Translate this page into:
Assessment of different salt concentrations on the growth and phytochemical change of the ice plants
⁎Corresponding author at: Department of Bio-systems Engineering, Gyeongsang National University (Institute of Smart Farm), Jinju 52828, Republic of Korea. Tel: +82-55-772-1896. bioani@gnu.ac.kr (Hyeon Tae Kim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The ice plant (Mesembryanthemum crystallinum L.) has become a halophyte model to study the plant photosynthetic responses C3 photosynthesis to crassulacean acid metabolism (CAM), which is accelerated by salt stress. However, this adaptive mechanism improves water use efficiency, and water stress tolerance is still poorly known. This study examined the effect of individual and mixture of NaCl and CaCl2 concentrations on morphological parameters and bioactive component contents of ice plants in a plant factory system. Eight salt treatments individually and a combination of sodium chloride (NaCl) and calcium chloride (CaCl2), and Hoagland solutions were applied after the transplanting of ice plants. Morphological parameters like the number of leaves and lateral stems, leaf chlorophyll content (SPAD value), fresh weight, and dry weight of shoots and roots were measured at the adult stage. Concurrently, in the juvenile phase, the area of a canopy was evaluated using an image processing technique in HSV (hue, saturation, value) colour space. Correspondingly, ice plant secondary metabolites such as cations, anions, and radical scavenging activity were assayed in the adult phase correlated to the salt stress. The effects of salt stress on the growth of ice plants and secondary metabolite production were analysed using completely randomized block designs through the variance by one-way ANOVA with a significance level of p < 0.05. This study demonstrated that 400 mM CaCl2 (T4) enhanced the biomass and high sodium (Na+) and calcium (Ca2+) accumulations, and 200 mM CaCl2 (T3) accelerated the potassium (K+), magnesium (Mg2+), phosphate (PO43−), and sulfate (SO42−) accumulations. Moreover, NaCl 400 mM (T1) and combination of 100 mM NaCl and 300 mM CaCl2 (T5) positively influenced the chloride (Cl−) deposition and combination of 200 mM NaCl and 200 mM CaCl2 (T6) improved nitrate (NO3–) accretion. Furthermore, 100 mM CaCl2 (T2) exhibited the highest antioxidant activity in ice plants grown under the plant factory system.
Keywords
Mesembryanthemum crystallinum L.
Photosynthesis
Image processing technique
Salt stress
Secondary metabolites
1 Introduction
The common ice plant (Mesembryanthemum crystallinum L.) has become synonymous with the salt stress response model. It is systematically developed under controlled and salinity environmental conditions. Furthermore, it can encompass the salinity by enhancing the water usage efficiency by switching the photosynthesis mechanism C3 to CAM (Adams et al., 1998). Moreover, salinity responses result in the compartmentalization of ions and compatible solutes, and physiologically active compounds, as reported by Kim et al., 2021. Correspondingly, these physiologically active substances, presumably phenolic compounds and flavonoids, are profusely consumed by humans to prevent detrimental health problems (Kim et al., 2021).
Consuming ice plant leaves helps treat diabetes by inhibiting increases in blood sugar levels, as mentioned by Kang et al., 2006. Consequently, consumer interest in common ice plants, effective against diabetes mellitus, has risen recently (Kim et al., 2018). The plant is regarded as the crystalline ice plant because it is surrounded by larger, glistering epidermal bladder cells (EBCs) that resemble ice crystals (Kim et al., 2021). A large central fluid vacuole distinguishes EBCs, which uses the acclimation process to regulate salt retention and water as reservoirs for suitable solutes and phytochemicals like flavonoids, pinitol, and myo-inositol (Agarie et al., 2007; Hasegawa et al., 2000).
In addition to that, myo-inositol has been involved in the cellular process in the plant, such as signal transduction, growth regulations, and membrane response. Correspondingly, myo-inositol plays an intriguing role in plants and animals by breaking down cholesterol and lipids causing panic disorder and disease depression and regulating blood pressure, as reported by Agarie et al., 2009. Salt stress induces the accumulating high concentrations of phytochemicals (d-pinitol, myo-inositol, d-ononitol) in the cytosol. Considering the above facts, evaluating antiradical and radical scavenging properties is vital to determine the basis of the phenolic concentration of ice plant extract. According to Shalaby and Shanab, 2013, the antioxidant activity of plant tissues is typically measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) to assess radical scavenging or antiradical activity, or the potential of compounds to react with free radicals.
Notably, the ice plant is a facultative halophyte that responds to extreme salinity by switching its metabolism from C3 to CAM photosynthesis, an ecophysiological adaptation that aids in water conservation by assimilating external CO2 with minimal water loss by opening its stomata at night, with lower CO2 compensation points during the diurnal cycle’s dark and early light periods and appreciably lower photorespiration rates than C3 plants (Agarie et al., 2009; Cushman et al 1990). In addition, CAM atmospheric CO2 is converted to organic acids (e.g., malic acid, citric acid) and stored in mesophyll cells, where phosphoenolpyruvate carboxylase (PEPC) remobilizes and decarboxylates the organic acids to provide CO2 during the day through the Calvin cycle, as revealed by Guan et al., 2020.
As mentioned above, M. crystallinum is a facultative halophyte for the study of C3 and CAM due to its ability to respond to salt stress. As a result, this plant is highly tolerant of salinity and drought. It completes its life cycle on soil with a NaCl concentration equivalent to seawater (500 mM) (Agarie et al., 2009). Notably, Guan et al., 2020 found that 500 mM NaCl showed a similar leaf growth rate as the control group (0.5 × Hoagland’s solution) within the initial 5 days of salt treatments and then growth delayed after 7 days. Considering the carbon fixation, salt-stressed ice plants have performed less carbon fixation compared to C3 plants.
Correspondingly, NaCl inducible myoinositol-1-phosphate synthase in roots and sodium absorption and transport through the xylem was coupled to a tenfold increase in myo-inositol and onitol in the xylem. Concurrently, myo-inositol serves as a substrate for producing compatible solutes and a signal for enhancing sodium absorption between leaves and roots (Nelson et al., 1999).
