Translate this page into:
Assessment of anticoagulant potential of Halocnemum strobilaceum (Pall.) against black rat (Rattus rattus Lin.) from Algeria
⁎Corresponding author. randa.mlik@yahoo.fr (Randa Mlik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Black rats (Rattus rattus; Rodentia: Muridae) cause significant damage to agricultural and human health, and they are considered a reservoir for a variety of diseases.
Objectives
The current study aimed to evaluate the toxicity of oral anticoagulants derived from Halocnemum strobilaceum (Pall.) M. Bieb. to these dangerous animals.
Method
Four selected doses (100, 150, 200 and 300 mg/day/kg of individual weight) and one control were utilized, with 10 repetitions of each dose (5 ♂ and 5 ♀). The extract was powdered on dates as bait.
Results
UPLC-ESI/MS-MS chromatography identified three distinct coumarins (coumaric acid, p-coumaric acid and 4-hydroxycoumarin). The results showed that the fourth dose used caused the highest number of deaths (100 % after three days of treatment). In contrast, after 48 h of therapy, all rats examined showed lethargy, anorexia and unconsciousness, followed by rapid death at 72 h in the treated individuals. The LD50 was recorded at 146.4 mg.kg−1 with a LT50 of 59.37 h. At the end of the experiment, the autopsies of all deceased individuals allowed us to observe the bleeding of their internal organs. The analysis of PT and aPTT demonstrated that the halophyte under investigation possesses anticoagulant activity that increased with the concentration. Histological sections of the liver revealed cellular alteration and significant necrosis. As well, the kidneys had vascular occlusion with an inflammatory filtrate.
Conclusions
To reduce food losses and wastes caused by these pests, H. strobilaceum will be an important plant for use in protecting against invasive rodents, especially in environmental and stored product programs focused at eradicating rodent pests.
Keywords
Anti-Vit-K
Biological treatment
Hemorrhage
Roof rat
Spontaneous plant
1 Introduction
Certain pest species, such as rats, constitute a severe threat to agriculture because they can damage crops, break husbandry rules, transfer diseases, and harm the environment (Mlik et al., 2018; 2022; Meddour et al., 2022; Mlik et al., 2024). Anthropization of natural ecosystems and the adaptability of certain euryetic species have caused the latter species to spread almost everywhere in the world (Hassell et al., 2021; Dalecky et al., 2023). The world's top three invasive rodent species include Rattus norvegicus, R. rattus and Mus musculus (Badou et al., 2023). Due to human migrations and international trade, especially maritime exchanges, they have expanded throughout the world (Badou et al., 2023; Skotnes-Brown, 2023). This has significant consequences for the spread of zoonotic pathogens e.g. Yersinia pestis, Leptospira, Hantavirus…etc. (Ganjeer et al., 2021; de Cock et al., 2023) and food destruction (Constant et al., 2020; Munawar et al., 2020; Jurišić et al., 2022; Jarzębowska, 2023). Rats and mice, along with their associated ectoparasites and rodent-borne diseases, are frequently found on large ships, from which they may be involuntarily introduced into seaports and eventually colonize new locations (Kuo et al., 2017; Rahelinirina et al., 2018). Nonetheless, black rats cause significant losses in several plant species, particularly cereals, where they can destroy crops like Meriones. In contrast, Mlik et al. (2017) and Alia et al. (2018) discovered that the black rat caused damage to spars and dates in southeastern Algeria, both in open fields and in storage. They also target domestic animals, particularly chickens and quails, and eat their eggs. Furthermore, they harbor a high community of parasitic arthropods capable of transmitting dangerous diseases to humans and animals (Mlik et al., 2022). Controlling these pests involves numerous strategies, including biological, mechanical, environmental, cultural, and chemical treatments (Constant et al., 2020). Other alternatives, such as plant-based natural extracts, have a diverse spectrum of bioactive compounds with various applications. When explored in a behavioral model, a range of plant extracts, either alone or in combination, can be tested to see if they could reduce rat infestation (Singh and Mirza, 2020). The primary anticoagulants containing coumarin derivatives are now utilized to manage commensal rodents in agricultural and urban environments (Mercer et al., 2022; Khidkhan et al., 2024), particularly in environmental programs to eradicate rodent pests on islands (Samaniego-Herrera et al., 2013; Wheeler et al., 2019), despite having the same mode of action; interference with coagulation factor synthesis, resulting in bleeding and death (Endepols et al., 2015). Moreover, it has been established that employing chemical anticoagulants (such as bromadiolone) to kill rodents has a deleterious influence on their predators-in this case, foxes (Jacquot et al., 2013; Rached et al., 2020).
