Translate this page into:
Assessment of antioxidant, anticancer, and antibacterial activities of the rhizome of ginger (Zingiber officinale)
⁎Corresponding authors. aalfuraydi@ksu.edu.sa (Akram A. Alfuraydi), majhdi@ksu.edu.sa (Fahad N. Almajhdi) vrg_ksu@yahoo.com (Fahad N. Almajhdi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Zingiber officinale rhizome is rich in phenolic and flavonoids contributing antioxidant and anticancer activity.
Methods
This study was designed to assess the in vitro antioxidant, anticancer, and antibacterial activities of Z. officinale rhizome. Ethanol extract of Z. officinale rhizome was tested for total phenolic and flavonoid content. The antioxidant activity was analyzed by DPPH scavenging and ABTS assay methods. The cytotoxic potential of ethanolic rhizome extract was tested against MCF-7 cell lines and anti-apoptotic genes were determined.
Results
Ethanol extract of Z. officinale rhizome exhibited phenols (266.57 ± 4.09 mg GAE/g dry weight of the dry extract) and flavonoid (114.04 ± 2.46 mg QE/g mg dry weight of the dry extract). The ethanol extract significantly affected the generation of free radicals and the IC50 values were 107.19 ± 1.7 μg/mL (DPPH method) and 121.94 ± 0.32 μg/mL (ABTS method) (p < 0.05). The rhizome extract showed statistically significant cytotoxic activity compared to the control (p < 0.05) with an IC50 value of 92.49 ± 3.14 μg/mL. It also showed bactericidal activity against Gram-positive and Gram-negative bacteria. The MIC values ranged from 3.12 ± 0.44 μg/mL to 25 ± 5.33 μg/mL. The most susceptible strain was S. epidermidis (MTCC 12228) the MIC value was 3.12 ± 0.44 μg/mL and the MBC value was 6.25 ± 3.23 μg/mL. The rhizome extract increased the mRNA levels of caspase-9, 8, and −3, Bax mRNA and subsequently reduced anti-apoptotic genes (Bcl-xL and Bcl-2) than control (P < 0.05). The MCF-7 cells, treated with rhizome extract showed upregulation of p21 and p53 mRNA than untreated control cells (p < 0.05).
Conclusion
The present finding revealed the antioxidant, anticancer, and antibacterial properties of Z. officinale rhizome.
Keywords
Alternate medicine
Rhizome
Bactericidal
Antioxidant
Apoptosis
Down-regulation
1 Introduction
The medicinal plant is a source of various natural compounds, including phenols, which may effectively scavenge free radicals and protect the cells against oxidative damage. Phenolic compounds such as phenolic acids, flavonoids, stilbenes, and lignans have antioxidant properties (Sun and Shahrajabian, 2023). The available phenolic compounds from medicinal plants react with the oxidation products derived from fatty acids prevto FVent oxidative stress and improve the quality of food and food products. Phenolic compounds maintain sensory properties and protect organoleptic compounds of food and food products (Ziarno et al., 2021).
Ethnomedicines are alternative medicine, cost-effective, less side effects on the cellular system, and are easy to manage. Antibacterial compounds from medicinal plants are alternatives to costly synthetic drugs to treat various infectious pathogens, including drug-resistant organisms (Gang et al., 2023). In humans, oxidative stress is one of the complicated pathogenic factors in the etiology of various chronic and degenerative diseases including, atherosclerosis, diabetes, renal disorders, parkinsons disease, cancer, inflammatory, cardiovascular, neurodegenerative, and autoimmune diseases. Medicinal plant extracts exhibit therapeutic properties and are the major resource of various secondary metabolites. Z officinale is one of the major medicinal plants consumed as a spice or medicine and the rhizome is generally used (Dhanik et al., 2017). The rhizome has antioxidant properties and prevents the formation of free radicals in the cellular system in humans. It has antibacterial, antifungal, antiprotozoal, and insecticidal activities. The phytochemicals extracted from the rhizome of Z. officinale are safe (Hosseinzadeh et al., 2017).
Cancer disease is caused by various factors, including, metabolic, environmental, physical, genetic, and chemical factors. An antioxidant diet is one of the major factors to control the development of cancers and prevent cancer spread. The available polyphenols from the medicinal plants exhibited anticancer activities. Quercetin extracted from medicinal plants is involved in the inhibition of cancer cell proliferation and affected signals (Bahrami and Tafrihi, 2023). The polyphenols extracted from medicinal plants inhibited the human cancer cell lines, including, colon (HT-29), oral (KB and CAL-27), breast (MCF-7), and prostate (LNCaP and DU-145) (Zhang et al., 2008, Thirthalli et al., 2016). It has been recently reported that the polyphenols (epicatechin, catechin, quercetin, resveratrol, and epigallocatechin) exhibited anticancer activities (Weisburg et al., 2004). The ginger extract contains phytochemical compounds, such as 6-shogaol and 6-gingerol and these compounds show anticancer properties. Recently, ginger has been recommended to treat rheumatoid arthritis and osteoarthritis (Lindler et al., 2020). The phytochemical components from ginger inhibited cancer cell proliferation and induced cancer cell death in a variety of cancer cell lines in vitro (Anusha et al., 2023). Moreover, the cytotoxic effects of ginger rhizome and its chemical components against breast cancer cell lines (MCF-7) were not completely elucidated. In this study, we used the rhizome of Z. officinale to assess the in vitro antioxidant, anticancer, and antibacterial activities.