In addition to sodium, calcium is rich in green vegetables, facilitating the calcium deficiency in human nutrients. Considering that calcium deficiency is the most frequent mineral shortfall in modern diets, boosting the calcium content of leafy vegetables in ice plants can help consumers acquire more nutrients (Yuan et al., 2018). In addition to that Ca2+ ions increased the membrane integrity and storage capacity (Xu et al., 2013). Further, calcium appears to play a protective function to salt-induced mechanisms, prompting growth regulators and other substances to alleviate the adverse effects and maintain normal features of plants (Xu et al., 2013). Furthermore, M. crystallinum stomatal movement and phenotypic changes are influenced by high salt concentrations in the soil; combining the leaf succulence assay during the transition stage to facultative CAM plants improves leaf succulence and facilitates photosynthesis (Guan et al., 2020). In this regard, analysis of the ion content of ice plants is of utmost importance to confirm previous predictions that EBCs in M. crystallinum under salt stress conditions may act as water reservoir organs for vacuolar ion homeostasis and salt retention, as shown by Agarie et al., 2007.
Following changes in salt stress conditions, morphological characteristics may appear on above and below ground parts such as leaves, stems, and roots (Buckleyl et al., 1997). In general, growth analysis reveals the primary productivity and physiological phenomena of plants.
Generally, mild salt concentration influenced the canopy area development compared to the lower concentration of salt treatments. Interestingly, CaCl2 has been involved in plant regulatory mechanisms that ensure plants adjust to unfavourable stress conditions and ameliorate the negative effects by altering physiological and biochemical mechanisms in leaves (Xu et al., 2013). Morphological parameters are based on raw data such as the number of leaves and lateral stems, SPAD value, fresh weight and dry weight of root and shoot, and canopy area, as revealed by Madhavi et al., 2021. The SPAD value is crucial for measuring the chlorophyll content of leaves, which largely determines photosynthetic capacity and plant growth (Li et al., 2018).
However, the investigation of ice plants’ growth and bioactive compounds (compatible solutes, myo-inositol, pinitol) are affected by environmental conditions. To overcome the aforementioned difficulties, cultivation methods must be developed in controlled environments, such as plant factories. A plant factory is an automated system that artificially controls environmental conditions, light, relative humidity, temperature, and CO2 concentration. It is possible to produce leafy vegetables similar to industrial products within a facility, regardless of the location (Takatsuji, 2008). According to Kim et al., 2018 various light sources have different effects on plant morphological changes. Considering this fact, in recent decades, researchers have conducted plant cultivation using light-emitting diodes (LEDs) because they have low high energy efficiency, heat emission, high power, and the capability of discrete emitting wavelengths with a narrow bandwidth. As a result, plant factories can produce high quality, higher annual yields with lower resource consumption and shorter production cycles for uniform plants without contamination (Lee et al., 2019).
Considerable research has been carried out with LEDs light treatment and sodium applications individually. Agarie et al., 2009 planted ice plants in underwater culture and added NaCl at 0, 100, 200, and 400 mM to the culture solution. This study found the maximum concentration of bioactive components, namely pinitol, ononitol, and myo-inositol, and high DPPH radical scavenging activity in NaCl 400 mM treatment, among other treatments. However, to our knowledge, a comparative evaluation of two types of salt and different concentrations, as well as the combined effect of salt on the development and growth of phytochemicals in ice plants, has not yet been carried out. Therefore, the current study attempted to investigate the effects of individual and combined concentrations of NaCl and CaCl2 on the morphological parameters and the content of bioactive compounds in ice plants in a closed plant production system.
2 Materials and methods
2.1 Plant growth conditions
During the summer of 2021, the current investigation was conducted in the controlled plant factory at Gyeongsang National University, Smart Farm Systems Laboratory, South Korea. The total duration of the experiment was 120 days (from early March to late June). The main environmental parameters, namely temperature, humidity, and CO2 concentration, were monitored daily using a specific high precision sensor unit (Hanam Engineering Co. ltd, South Korea) (Madhavi et al., 2022).
Seeds of the ice plant (Mesembryanthemum crystallinum L.) were sown in 50 cell plug trays [(54 cm × 28 cm × 4 cm (L × W × H) ] using bio plus compost soil (cocopeat 68.86%, peat moss 11.00%, perlite 11.00%, and zeolite 9.00%) as a growing medium (Madhavi et al., 2022). Germinated seedlings were grown in a plant factory system for 28 days at 25 ± 1 °C, 60 ± 10% humidity, 1000 µmolmol −1 CO2, and under 120 µmolm−2s−1 photosynthetic photon flux density (PPFD) using red, blue, and green LED treatment (Hanam Engineering Co. ltd, South Korea) with 16 h/8h (light/dark) photoperiod as shown in Fig. 1.
Seedling stage (30 days after planting) of M. crystallinum grown under the plant factory system.
Plants were watered three times a week with 50 ml of 0.5 × Hoagland’s solution (Hoagland and Arnon, 1950). After appearing 4 leaves (28 days mature plant), 9 seedlings per treatment were transplanted in bio plus compost containing pots [(60 cm × 20 cm × 12 cm (L × W × H)] (Guan et al., 2020). For each treatment, 3 pots were used for 9 plants (20 cm apart; 3 plants per pot), with the single plant representing an independent biological replicate.
The different number of replicates were utilized for evaluating the effect of salt treatments on morphological growth, such as canopy area in the juvenile stage (n = 3), and the number of leaves, lateral stems, SPAD value, fresh weight, and dry weight of shoot and root in the adult stage (n = 9). Eventually, the cation and anion contents and DPPH radical scavenging activity(%) in the adult stage, three replicates were used.
Following the protocol by Cushman et al., 1990, plants in the control group were watered daily with 50 ml of 0.5 × Hoagland’s solution (application into one pot; 3 plants), while those in the treatment group were also watered daily with 0.5 × Hoagland’s solution containing the different salt treatments per pot as demonstrated in Table 1 (Guan et al., 2020). Previous studies reported that 400 mM NaCl exhibited the highest growth performance and bioactive component accumulation (Agarie et al., 2009). In this study, individual CaCl2 concentrations were selected as 100 mM, 200 mM, and 400 mM as previous studies' NaCl individual concentrations (Agarie et al., 2007; Agarie et al., 2009). In addition to that, NaCl and a mixture of CaCl2 and NaCl solution concentrations were maintained up to 400 mM in each salt treatment, and electrical conductivity (EC) was evaluated. EC was maintained at the same level for each solution, as denoted in Table 1. Initially, salt solutions were prepared and monitored EC (“Measuring soil salinity: plant stress, n.d.”). Simultaneously, the salt solution (25 ml) was mixed with 0.5 × Hoagland’s solution (25 ml) and applied to the pot, as mentioned previously. EC values of final salt solutions with 0.5 × Hoagland’s solution were indicated in Table 1. Eventually, the influence of salt stress on ice plant growth, and phytochemical changes were measured. The facts mentioned above affect the selection of eight salt treatments for our study.