The current proposal is to evaluate the anticoagulant potential of coumarin derivative with rodent-killing ability caused by vitamin K inhibition (as an anticoagulant) using a fraction of a spontaneous halophyte from south-eastern Algeria, Halocnemum strobilaceum, on the roof rat Rattus rattus.
2 Materials and methods
2.1 Plant material
The plant material employed in this experiment was Halocnemum strobilaceum (Pall. 1819), a species of the Amaranthaceae family, also known as “Grina” locally. In May 2020, this plant's aerial part was collected in the province of Ouargla (32°23’49.22”N; 5°22’27.45”E) in Southeast Algeria. The plant was crushed after seven days of air-drying at room temperature (25–30 °C). 30 g of the plant's powder was then combined with 100 mL of MeOH/H2O (20/80 mL: v/v) and macerated for five days. The resulting solution was filtered and evaporated using a rotary evaporator, then liquid–liquid extracted with ethyl acetate (EtOAc) and stored at 4 °C until further analysis and activities. Total phenols, flavonoids, and tannins were analyzed to identify their abundance in this halophyte's extracts.
2.2 High-performance liquid chromatography (HPLC)
The resulting ethyl acetate fraction was analyzed using a Shimadzu LC 20 AL HPLC with a universal injector (Hamilton 25l). The phenolic components of the selected fraction were detected using an analytical column Shim-pack VP-ODS C18 (4.6 mm, 250 mm, 5 m) with a UV–VIS detector type SPD 20A (Shimadzu). The mobile phase (acetonitrile/acetic acid 0.1 % v/v) used gradient elution and the reverse chromatographic phase analyses used non-polar aliphatic residues. The flow rate was 1 mL/min, whereas the injection volume was 0.45 l. The monitoring wavelength was 268 nm, and the sample and standard phase injection volumes were 20 l each. To identify the compounds, their retention times and UV absorbance were compared to the standards.
2.3 Ultra-performance liquid chromatography-mass spectrometry (UPLC/MS-MS)
To optimize standard polyphenols, three coumarins were separated on a SHIMADZU 8040 Triple Quad UPLC/MS with electrospray ionization (ESI). LC featured the binary bump (Nexera XR LC-20AD). Gradient elution was used with the mobile phase consisting of 30 % H2O, 0.1 % HCOOH and 70 % MeOH with a total flow of 0.2 mL/min and an injection volume of 6 l and positive and negative ion (ESI) proven. In multiple reaction monitoring (MRM) modes. The ionization source was set up with the following parameters: 6000 V for the spray voltage, 250 °C for the source temperature, and 350 °C for the desolvation temperature. The nebulizer gas utilized was nitrogen, with a flow rate of 3 l/min. High-purity nitrogen was under 0.23 MPa of pressure during collision-induced dissociation (CID). Quantification was performed using multiple reaction monitoring (MRM) modes. A summary of MS/MS detection parameters is presented in Table 1.
Compound
Charge
(+/-)
Precursor
(m/z)
Product
(m/z)
Dwell time (msec)
Q1 Pre Bias
(v)
CE
(v)
Q3 Pre Bias
(v)
Coumaric acid
+
165.100
101.200
100
−17
−14
−10
69.150
100
−18
23
−27
4-hydroxy coumarin
−
160.800
117.100
100
18
22
20
89.050
100
17
11
16
P-coumaric acid
+
165.100
59.100
100
−12
−17
–22
119.100
100
18
16
22
93.150
100
19
31
17
−
162.800
2.4 Test animals
The current experiment used adult black rats (Rattus rattus) weighing between 150 and 180 g. All of the individuals included in the current study were captured by farmers as part of the pest management strategy. For three months, the animals were acclimatized at 25 ± 2 °C and 40 ± 10 % relative humidity, with a 12 h light/ 12 dark cycle to prevent mortality and abnormal behavior. Four doses (100, 150, 200 and 300 mg/day/kg of individual weight) and one control were chosen, with six replicates of each dose (5 ♂ and 5 ♀). The present experiment followed the OECD Guideline 423 (OECD 2001). This work has been authorized by the ethical committee of the University of Kasdi-Merbah in Ouargla.