2 Methodology
2.1 Rhizome and extraction
Dried Z. officinale rhizomes were collected from the market in Riyadh, Saudi Arabia market in Riyadh, Saudi Arabia, in March 2023. It was dried and powdered. 10 g dried powder was weighed and 100 mL ethanol was added. It was sonicated for 30 min and the extract was filtered. The extracted sample was evaporated at room temperature (32 ± 1 °C) and maintained at 80 °C until constant weight was achieved. The dried extract was further suspended in 0.1 % dimethylsulfoxide (DMSO) or ethanol at 1 mg/mL concentration and stored in amber-colored vials and stored at 4 °C until further use.
2.2 Analysis of total phenolic compounds
The total phenol level of the rhizome was tested using the Folin-Ciocalteu method as described previously by (Wolfe and Liu, 2003). Briefly, 0.05 mL extract was mixed with 0.25 mL Folin-Ciocalteau reagent (0.5 N) and incubated for 10 min. Then, 2.5 mL of 0.5 M sodium carbonate solution was added and incubated for 30 min and vortexed. The optical density of the sample was read at 765 nm using VR-2000.0 spectrophotometer (JP Selecta, Barcelona, Spain) against the reagent blank. Total phenolic content was estimated using gallic acid standard prepared at various concentrations (10–100 mg/mL). The result was expressed gallic acid equivalents (GAE)/g extract.
2.3 Analysis of total flavonoid content
The total flavonoid content of the sample was estimated from the sample as described earlier by (Ordonez et al., 2006). Briefly, 0.1 mL extract was incubated with 2.0 mL of 2 % AlCl3 solution and incubated for 30 min. After 30 min, the absorbance of the sample was tested at 420 nm using an ELX-808 microplate reader (BioTek Laboratories, LL, Shoreline, W.A, USA). Quercetin was prepared at various concentrations (10–100 mg/mL) and used for the preparation of a standard curve. The flavonoid content was estimated using a standard quercetin curve.
2.4 Antioxidant assay
1,1-diphenyl-2-picryl hydrazyl (DPPH) scavenging assay. The DPPH free radical scavenging activity assay was performed. Briefly, the rhizome extract was prepared at four different concentrations (100–900 μg/mL. Half mL extract was mixed with 2 mL DPPH solution (0.08 mM) and incubated in the dark for 10 min. The optical density of the sample was read at 517 nm with a VR-2000 spectrophotometer (JP Selecta, Barcelona, Spain). against the reagent blank. Butylated hydroxytoluene (BHT) and ascorbic acid were used as the standards. The result was calculated the IC50 values were obtained and the % DPPH scavenging activity was determined.
2.5 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay
The ABTS free radical scavenging assay was performed as described previously (Yu et al., 2013). ABTS·+ cation radical was generated by the reaction between 2.45 mM K2S2O8 solution and 7 mM aqueous ABTS (1:1) stored for 12 h before use in the dark. The working solution was prepared by diluting the ABTS solution in methanol until reached the OD value of 0.7 at 734 nm. 5 μL plant extract was added with 2.995 μL ABTS solution and incubated in the dark for 30 min. The absorbance of read at 734 nm using a VR-2000 spectrophotometer (JP Selecta, Barcelona, Spain) against the blank and the scavenging activity (%) was calculated. Trolex was used as the positive standard.
2.6 Anticancer activity analysis
The breast cancer cell lines (MCF-7) were cultured in a microtiter plate using a CO2 incubator with Dulbecco's Modified Eagle Medium (DMEM) (Qiagen, USA). Briefly, the cell culture was performed with DMEM medium containing Pyruvate, L-glutamine, and Glucose (4.5 g/L), 10 % Fetal Bovine Serum with antibiotics (streptomycin/penicillin; 100 U/mL). The cell lines were subcultured in 25 mL vials and maintained in a CO2 and the culture medium was changed every 2–3 days.