Treatments
Concentration levels of salt treatment with 0.5 × Hoagland’s solution
EC value
(dS/m) at 25 °C
CS
Control
4
T1
400 mM NaCl
39.6
T2
100 mM CaCl2
11
T3
200 mM CaCl2
22
T4
400 mM CaCl2
44
T5
100 mM NaCl + 300 mM CaCl2
42
T6
200 mM NaCl + 200 mMCaCl2
40
T7
300 mM NaCl + 100 mM CaCl2
38
2.1.1 Measurements of plant growth and morphology
Plant morphological parameters, namely number of leaves, number of lateral stems, and, SPAD value, were measured during 120 days of mature (adult phase) plants. Typically, the canopy area was high in the early stage, decreasing the later growth stage of all treatments. Therefore, canopy area measurement in the juvenile stage is crucial. Otherwise, all leaves drop and become small in the reproductive stage, as revealed by Bohnert and Cushman, 2000. The canopy area was measured during 60 days (juvenile phase) of mature plants by creating a python program with the green pixels against the reference reported by Guan et al., 2020.
The number of leaves was counted for all plant leaves except the cotyledons in each of the nine plants per treatment. The number of lateral stems was counted in every-nine plants. A portable chlorophyll meter (SPAD-502, Konica Minolta Inc., Osaka, Japan) was used to measure the value SPAD, indicating the chlorophyll content of the third leaf pair from the bottom leaf pair, in each of the nine plants per treatment (Kim et al., 2018). Subsequently, the canopy area was determined using the image processing algorithm developed by python program from Google Colaboratory using the ratio of green pixels against 4 cm2 purple square reference paper with images taken by RGB camera (SONY DSC-RX100 vii, Korea) (Chaudhary et al., 2012). Three plant images were photographed in each control and salt treatment group, as demonstrated in Fig. 2. Each image of three plants was taken at 2 pm on Friday to track canopy growth and use the python program to measure canopy area (Fig. 2). Each image was captured using the RGB camera, cropped, and downscaled to a ratio of 0.25; the green, yellow and cyan colour threshold was extracted using the HSV colour model and converted to a black and white image shown by Madhavi et al., 2021. Precisely, plant and reference black and white image pixel values were calculated separately and evaluated the canopy area relevant to the reference area.
Images acquisition for the canopy area measurement (60 days mature plant) of M. crystallinum grown under the plant factory system. The purple-coloured square paper demonstrated the reference for calculating the canopy area.
Eventually, the plants (120 days mature plant) were harvested three months after salt application. Fresh weights of shoots and roots were measured immediately after harvest using a digital balance (Model-FX-300iWP, A&D Company Limited, Tokyo, Japan). The dry weights were determined after drying the tissues in a drying oven (Shelves for 5E-DHG6310: 2 layers, Changsha Kaiyuan Instruments Co., ltd, Changsha 410100, PR China) at 80 °C for 48 h (Agarie et al., 2009). Generally, all weight measurements were taken in every-nine plants per treatment.
2.2 Ion content determination
2.2.1 Cation content determination
The dried leaves of the ice plant (0.1 g) were kept in the centrifuge tubes (15 ml), and 6 ml of HNO3 was added to each tube. The samples were filled into digestion tubes (50 °C to 220 °C) and kept for 1 h and 20 min (heating time- 30 min, holding time-20 min and cooling time-30 min) for microwave digestion in the advanced microwave digestion system (Milestone, IT/ETHOS EASY, Italy). Four millilitres of distilled water were added to the digested samples and filtered with the 0.45 µm membrane filter. One millilitre of the filtrate was taken and again diluted with distilled water up to 10 ml. Subsequently, the solution’s cation (Na+, K+, Ca2+, and Mg2+) concentration was determined using an inductively coupled plasma spectrophotometer (Optima 8300, ICP-OES Optical System, and SCD Detector, Singapore) (Wilschefski and Baxter, 2019).
2.2.2 Anion content determination
One gram of dry leaves was ground with 20 ml of distilled water using a mortar and pestle and centrifuged at 3000×g for 15 min (Eppendorf Microcentrifuge 5430 R, North America). The supernatant was filtered through Whatman 120 MM no 1 filter paper (Agarie et al., 2007). The filtrate was used for anion (Cl−, NO3–, PO43− and SO42−) concentration determination by ion combustion chromatography (930 Compact IC Flex, Philippines) (Lopez-Ruiz, 2000).
2.3 DPPH radical scavenging activity(%)
Dried ice plant leaves (1 g) were ground with a mortar and pestle and placed in a centrifuge tube (15 ml) with 5 ml of 80% methanol. The mixture was shaken at room temperature for 12 h and centrifuged at 10,509×g for 10 min. After centrifugation, the supernatant was filtered through Whatman 120 MM no 1 filter paper (Lee et al., 2015). The 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma-Aldrich Co., MO, USA) determined the free radical scavenging activity of the ice plants modified method described by Kim et al 2021. The filtrates were used for analyzing the free radical scavenging activity. The reaction mixture containing 1 ml of extract and 3 ml of 0.3 mM DPPH was shaken vigorously for 5 s and incubated in the dark at room temperature for 20 min. After incubation, the absorbance of the extract was measured at 517 nm using an Ultraviolet-VIS-NIR Spectrophotometer (U-4100, Hitachi, America), with methanol as the blank and methanol and DPPH solution as the control (Weeplian et al., 2018). The scavenging effect for DPPH radical was calculated using the following equation:
Scavenging effect (%) = (1- As/Ac) × 100 (Weeplian et al., 2018).
As and Ac absorbance of sample and absorbance of the control respectively, at 30 min.
2.4 Statistical analysis
Data on morphology, ion content, and DPPH radical scavenging of ice plants were collected in MS Excel (Microsoft Office 2019, Seattle, WA, USA). Moreover, for data analysis, statistical methods in the Statistical Package for the Social Sciences (SPSS) were used, including analysis of variance (one-way ANOVA) to practice with a significance level of p < 0.05. Significant differences between the means of the experimental data were tested using a Post- Hoc Tukey's HSD test in Statistics 10 (SPSS version: 25.0.0, IBM, New York, USA). All graphical plots were illustrated using OriginPro 9.0 (Origin Lab Corporation Northampton, MA, USA).
