;1196:88-95.
- [Google Scholar]
- Quantitative analysis of glucose by using (PVP and MA) capped silver nanoparticles for biosensing applications. Physica B. 2021;602:412564
- [Google Scholar]
- Anticancer and antibacterial potential of Rhus punjabensis and CuO nanoparticles. Nat. Prod. Res.. 2020;34(5):720-725.
- [Google Scholar]
- Toxicity of copper oxide nanoparticles: a review study. IET Nanobiotechnol.. 2020;14(1):1-13.
- [Google Scholar]
- Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed.. 2013;8:889.
- [Google Scholar]
- Potentiating effect of ecofriendly synthesis of copper oxide nanoparticles using brown alga: antimicrobial and anticancer activities. Bull. Mater. Sci.. 2016;39(2):361-364.
- [Google Scholar]
- Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother.. 2017;89:1067-1077.
- [Google Scholar]
- Fabrication and characterization of Zinc Telluride (ZnTe) thin films grown on glass substrates. Physica B. 2019;560:204-207.
- [Google Scholar]
- Influence of temperature on Sr 0.35 La 0.40 Ca. 25 Fe 11.6 Co 0.4 O 19 hexagonal ferrites against structural, morphological and magnetic properties prepared by conventional ceramic reaction methodology. J. Supercond. Novel Magn.. 2018;31(3):925-932.
- [Google Scholar]
- An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods. 2020;74:104171.
- [CrossRef] [Google Scholar]
- Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng., C. 2014;44:234-239.
- [Google Scholar]
- An in vitro study of the photodynamic effectiveness of GO-ag nanocomposites against human breast cancer cells. Nanomaterials. 2017;7(11):401.
- [Google Scholar]
- Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloy. Compd.. 2015;632:321-325.
- [Google Scholar]
- Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2014;129:255-258.
- [Google Scholar]
- A green synthesis of copper oxide nanoparticles by mechanochemical method. Curr. Chem. Lett.. 2014;3(4):215-220.
- [Google Scholar]
- Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol., B. 2019;191:143-149.
- [Google Scholar]
- Synthesis and biomedical applications of copper oxide nanoparticles: an expanding horizon. ACS Biomater. Sci. Eng.. 2019;5(3):1170-1188.
- [Google Scholar]
- Antitumor activities of metal oxide nanoparticles. Nanomaterials. 2015;5(2):1004-1021.
- [Google Scholar]
- Synthesis of copper oxide nanoparticles using carbon nanotubes as templates. Chem. Phys. Lett.. 2002;364(1-2):152-156.
- [Google Scholar]
- Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Applied Nanoscience. 2017;7(7):417-427.
- [Google Scholar]
- Mitigation of CuO nanoparticle-induced bacterial membrane damage by dissolved organic matter. Water Res.. 2013;47(12):4169-4178.
- [Google Scholar]