2.7 Cytotoxicity analysis
The cytotoxicity analysis was performed as described previously with minor modifications. MCF-7 cell lines were cultured in 96-well microtiter plates and exponential phase was determined. The cells were collected using 0.25 % Trypsin-EDTA and loaded into a microtiter plate at 1 × 104 cells/ well and a fresh tissue culture medium was added. The wells were treated with various concentrations of rhizome extract (25–400 μg/mL) and incubated at 37 °C for 24 h in a humidified chamber containing 5 % CO2. After 24 h, 10 µL MTT reagent (20 mg/20 mL) was added and incubated for 30 min. To the wells, 100 μL DMSO was added and incubated for 10 min. The absorbance of the sample was read using a microtiter plate reader at 590 nm using an ELX-808 microplate reader (BioTek Laboratories, LL, Shoreline, W.A, USA) against positive and negative controls. The viability of the cells (%) was determined and a triplicate experiment was performed. The mean value was considered for data processing and the half maximal inhibitory concentration (IC50) value was determined.
2.8 Gene expression analysis
Analysis of apoptosis coding genes was performed. The MCF-7 cells were grown in a microtiter plate and the culture medium was transferred every two days. It was incubated with various concentrations of rhizome extract. After 24 h, the cells were digested with trypsin (0.02 %) and centrifuged at 8000 × g for 10 min at 4 °C. The clear supernatant was removed and the pellet was reconstituted with PCR buffer and used for rRT-PCR analysis. To determine the apoptosis coding genes, RNA extraction was performed using a RNeasy as described by the manufacturer’s instructions (QIAGEN, Germany). The extracted RNA was used for qPCR analysis. The master mix (25 µL) was prepared using Go TaqQpcrmastermix and F and R primers and the purified RNA was used as the template. RT2 PCR Array was performed using a real-time instrument (Applied Biosystem). A Fast-real-time PCR was used for the determination of apoptosis coding genes in the rRT-PCR machine (Applied Biosystems). The results were obtained using the 2−ΔΔCq method (Schmittgen and Livak, 2008), and delta Cq (ΔCq) values obtained for the different genes were normalized based on the value of GAPDH amplified from the same genes, and the fold-change in expression was calculated as referenced to the expression of the untreated control cells. The list of primers is listed in Table 1.
Gene name
Primers sequence
References
Caspase-3
F: 5′-GCTGGATGCCGTCTAGAGTC-3′
(Alotaibi et al., 2018)
R: 5′-ATGTGTGGATGATGCTGCCA-3′
Caspase-8
F: 5′-AGAAGAGGGTCATCCTGGGAGA-3′
(Honarpisheh et al., 2016)
R: 5′- TCAGGACTTCCTTCAAGGCTGC-3′
Caspase-9
F: 5′- ATTGCACAGCACGTTCACAC-3′
(Alotaibi et al., 2018)
R: 5′-TATCCCATCCCAGGAAGGCA-3′
Bax
F: 5′-GAGCTAGGGTCAGAGGGTCA-3′
(Alotaibi et al., 2018)
R: 5′-CCCCGATTCATCTACCCTGC-3′
Bcl-2
F: 5′-ACCTACCCAGCCTCCGTTAT-3′
(Alotaibi et al., 2018)
R: 5′-GAACTGGGGGAGGATTGTGG-3′
Bcl-XL
F: 5′-CAGAGCTTTGAACAGGTAG-3′
(Buskaran et al., 2021)
R: 5′-GCTCTCGGGTGCTGTATTG-3′
p53
F: 5′-GCTCTGACTGTACCACCATCC-3′
(Jiang et al., 2017)
F: 5′-CTCTCGGAACATCTCGAAGCG-3′
p21
F: 5′- CTCAGAGGAGGCGCCATG-3′
(Jiang et al., 2017)
R: 5′- GGGCGGATTAGGGCTTCC-3′
GAPDH
F: 5′- CGGAGTCAACGGATTTGGTC −3′
(Jiang et al., 2017)
R: 5′- AGCCTTCTCCATGGTCGTGA −3′
2.9 Antibacterial activity
Gram-positive and Gram-negative bacterial strains were used for the determination of antimicrobial activities. The slected Gram-positive bacteria were: Bacillus subtilis (MTCC-10400), Staphylococcus aureus (MTCC-29213) and Staphylococcus epidermidis (MTCC-12228). A total of three Gram-negative bacteria (Klebsiella pneumonia (MTCC-13883), Escherichia coli (ATCC-25922), and Pseudomonas aeruginosa (MTCC-27853) were used. All these bacterial strains were obtained from the King Khalid University Hospital, KSA.
2.9.1 Disc diffusion method
The antibacterial activity assay was performed using the disc diffusion method as indicated as previously reported by (Salem et al., 2018). Briefly, 20 µg of rhizome extract was placed on a Whatman No. 1 filter paper (6 mm diameter). The antimicrobial activity study was carried out using Gram-positive and Gram-negative strains using Mueller Hinton Agar MHA) medium. About 0.1 mL bacterial inoculums were spread on MHA plates using an “L” rod. The sample and standard disc were placed on MHA plates and incubated for 24 h. After 24 h incubation, the zone of inhibition was analyzed. Chloramphenicol (25 μg) was used as the positive control.