3 Results and discussion
3.1 Measurements of plant growth and morphology
Ice plants grown under different salt treatments showed appreciable differences in morphological parameters such as number of leaves, number of lateral stems, SPAD value, fresh and dry weight of shoots and roots, and canopy area. Subsequently, the number of leaves was significantly higher in treatment T4, whereas there was less leaf production with T5 and T6 treatments, as shown in Table 2. Consequently, higher leaf production was observed in CaCl2 treatments. This is because calcium plays a direct role in the regulatory mechanisms that activate plants under salt stress and enhance the stimulation of growth regulators and other substances in plants (Xu et al., 2013). However, the CS, T1, T2, T3, and T7 showed no significant differences in total leaf numbers due to the different concentrations of NaCl and CaCl2 salt treatments. Different letters indicate significant differences between groups (p < 0.05). Values represented means ± SD (n = 9).
Salt treatments
Number of leaves
Number of lateral stems
SPAD value
Fresh weight (g/plant)
Dry weight (g/plant)
Shoot
Root
Shoot
Root
CS
44.67
±2.45b
9.78
±1.64 ab
50.18
±4.54b
21.29
±1.08d
2.60 ± 0.35c
0.68
±0.01d
0.56
±0.03d
T1
46.78
±2.54b
6.89
±1.90b
42.54
±3.86bc
15.67
±0.28f
1.29
±0.15e
0.54
±0.01f
0.37
±0.03f
T2
47.56
±4.69b
10.56
±1.13ab
39.92
± 2.76c
24.76
±0.42c
3.56
±0.34b
0.83
±0.02c
0.65
±0.03c
T3
46.33
±4.85b
7.22
±1.99b
39.90
± 2.66c
17.49
±0.60e
1.69
±0.16d
0.60
±0.03e
0.45
±0.03e
T4
64.89
±5.40a
13.33
±2.24a
48.02
± 2.20b
30.84
±0.74a
4.17
±0.08a
0.93
±0.02a
0.86
±0.03a
T5
25.44
±2.24c
8.11
± 1.45b
49.73
±4.71b
20.14
±0.75d
0.80
±0.06f
0.52
±0.01f
0.07
±0.01 g
T6
29.44
±2.24c
5.22
±1.99b
65.08
± 2.03a
12.34
±0.89 g
0.60
±0.06 g
0.47
±0.01 g
0.04
±0.01 h
T7
51.11
±4.59b
11.11
±1.54a
47.89
±1.81b
28.59
±1.08b
3.76
±0.15b
0.87
±0.01b
0.74
±0.03b
Correspondingly, the number of lateral stems was promoted by T4 and T7 treatments, whereas lateral stem production was suppressed by the T6 treatment compared to other salt treatments (Table 2). Bohnert et al., 2000 found that this species is a salt accumulator, storing a high amount of NaCl and CaCl2 in a gradient from roots to developing shoots. Furthermore, at adequate salinity levels ranging from 100 to 400 mM, this salt-loving plant grew at its best (Agarie et al., 2007). Therefore, lateral stem growth was stimulated by the high concentration of salt treatment.
Interestingly, fresh weight and dry weight of shoots and roots tended to be higher in treatments T4 and T7, as shown in Table 2. Furthermore, a positive correlation was observed with the number of leaves, lateral stems, and canopy area related to the ice plant's shoot and root biomass. Kim et al., 2021 also exhibited that the number of leaves, lateral stems, and leaf area of ice plants positively correlated with the root and shoot biomass. Thus, it concluded that the highest morphological growth is appreciably related to the biomass of ice plants. Agarie et al., 2007 reported that 400 mM NaCl increased the dry weight of wild-type ice plants by almost 2-fold compared to the mutant ice plants. Therefore, the higher concentration of salt impacts the biomass enhancement of ice plants.
Different salt concentrations affected the SPAD value and photosynthetic rate inside the ice plant leaves (Table 2). Moreover, the SPAD value was significantly higher in T6 than within the other different treatments. The photosynthetic rate was not appreciably different within the salt treatments CS, T1, T4, T5, and T7. A low photosynthetic rate was observed in salt treatments T3 and T2. However, the SPAD value and photosynthetic rate of ice plant leaves showed a considerably high value in T6 treatment because the similar concentration of NaCl and CaCl2 increased chlorophylls and net photosynthetic rate (Trajkova et al., 2006). The higher concentration of NaCl and CaCl2 decreased the contents of chlorophylls and the net photosynthetic rate. It accelerates the respiration rate and CO2 compensation concentration in leaves, as revealed by Khavari-Nejad and Chaparzadeh (1998).
The T4 (258.08 ± 2.09 cm2) and T7 (207.74 ± 1.21 cm2) salt treatments produced a larger canopy area during the early growth stage, while the T3 treatment produced a lower canopy area (60.26 ± 2.57 cm2). Besides, salt application induces cell expansion and protein synthesis in ice plants, facilitating leaf expansion and a high growth rate (Yang and Yen, 2002). The response of canopy area for the other salt treatments in the juvenile stage was observed in T2 (183.01 ± 2.47 cm2) followed by T1 (174.78 ± 3.41 cm2), T5 (133.11 ± 2.52 cm2), T6 (103.68 ± 2.06 cm2), and CS (66.36 ± 1.42 cm2) as in Fig. 4. There were no appreciable differences between CS and T3 during the early stage of the slow uptake of salt to plant (Figs. 3, 4).
HSV developed images for calculating the canopy area of ice plants in their juvenile growth stage.
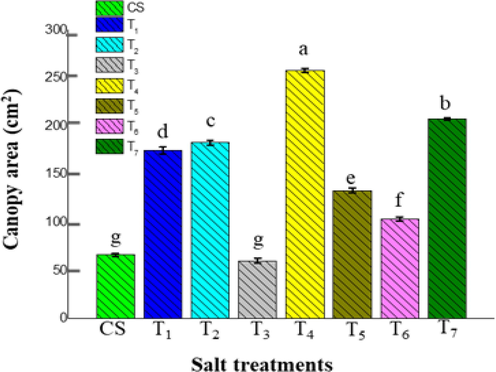
The impact of various salt treatments on the canopy area of juvenile stage ice plants (60-days mature plants). The data indicate the means ± SD (n = 3). Different letters above bars indicate significant differences at p < 0.05.
3.2 Ion content determination
3.2.1 Cation content determination
The results of cation content clearly showed that the ice plant accumulated comparatively much Na+ in T4 (210.72 ± 9.24 mg g−1) and less in T1 (89.58 ± 1.33 mg g−1) was expressed on a dry weight basis (Fig. 5A). There were no significant differences for Na+ storage in ice plant leaves among T5 and T7 treatments and between T1 and T2 salt treatments. The Na+ storage in ice plant leaves response to other salt treatments were T4 (210.72 ± 9.24 mg g−1), T6 (204.08 ± 6.80 mg g−1), T7 (187.34 ± 8.97 mg g−1), T5 (181.06 ± 9.43 mg g−1), T3 (156.91 ± 4.12 mg g−1), CS (104.40 ± 2.89 mg g−1) and T2 (93.69 ± 3.02 mg g−1) in respectively. Furthermore, T4 and T6 exhibited the highest Na+ content due to the salt transport from root cells into leaf mesophyll relying on myo-inositol, and sodium interaction between myo-inositol facilitates the sodium uptake and long-distance transport. Moreover, the synergy between Ca2+ ion accumulation leads to the synthesis of myo-inositol and the uptake of Na+ (Nelson et al., 1999). As a result, sodium is stored in EBCs and plant younger tissues, and pinitol production in the ice plant shoot top is enhanced by salt. A similar tendency was observed in the Ca2+ ion results, as demonstrated in Fig. 5C.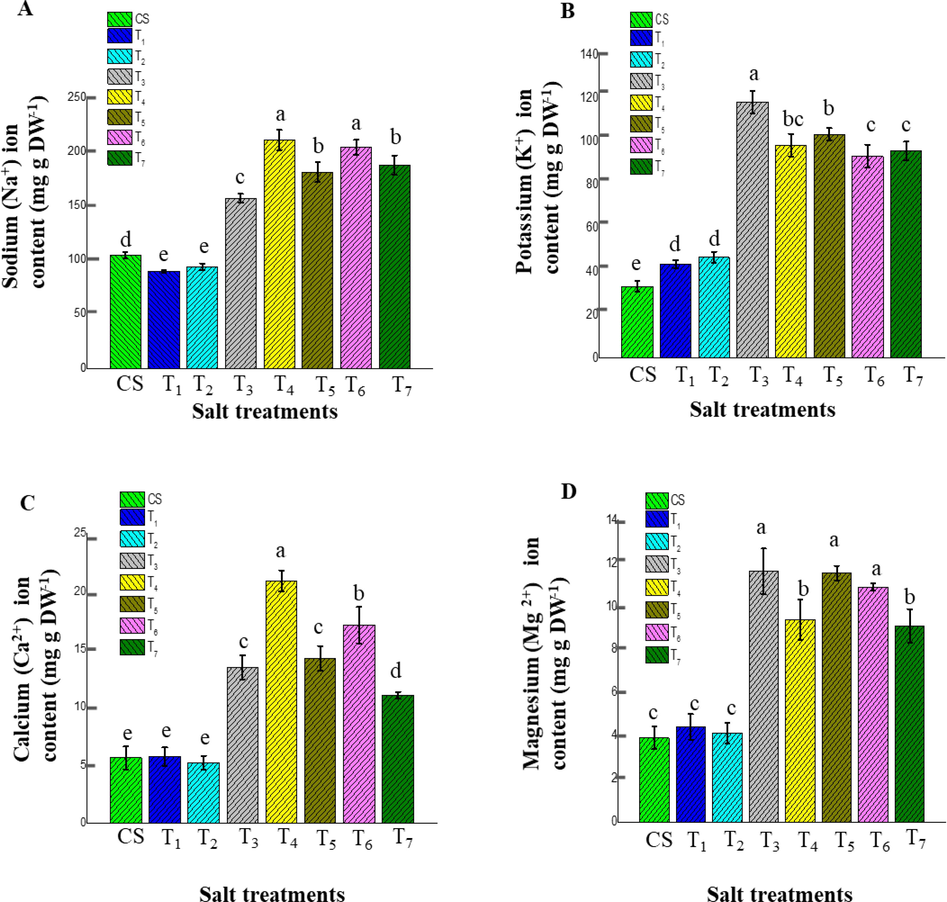
The effect of different salt treatments on cation content of ice plant leaves in the adult stage (120 days mature plant). Data indicate the means ± SD (n = 3). Different letters above bars indicate significant differences at p < 0.05. (A) Na+, (B) K+, (C) Ca2+, (D) Mg2+ indicated the ion content measurement based on dry weight, respectively.
According to the K+ ion results, the highest value was measured in T3 (118.23 ± 5.26 mg g−1), and the lowest value CS (33.04 ± 2.46 mg g−1) represented as a percentage of dry weight basis, respectively. There was no appreciable difference noted among K+ ion accretion among T4 and T5 treatments, T6 and T7 treatments, and T1 and T2.
Based on the K+ ion results the remaining treatment showed the storage such as T5 (103.37 ± 2.75 mg g−1), T4 (98.36 ± 5.13 mg g−1), T7 (95.62 ± 4.35 mg g−1), T6 (93.20 ± 5.36 mg g−1), T2 (46.50 ± 2.52 mg g−1) and T1 (43.40 ± 1.82 mg g−1) respectively. These results have revealed that a lower concentration of CaCl2 influences the accumulation of K+ ions in tissues of leaves and EBCs.
Moreover, increasing NaCl up to 400 mM reduced the K+ ion accumulation of photosynthetically active leaf tissues of ice plants, as illustrated in Fig. 5B. However, considering the Ca2+ ion, the significantly high concentration existed in T4 in dry weight basis (20.90 ± 0.90 mg g−1) and less in T2 (5.19 ± 0.61 mg g−1) respectively (Fig. 5C). No significant difference was observed in the Ca2+ ion accumulation among T3 and T5 treatments, and CS, T1, and T2 treatments. According to the results, Ca2+ ions in other salt treatments were observed T6 (17.08 ± 1.57 mg g−1), T5 (14.21 ± 1.06 mg g−1), T3 (13.44 ± 1.04 mg g−1), T7 (11.01 ± 0.30 mg g−1), T1 (5.72 ± 0.79 mg g−1), CS (5.62 ± 0.98 mg g−1) dry weight basis in consecutively. Thus, results showed an increasing trend in salt treatments and accumulation of Ca2+ ions in leaf tissues in ice plants.