2.9.2 Analysis of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The rhizome extract was used for the determination of MIC and MBC against the selected pathogenic bacterial strains by broth dilution method as described earlier by (Basri and Sandra, 2016) with minor modifications. To the microtiter plates, 300 µL nutrient broth medium was supplemented. The antimicrobial plant extract (1.56 to 800 μg/mL) was diluted with a nutrient broth medium. Then, 20 µL inoculums (1 × 105 CFU/mL) were added to all wells. The microtiter plates were incubated for 18 h at 37 °C and the bacterial growth was visually examined. Chloramphenicol (20 μg/mL) was used as the positive control and 0.1 % DMSO was considered as the negative control. The culture was spread on nutrient agar plates and MBC values were determined (Aljeldah et al., 2022).
2.10 Statistical analysis
Analysis of variance (ANOVA) was performed and a significant level was observed. All experiments were performed in triplicates and the resulting data were then expressed as mean ± SD, and a comprehensive statistical analysis was carried out using One-way ANOVA. The “p” value >0.05 was considered statistically significant.
3 Results
3.1 Phenolic and flavonoid content of rhizome
In this study, the total phenolic content of the ethanol extract of dried ginger rhizome was determined spectrophotometrically and was 266.57 ± 4.09 mg GAE/g dry weight of the dry extract. Total flavonoid content was estimated and was found to be 114.04 ± 2.46 mg QE/g dry weight of the dry extract.
3.2 Antioxidant activity
The antioxidant capacity of the phytochemicals extracted from the rhizome was analyzed using two different methods (DPPH and ABTS radical scavenging activity). DPPH and ABTS radical scavenging activity results are described in Fig. 1. The results were compared with standard antioxidants (vitamin C). At increased extract concentrations, DPPH and ABTS radical activities improved. The IC50 value was 107.19 ± 1.7 in the DPPH assay, whereas the ABTS assay showed 121.94 ± 0.32 μg/mL.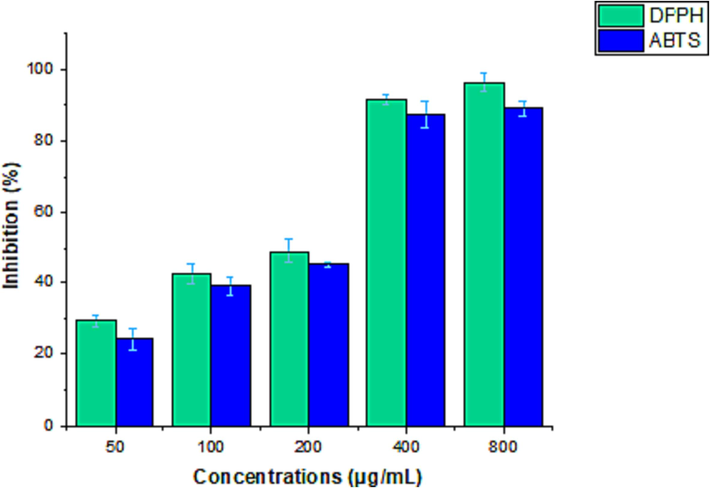
Antioxidant activity (DPPH reducing power and ABTS scavenging activity) of rhizome extract at various concentrations. values represent the mean SD of three independent experiments.
3.3 Cytotoxic activity
The cytotoxicity activity of the rhizome extract was analyzed and the extract exhibited dose-dependent activity on MCF-7 cells (Fig. 2). The rhizome extract showed more potent cytotoxic activity than the control and was statistically significant (p < 0.05). After 100 μg/mL extract concentration, cytotoxic activity was less. However, at increasing concentrations showed improved cytotoxic activity. The IC50 value was 92.49 ± 3.14 μg/mL.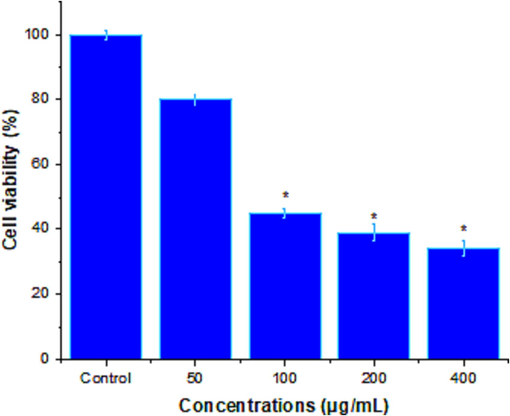
Cytotoxic activity of rhizome extract of Z. officinale at various concentrations (0–800 μg/mL) after 24 h. The results were the mean ± SD of three different experiments. At higher concentrations, rhizome extract significantly decreased (*) = A significant difference from the untreated control cells (* = p < 0.05) is indicated.
3.4 Analysis of rhizome extract on MCF-7-induced apoptosis signaling
The effect of rhizome extract on MCF-7-induced apoptosis signaling was evaluated using RT-PCR after 24 h treatment. The rhizome extract increased the mRNA levels of caspase-9, caspase-8, and caspase-3 (Fig. 3A). The result was compared with the untreated control. As described in (Fig. 3B), rhizome extract treated with MCF-7 after 24 h showed an increased level of Bax mRNA and subsequently reduced anti-apoptotic genes (Bcl-xL and Bcl-2) than control (p < 0.05). The MCF-7 cells, treated with rhizome extract showed upregulation of p21 and p53 mRNA than untreated control cells (p < 0.05) (Fig. 3C).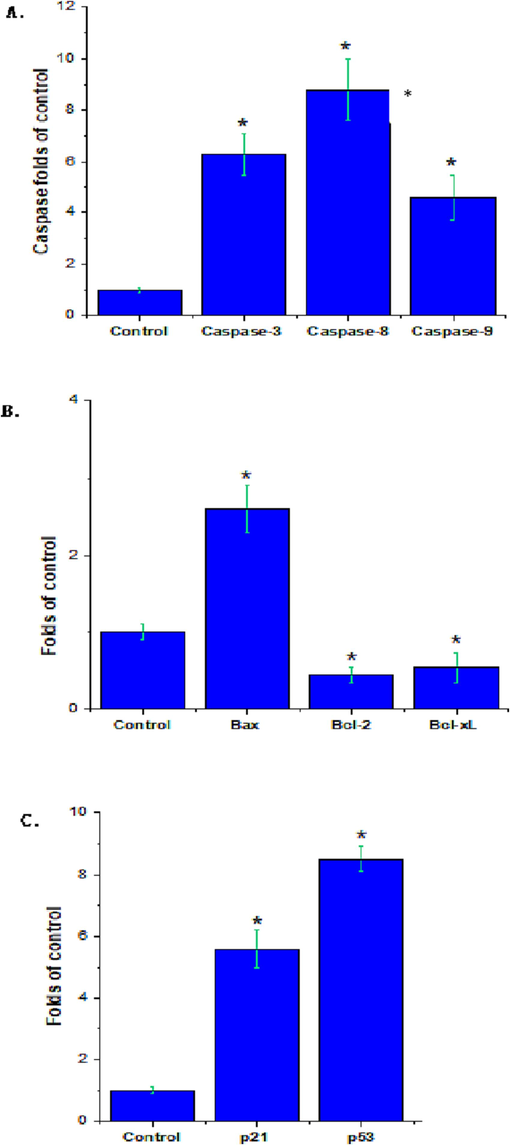
Effect of Z. officinale rhizome extract on A549 cells and analysis of pro- and anti-apoptosis marker genes. (A) caspase-3, 8 and 9 genes; (B) Bax, Bcl-2 and Bcl-Xl genes; (C) p53 and p21 genes. Expression of genes was a statistically significant difference from the untreated control cells (* = p < 0.05) indicated.
3.5 Antibacterial activity of Z. Officinale rhizome extract
The rhizome extract was used to test antibacterial activity against six selected bacterial pathogens. Disc diffusion analysis revealed broad spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria. S. epidermidis was highly susceptible to rhizome extract, followed by B. subtilis, and the zone of inhibition (Fig. 4). The MIC and MBC value was determined against selected six bacterial pathogens (Gram-positive and Gram-negative) strains and the result was depicted in (Table 2). The MIC values ranged from 3.12 ± 0.44 μg/mL to 25 ± 5.33 μg/mL. The most susceptible strain was S. epidermidis (MTCC 12228) the MIC value was 3.12 ± 0.44 μg/mL and the MBC value was 6.25 ± 3.23 μg/mL. Moreover, bactericidal activity was minimal against K. pneumoniae (MTCC 13883) with a MIC value was 25 ± 3.44 μg/mL and a MBC value of were100 ± 4.24 μg/mL.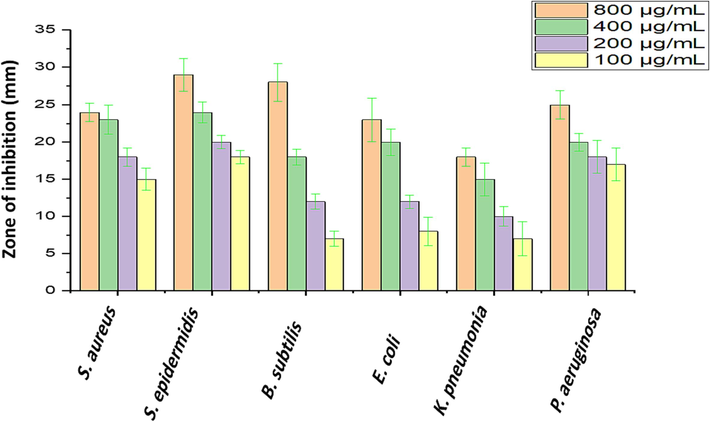
Antibacterial activity of rhizome extract against Gram-positive and Gram-negative bacteria.