Regarding the Mg2+ ion, the highest accumulation was observed in T3 as a dry weight basis (11.62 ± 1.06 mg g−1) and the lowest in CS (3.90 ± 0.53 mg g−1) orderly (Fig. 5D). Based on the results no significant difference was observed among T3, T5 (11.54 ± 0.33 mg g−1) and T6 (10.89 ± 0.17 mg g−1) treatments and between T4 (9.37 ± 0.93 mg g−1) and T7 (9.09 ± 0.78 mg g−1) treatments and among T1 (4.41 ± 0.60 mg g−1), and T2 (4.13 ± 0.48 mg g−1) treatments in respectively. According to the results mentioned above, Na+ and Ca2+ ions showed a positive tendency for accumulation. In contrast, similar trends were observed K+ and Mg2+ ions deposition in ice plant leaves. However, Na+, K+, and Mg2+ absorption were high in the presence of CaCl2 salt treatment due to the NaCl suppressing the K+ ion concentration and CaCl2 enhancing the leaf Cl− and Ca2+ level, which leads to the uptake of other cations (Trajkova et al., 2006). A high concentration of NaCl worked on the lack of absorption of K+, Mg2+, Ca2+, also Na+ competes with K+ and replaces it in the cell, as reported by Mohamed and Basalah, 2015.
3.2.2 Anion content determination
To assess the possible content of anion sequestration on a dry weight basis, the Cl− stored high in T1 (57.35 ± 0.63 mg g−1) and less in CS (26.06 ± 1.96 mg g−1) consecutively. According to the anion storage in ice plant leaves, no significant differences were observed among T1 and T5 (57.28 ± 3.58 mg g−1) treatments and T4 (35.74 ± 2.73 mg g−1), T6 (38.46 ± 1.63 mg g−1) and T7 (37.23 ± 2.00 mg g−1) treatments. Following the Cl− sequestration appreciable difference was observed T3 (47.45 ± 2.18 mg g−1) and T2 (31.45 ± 1.24 mg g−1) treatments in orderly. According to the individual (NaCl) and combination effect (NaCl and CaCl2) results, the highest concentration, 400 mM, exhibited the highest leaf Cl− accumulation compared to the lower concentration of salt treatment (T2) and control samples, as shown in Fig. 6A.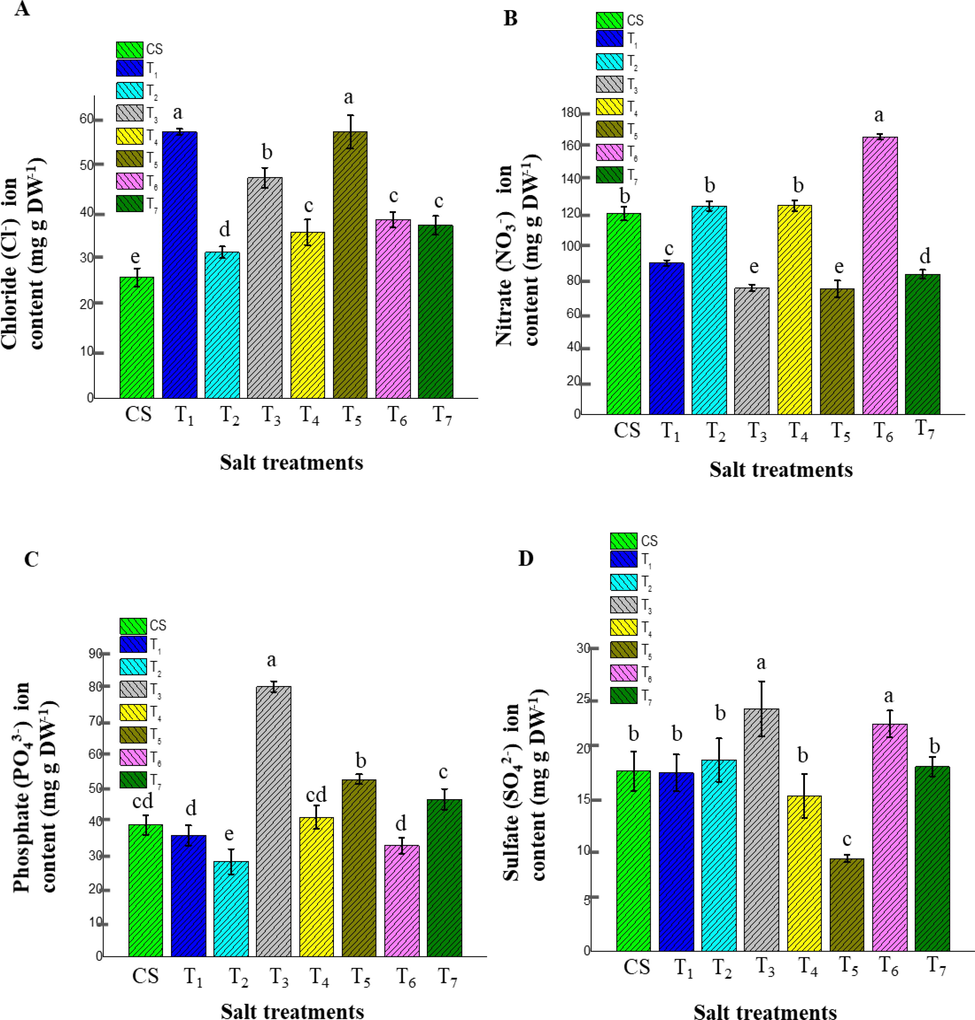
The effect of different salt treatments on the anion content of adult stage ice plant leaves (120 days old plant). The data indicate the means ± SD (n = 3). Different letters above bars indicate significant differences at p < 0.05. (A) Cl−, (B) NO3–, (C) PO43−, (D) SO42− denoted the ion content measurement based on dry weight, respectively.
Correspondingly, NO3– and SO42− are vital for amino acid synthesis and metabolic reactions of halophytic plants. According to the NO3– ion results from the highest value obtained in T6 as dry weight basis (165.88 ± 1.63 mg g−1) and lowest results followed by T1 (90.58 ± 1.77 mg g−1), T7 (83.82 ± 2.65 mg g−1) and T5 (75.08 ± 4.99 mg g−1) respectively (Fig. 6 B). However, no significant difference was shown among CS (120.12 ± 4.23 mg g-1), T2 (124.58 ± 2.90 mg g−1) and T4 (124.76 ± 3.11 mg g−1) treatments, and T3 (75.69 ± 2.01 mg g−1) and T5 (75.08 ± 4.99 mg g−1) treatments in respectively.