Bacteria/Dilution
MIC (μg/mL)
MBC (μg/mL)
S. aureus (MTCC 29213)
6.25 ± 1.27
12.5 ± 2.12
S. epidermidis (MTCC 12228)
3.12 ± 0.44
6.25 ± 3.23
B. subtilis (MTCC 10400)
6.25 ± 1.33
12.5 ± 1.91
E. coli (ATCC 25922)
25 ± 5.33
50 ± 0.97
K. pneumoniae (MTCC 13883)
25 ± 3.44
100 ± 4.24
P. aeruginosa (MTCC 27853)
12.5 ± 2.14
25 ± 3.43
4 Discussion
Medicinal plants produce various secondary metabolites with novel biological properties and are used to treat various diseases and disorders (Zandavar and Babazad, 2023). The medicinal value of plants varies based on sources, season, and plant parts. In our study, the phytochemical compound of the rhizome of Z. officinale was analyzed. The ethanol extract showed the presence of phenols and flavonoids (266.57 ± 4.09 mg GAE/g dry weight of the dry extract and was found to be 114.04 ± 2.46 mg QE/dry weight of the dry extract respectively). Moreover, phytochemical compounds such as anthraquinone, terpenoids, glycoside, saponins, alkaloids, and tannins were reported previously and these compounds exhibited antioxidant activity. The amount and the number of phytochemicals in the extract varied based on the type of solvent used for extraction and the selected extraction methods. Flavonoids and saponin are commonly presented in the aqueous extract, however, anthraquinones, terpenoids, phlobatanins, alkaloids, steroids, glycosides, and tannins are uncommon in Z. officinale (Sulaiman et al., 2014).
An early study stated that phytochemicals varied based on the organ of the plant, solvent, and extraction method selected in the extraction steps. Z. officinale is used to treat oxidative stress-mediated diseases. It involved in inhibition of DPPH free radicals and the conversion of Fe3+ to Fe2+ and this process was dose-dependent. At increased concentrations of Z. officinale rhizome extract, maximum activity was observed (Shittu and Abubakar, 2014). The IC50 value observed in our study was similar to previous reports from Iraq Z. officinale (Aziz et al., 2015). In our study, the presence of significant levels of phenols and flavonoids in the ethanolic extract could be responsible for free radical scavenging activity.
The extract exhibited cytotoxicity against MCF-7, HT116, and HT29 cell lines. The extract was associated with the presence of 6-gingerol and 6-shogaol and these compounds have cytotoxic effects (Ali et al., 2022). The ginger rhizome extract exhibited cytotoxic activity against HepG2 cell lines and the IC50 values were < 50 μg/mL (Shalaby et al., 2023). In our study, ginger rhizome extracts induced apoptosis in MCF-7 cancer cell lines. The rhizome extracts arrested cell cycles and triggered apoptosis. Ginger extract affected cell cycles in HT 29 and HCT 116 colon cancer cell lines (Abdullah et al., 2010), induced apoptosis in endometrial cancer cells (Liu et al., 2012) and ovarian cancer cell line (Pashaei-Asl et al., 2017).
Cancer cells can avoid apoptosis; thus, inducing apoptosis is an important technique for cancer therapy. A significant increase in caspase-3, −8, and −9 mRNA levels was observed in Z. officinale rhizome extract treated MCF-7 cells compared to untreated control cells (p < 0.05). Apoptosis is a type of genetically controlled cell death that results in the development of particular morphological features such as membrane blebbing and the formation of apoptosis bodies. Intrinsic (mitochondrial-mediated), extrinsic (death receptor-mediated), and endoplasmic reticulum stress-dependent (ERS) signaling transduction are the three primary pathways that lead to apoptosis. Caspase-8 is a key extrinsic pathway mediator; when activated, it causes apoptosis by activating the executioner caspases −3, −6, and −7. Caspase-9 is an important upstream mediator in the intrinsic route that activates caspases −3 and −7 to cause apoptosis. Through interactions with caspase-8 and −9, Caspase-3 was found to exhibit both exogenous and endogenous apoptotic characteristics (Jiang et al., 2020). Furthermore, our qPCR data show that A in Z. officinale rhizome extract significantly reduces the mRNA levels of anti-apoptotic genes Bcl-2 and Bcl-xL while increasing the levels of apoptosis-promoting genes (Bax, p53, and p21). Bcl2, an anti-apoptotic gene, fights apoptosis by preserving the integrity of the mitochondrial membrane. The Bax/Bcl2 ratio appears to have an effect on apoptotic susceptibility. Activated p53 triggers cell cycle arrest via modifying the activity of Bcl-2 family members, allowing for DNA repair and/or apoptosis (Pal et al., 2019).
The antibacterial activity of an ethanol extract of Z. officinale rhizome showed the greatest activity against Gram-positive bacteria such as S. epidermidis and B. subtilis and the least activity against K. pneumoniae, indicating that Gram-negative bacteria are more sensitive to Z. officinale than Gram-negative bacteria. These findings are consistent with an earlier study that observed Z. officinale essential oils to have moderate activity against Gram-positive bacteria, with MIC values ranging from 0.16 to 0.63 mg/mL, indicating that Gram-negative bacteria are more resistant to Z. officinale essential oils than Gram-positive bacteria (Teles et al., 2019). These results are expected due to the structure of the Gram-negative cell wall (Singh et al., 2008). The zone of inhibition against the bacteria pathogens in this study revealed better antibacterial activity than previous reports (Sulaiman et al., 2014). It has been previously reported that most of the bioactive compounds were not soluble in water and organic solvent with low polarity is recommended to extract various bioactive compounds to improve the compound yield (Afolayan et al., 2017). The methanol or ethanol extract exhibited improved antimicrobial activities than the aqueous extract which exhibited few antimicrobial compounds (Aliero et al., 2006, Ashafa et al., 2008). The phytochemicals extracted from the rhizome of Z. montanum exhibited antibacterial activity against K. pneumoniae, E. coli, Shigella flexneri, Salmonella typhi, and S. paratyphi (Amatayakul et al., 1979). The phytochemicals extracted from Z. montanum showed antibacterial activity against various bacterial strains including, E. coli, Bacillus subtilis, and S. typhi (Pithayanukul et al., 2007). The present findings revealed that the tested Z. officinale rhizome extract affected bacterial growth and the bactericidal activity varied widely.
5 Conclusion
Medicinal plants contain valuable phytochemicals that are applied in various research areas, especially because of their phenols and flavonoids they showed various pharmacological effects. Phenolic compounds and flavonoids were determined in our study, which are involved in antimicrobial effects. The rhizome of Z. officinale exhibited antibacterial activity against both Gram-positive and Gram-negative bacterial pathogens. Antioxidant activity was determined using two different methods (DPPH and ABTS) and was concentration-dependent. The ethanol extract showed cytotoxic activity against cancer cell lines and induced apoptosis. The present finding revealed that the rhizome from the family Zingiberaceae can be a good source of anticancer and antimicrobial compounds. One limitation could be the lack of in vivo assays, which were not the primary focus of this study. Additional in vitro and in vivo investigations are needed to study the supposed bioactivity of Z. officinale rhizome against cancer and infectious diseases.
Acknowledgement
The authors thank the Researchers Supporting Project number (RSPD2024R536), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ginger extract (Zingiber officinale) triggers apoptosis and G0/G1 cells arrest in HCT 116 and HT 29 colon cancer cell lines. Afr. J. Biochem. Res.. 2010;4:134-142.
- [Google Scholar]
- Antimicrobial activity of Emilia pratermissa leaf extracts on organisms isolated from patients with otitis media attending Federal Medical Centre Owo and Ondo states specialist hospital Akure. Microbiol. Res. J. Int.. 2017;19:1-8.
- [Google Scholar]
- Cytotoxicity, phytochemical screening and genetic analysis of ginger (Zingiber officinale Rosc.) Callus and Rhizome. S. Afr. J. Bot.. 2022;151:54-59.
- [Google Scholar]
- Synergistic antibacterial potential of greenly synthesized silver nanoparticles with fosfomycin against some nosocomial bacterial pathogens. Infect. Drug Resistance 2022:125-142.
- [Google Scholar]
- Characterization of apoptosis in a breast cancer cell line after IL-10 silencing. Asian Pac. J. Cancer Prev.. 2018;19:777.
- [Google Scholar]
- Chemistry and crystal structures of some constituents of Zingiber cassumunar. Aust. J. Chem.. 1979;32:71-88.
- [Google Scholar]
- Ginger exosome-like nanoparticles (GELNs) induced apoptosis, cell cycle arrest, and anti-metastatic effects in triple-negative breast cancer MDA-MB-231 cells. Food Chem. Toxicol.. 2023;182:114102
- [Google Scholar]
- Antimicrobial activity of extract from Felicia muricata Thunb. J. Biol. Sci.. 2008;8:1062-1066.
- [Google Scholar]
- Antimicrobial and antioxidant activities of extracts from medicinal plant ginger (Zingiber officinale) and identification of components by gas chromatography. Afr. J. Plant Sci.. 2015;9:412-420.