Concurrently, a similar pattern was discovered in SO42− ion accumulation. The maximum value for SO42− storage in leaves tissue was observed in T3 (24.08 ± 2.73 mg g−1) and the minimum in T5 (9.23 ± 0.38 mg g−1) on a dry weight basis orderly (Fig. 6 D). Based on the results, there were no significant differences among CS (17.89 ± 1.94 mg g−1), T1 (17.73 ± 1.84 mg g−1), T2 (18.99 ± 2.15 mg g−1), T7 (18.34 ± 0.97 mg g−1) and T4 (15.43 ± 2.20 mg g−1) treatments in respectively.
Changes in NO3– and SO42− concentrations in the leaves of the ice plant confirmed a comparable absorption trend (Fig. 6 B, D). Increased Cl− concentrations competed with NO3– uptake, and accumulation of SO42−. Generally, NO3– and SO42− showed the same ion exchange and sorption pattern described by Stefan et al., 2014. Furthermore and even more importantly, PO43− is vital to plant growth and involved in several plant functions such as photosynthesis, the transformation of sugars, energy transfer, and nutrient movement, as reported by Mullins (2009). Therefore, elucidating the relationship between the energy state and stress tolerance PO43− ion is vital for growth hormone presumably gibberellin production and regulates the abiotic stress in halophytes (Zhu et al., 2012).
Based on the PO43− ion deposition results, the appreciable differences depicted T3 as dry weight basis (80.41 ± 1.57 mg g−1) followed by T5 (52.84 ± 1.47 mg g−1), T7 (46.92 ± 3.06 mg g−1), and T2 (28.37 ± 3.73 mg g−1) respectively (Fig. 6 C). Nonetheless, there was no significant difference obtained for the PO43− ion deposition among in T4 (41.58 ± 3.52 mg g−1), CS (39.24 ± 2.83 mg g−1), T1 (36.17 ± 3.02 mg g−1), T6 (33.10 ± 2.40 mg g−1) in consecutively.
As aforementioned, the results have revealed that a lower concentration of CaCl2 significantly influences the PO43− deposit in ice plant leaves.
3.3 DPPH radical scavenging activity(%)
The DPPH assay was used to assess the scavenging activity of ice plants under various salt stresses, as presented in Fig. 7. The lower value indicates the higher antioxidant activity, and the value of DPPH radical scavenging activity denoted the negative correlation with the antioxidant activity (Agarie et al., 2009). However, a scavenging activity among the salt treatments, no appreciable difference was denoted among T5 (89.38 ± 1.21%), T7 (87.96 ± 2.33%), CS (87.05 ± 5.23%) and T4 (86.35 ± 4.88%) treatments and T3 (45.98 ± 3.96%) and T2 (44.93 ± 3.70%) treatments. There was significant radical scavenging activity was observed among T6 (77.90 ± 2.10%), T1 (52.59 ± 3.11%), T2 treatments respectively.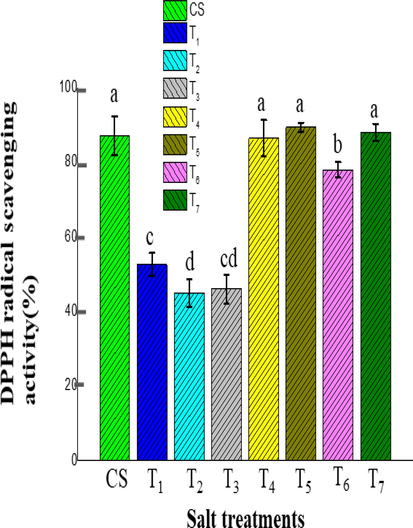
The effect of different salt treatments on DPPH radical scavenging activity(%) of ice plants in the adult stage (120 days old plant). Data indicate the means ± SD (n = 3). Different letters above bars indicate significant differences at p < 0.05.
According to the results, higher amounts of phenolic compounds are markedly promoted in the T2 salt treatment, which has a minimum radical scavenging effect.
Salt stress was successful in promoting pinitol/ononitol accumulation in M. crystallinum halophytic characteristics. It was speculated that the combined salt created a turgor gradient along the axes of the mature ice plant, which resulted in the accelerated growth of new cells. Photosynthesis and photorespiration were associated with the concentration gradient from the root to the mesophyll cell. The methylation of myo-inositol to the intermediate ononitol produces pinitol, which appears to be epimerized to pinitol. These phenolic chemicals are primarily responsible for the colour, flavour, and aroma of plants, and they are also high in antioxidant activity (Hichri et al., 2011). A similar observation was obtained from the present study from T1 treatment as low radical scavenging activity and high antioxidant activity by decomposition of phenolic compounds.
Antioxidant activity plays a fascinating role in removing reactive oxygen species that are harmful to the human body. According to Kim et al., 2021, it helps to avoid chronic diseases and ageing. As a result, several efforts to synthesize particular phytochemicals with antioxidant activity have been conducted. The bioactive concentrations in ice plants in T1, T2, and T3 treatments were high due to the mild concentration of CaCl2, and the high NaCl concentration affects the biosynthetic pathway through the accumulation of phenolic compounds. Based on the results, the combined impact of NaCl and CaCl2 and a higher concentration of CaCl2 were not affected by the increased antioxidant material as fewer secondary metabolites accumulated (Agarie et al., 2009).
Specifically, Agarie et al., 2009 revealed that the radical scavenging activity determined by the DPPH assay and the antioxidant activity of ice plants increased twofold by treatment with 400 mM NaCl, which was twice that of the lettuce plants. This study obtained a similar tendency of high antioxidant activity from the high concentration of NaCl and low CaCl2 salt treatments such as T1, T2, and T3. These results indicate the high potential of ice plants as polyol-rich, highly functional foods.
4 Conclusion
The present study revealed that T4 (400 mM CaCl2) salt treatment considerably improved ice plant morphological parameters such as number of leaves, number of lateral stems, fresh and dry weight of shoot and root, and leaf canopy area. It has a high potential for enhancing the biomass of the ice plant's accumulation of functional materials. Consequentially, T4 salt treatment exhibited a similar trend for the Na+ and Ca2+ deposition in ice plant leaves. Moreover, K+ and Mg2+ accumulation were high in T3 (200 mM CaCl2) salt treatment, with a mild concentration of CaCl2. A negative trend was denoted by cation accumulation in ice plant leaves regarding anion deposition.