- [Google Scholar]
- Global trends of cancer: The role of diet, lifestyle, and environmental factors. Cancer Innovation. 2023;2:290-301.
- [Google Scholar]
- Synergistic interaction of methanol extract from Canarium odontophyllum Miq. Leaf in combination with oxacillin against methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33591. Int. J. Microbiol. 2016
- [Google Scholar]
- Anticancer molecular mechanism of protocatechuic acid loaded on folate coated functionalized graphene oxide nanocomposite delivery system in human hepatocellular carcinoma. Materials. 2021;14:817.
- [Google Scholar]
- Ethnomedicine and ethnopharmacology of medicinal plants used in the treatment of diabetes mellitus in Uganda. Appl. Biol. Chem.. 2023;66:39.
- [Google Scholar]
- Regulated necrosis-related molecule mRNA expression in humans and mice and in murine acute tissue injury and systemic autoimmunity leading to progressive organ damage, and progressive fibrosis. Biosci. Rep.. 2016;36:e00425.
- [Google Scholar]
- Protective effect of ginger (Zingiber officinale roscoe) extract against oxidative stress and mitochondrial apoptosis induced by interleukin-1β in cultured chondrocytes. Cells Tissues Organs. 2017;204:241-250.
- [Google Scholar]
- The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discovery. 2020;6:112.
- [Google Scholar]
- Resveratrol enhances anticancer effects of paclitaxel in HepG2 human liver cancer cells. BMC Complement. Altern. Med.. 2017;17:1-12.
- [Google Scholar]
- Use of herbal medications for treatment of osteoarthritis and rheumatoid arthritis. Medicines. 2020;7:67.
- [Google Scholar]
- Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem.. 2006;97:452-458.
- [Google Scholar]
- Absence of the glycosyltransferase WcaJ in Klebsiella pneumoniae ATCC13883 affects biofilm formation, increases polymyxin resistance and reduces murine macrophage activation. Microbiology. 2019;165:891-904.
- [Google Scholar]
- The inhibitory effect of ginger extract on ovarian cancer cell line; application of systems biology. Adv. Pharm. Bull.. 2017;7:241.
- [Google Scholar]
- In vitro antimicrobial activity of Zingiber cassumunar (Plai) oil and a 5% Plai oil gel. Phytother. Res.: Int. J. Devoted Pharmacol. Toxicol. Evaluation Natural Product Derivatives. 2007;21:164-169.
- [Google Scholar]
- Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crop. Prod.. 2018;124:115-125.
- [Google Scholar]
- Analyzing real-time PCR data by the comparative CT method. Nat. Protoc.. 2008;3:1101-1108.
- [Google Scholar]
- Chemical constituents and biological activities of different extracts from ginger plant (Zingiber officinale) Chem. Biol. Technol. Agric.. 2023;10:14.
- [Google Scholar]
- Shittu, O.K., Abubakar, A., 2014. Evaluation of Phytochemicals, Proximate, Minerals and Anti-nutritional composition of Yam peel, Maize chaff and Bean coat.
- Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem. Toxicol.. 2008;46:3295-3302.
- [Google Scholar]
- Antimicrobial and toxic potential of aqueous extracts of Allium sativum, Hibiscus sabdariffa and Zingiber officinale in Wistar rats. J. Taibah Univ. Sci.. 2014;8:315-322.
- [Google Scholar]
- Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules. 2023;28:1845.
- [Google Scholar]
- Ginger (Zingiber officinale) antimicrobial potential: a review. Ginger Cultivation Antimicrob. Pharmacol Potentials 2019
- [Google Scholar]
- Traditional, complementary, and alternative medicine approaches to mental health care and psychological wellbeing in India and China. Lancet Psychiatry. 2016;3:660-672.
- [Google Scholar]
- In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic Clin. Paharmacol. Toxicol.. 2004;95:191-200.
- [Google Scholar]
- Apple peels as a value-added food ingredient. J. Agric. Food Chem.. 2003;51:1676-1683.
- [Google Scholar]
- Antioxidants in volatile Maillard reaction products: identification and interaction. LWT-Food Sci. Technol.. 2013;53:22-28.
- [Google Scholar]
- Zandavar, H., Babazad, M.A., 2023. Secondary Metabolites: Alkaloids and Flavonoids in Medicinal Plants. In: Herbs and Spices-New Advances. IntechOpen.
- Layer-by-layer assembly of multicolored semiconductor quantum dots towards efficient blue, green, red and full color optical films. Nanotechnology. 2008;19:435606
- [Google Scholar]
- The effect of selected herbal extracts on lactic acid bacteria activity. Appl. Sci.. 2021;11:3898.
- [Google Scholar]