Furthermore, NaCl, including salt treatments such as T1 (400 mM NaCl) and T5 (combination of 100 mM NaCl and 300 mM CaCl2), significantly influenced the Cl− deposition, and T6 (mixture of 200 mM NaCl and 200 mM CaCl2) affected NO3– accumulation. Conversely, a mild concentration of CaCl2 as T3 salt treatment positively affected the PO43− and SO42− high accumulation in the ice plant leaves. Simultaneously, DPPH radical scavenging activity(%) results negatively correlated with the antioxidant activity, and a high antioxidant value was observed in T2 (100 mM CaCl2), which has a higher potential of inducing polyol synthesis. In conclusion, T4 implied better performance of ice plants in terms of biomass and induced cation (Na+, Ca2+) accumulation. In addition, further studies seem to be required to clarify the effect of different types of salt treatments on the growth and secondary metabolism of ice plants.
Acknowledgement
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (717001-7).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Growth and development of Mesembryanthemum crystallinum (Aizoaceae) New Phytol.. 1998;138(2):171-190.
- [Google Scholar]
- Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J. Exp. Bot.. 2007;58(8):1957-1967.
- [Google Scholar]
- Potential of the common ice plant, Mesembryanthemum crystallinum as a new high-functional food as evaluated by polyol accumulation. Plant Prod. Sci.. 2009;12(1):37-46.
- [Google Scholar]
- The ice plant cometh: lessons in abiotic stress tolerance. J. Plant Growth Regul.. 2000;19(3):334-346.
- [Google Scholar]
- Plant morphological characteristics as a tool in monitoring response to silvicultural activities. In: Communicating the Role of Silviculture in Managing the National Forests. 1997. p. :37.
- [Google Scholar]
- Fast and accurate method for leaf area measurement. Internat. J. Comput. Appl.. 2012;49(9):22-25.
- [Google Scholar]
- Developmental control of Crassulacean acid metabolism inducibility by salt stress in the common ice plant. Plant Physiol.. 1990;94(3):1137-1142.
- [Google Scholar]
- Physiological changes in Mesembryanthemum crystallinum during the C3 to CAM transition induced by salt stress. Front. Plant Sci.. 2020;11:283.
- [Google Scholar]
- Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol.. 2000;51(1):463-499.
- [Google Scholar]
- The water-culture method for growing plants without soil. In: Circular (2nd ed). California Agricultural Experiment Station; 1950. p. :347.
- [Google Scholar]
- Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot.. 2011;62(8):2465-2483.
- [Google Scholar]
- Pinitol from soybeans reduces postprandial blood glucose in patients with type 2 diabetes mellitus. J. Med. Food. 2006;9(2):182-186.
- [Google Scholar]
- The effects of NaCl and CaCl2 on photosynthesis and growth of alfalfa plants. Photosynthetica. 1998;35(3):461-466.
- [Google Scholar]
- Ice plant growth and phytochemical concentrations are affected by light quality and intensity of monochromatic light-emitting diodes. Hortic. Environ. Biotechnol.. 2018;59(4):529-536.
- [Google Scholar]
- Growth and phytochemicals of ice plant (Mesembryanthemum crystallinum L.) as affected by various combined ratios of red and blue LEDs in a closed-type plant production system. J. Appl. Res. Med. Aromat. Plants. 2021;20:100267
- [Google Scholar]
- Biological activities of Mesembryanthemum crystallinum (ice plant) extract. J. Life Sci.. 2015;25(6):638-645.
- [Google Scholar]
- Lee, J. W., Son, K. H., Lee, J. H., Kim, Y. J., & Oh, M. M. (2019). Growth and biochemical responses of ice plant irradiated by various visible light spectra in plant factories.
- Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evolut.. 2018;6
- [Google Scholar]
- Advances in the determination of inorganic anions by ion chromatography. J. Chromatogr. A. 2000;881(1–2):607-627.
- [Google Scholar]
- Prediction of Strawberry Leaf Color Using RGB Mean Values Based on Soil Physicochemical Parameters Using Machine Learning Models. Agronomy. 2022;12(5):981.
- [Google Scholar]
- Influence of different growing media on the growth and development of strawberry plants. Heliyon 2021:e07170.
- [Google Scholar]
- Measuring Soil Salinity – plant stress. (n.d.). plant stress. https://plantstress.com/measuring-soil-salinity.
- The active role of calcium chloride on growth and photosynthetic pigments of cowpea“ Vigna unguiculata L. (Walp)” under salinity stress conditions. Am.-Euras. J. Agric. & Environ. Sci. 2015;15(10):2011-2020.
- [Google Scholar]
- Myo-inositol-dependent sodium uptake in ice plant. Plant Physiol.. 1999;119(1):165-172.
- [Google Scholar]
- Mullins, G. L. (2009). Phosphorus, agriculture & the environment.
- Shalaby, E. A., & Shanab, S. M. (2013). Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis.
- Influence of sulfate and nitrate uptake from aqueous solutions on surface exchange in Purolite A-520E resin. Comptes Rendus Chimie. 2014;17(7–8):738-745.
- [Google Scholar]
- Definition and meaning of the plant factory. In: Plant factory. Seoul: World Science Publishment; 2008. p. :8-13.
- [Google Scholar]
- Comparative effects of NaCl and CaCl2 salinity on cucumber grown in a closed hydroponic system. HortScience. 2006;41(2):437-441.
- [Google Scholar]
- Growth, development, and chemical constituents of edible ice plant (Mesembryanthemum crystallinum L.) produced under combinations of light-emitting diode lights. HortScience. 2018;53(6):865-874.
- [Google Scholar]
- Inductively coupled plasma mass spectrometry: introduction to analytical aspects. Clin. Biochem. Rev.. 2019;40(3):115-133.
- [Google Scholar]
- The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PloS one. 2013;8(7)
- [Google Scholar]
- Early salt stress effects on the changes in chemical composition in leaves of ice plant and Arabidopsis. A Fourier transform infrared spectroscopy study. Plant Physiol.. 2002;130(2):1032-1042.
- [Google Scholar]
- Effect of foliar application of CaCl2 on lettuce growth and calcium concentrations with organic and conventional fertilization. HortScience. 2018;53(6):891-894.
- [Google Scholar]
- The mitochondrial phosphate transporters modulate plant responses to salt stress via affecting ATP and gibberellin metabolism in Arabidopsis thaliana. PLoS ONE. 2012;7(8):e43530.
- [Google Scholar]