2.5 Preparation of rodenticide baits
The bait was formulated by removing the pits of dates, adding the extract, and closing it with some crushed dates. Fasting for 24 h before testing was required to verify that these animals consumed treated dates daily. Following administration of the appropriate doses, the animals were monitored at least once during the first 30 min, regularly throughout the first 24 h following treatment, with special attention during the first 4 h, and then daily for the next four days (until individual death). This was done to analyze the animals' general physical state, including mortality rates, toxicological signs, and other symptoms including morphological, physiological, and behavioral changes. Lethal dose and time (LD50 and LT50) were determined using the Probit method.
2.6 Biochemical analysis
To assess the anticoagulant potential of this extract, biochemical analyses of the two primary factors responsible for blood clotting in the body were undertaken e.g. extrinsic (prothrombin time: PT) and intrinsic pathway (activated partial thromboplastin time: aPTT). Blood samples were brought to the laboratory for analysis. At the end of the experiment, the rats were sacrificed, and liver and kidney tissues were removed for histological examination.
2.7 Statistical data
Statistica v. 10.0 (StatSoft) software was used to interpret the data analysis. The results obtained were presented as mean ± SD and analyzed using parametric tests for normal results (ANOVA) and non-parametric tests (Kruskal-Wallis) for abnormal data when comparing doses. Linear regression was performed to correlate the coagulation factors (PT and aPTT).
3 Results
3.1 Chemical characterization
The obtained results indicated that the phenolic contents of H. strobilaceum's ethyl acetate fraction were 17.1 ± 0.013 mg.g−1 DW. In contrast, flavonoids had a value of 12 ± 0.2 mg.g−1 DW and tannins had a value of 28.5 ± 0.01 mg.g−1 DW.
3.2 HPLC and UPLC/MS analyses
Table 2 lists the phenolic chemicals that HPLC identified in the H. strobilaceum fraction.
Compounds (µg.mL−1)
Retention time
EtOAc fraction (µg.mg−1)
Gallic Acid
5.29
0.23
Chlorogenic Acid
13.392
69.99
Caffiec Acid
16.277
8.14
Vanilin
21.46
0.45
p-Comaric Acid
23.817
9.87
Rutin
28.37
2.73
Quercetin
45.047
23.55
HPLC analyses showed seven phenolic components in the ethyl acetate fraction of H. strobilaceum: gallic acid, chlorogenic acid, vanillin, p-coumaric acid, rutin, and quercetin. (Table 2, Fig. 1).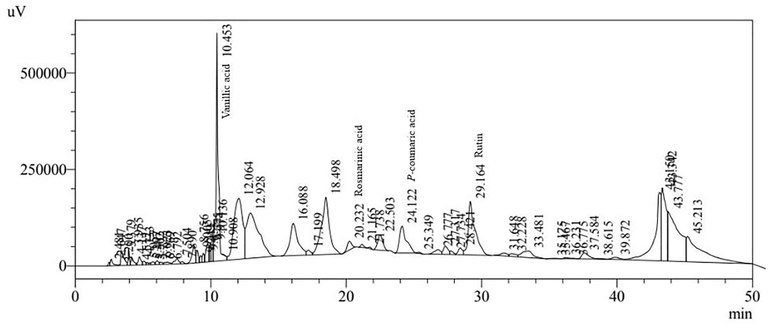
HPLC profile of the ethyl acetate fraction of H. strobilaceum.
Depending on the flavonoid content of the EtOAc fraction, a multiple reaction mode analysis (MRM) was used to identify coumarin derivatives having anticoagulant characteristics. Fig. 2 depicts the UPLC/MS chromatographic profiles for detecting and quantifying the three separated coumarins are shown in.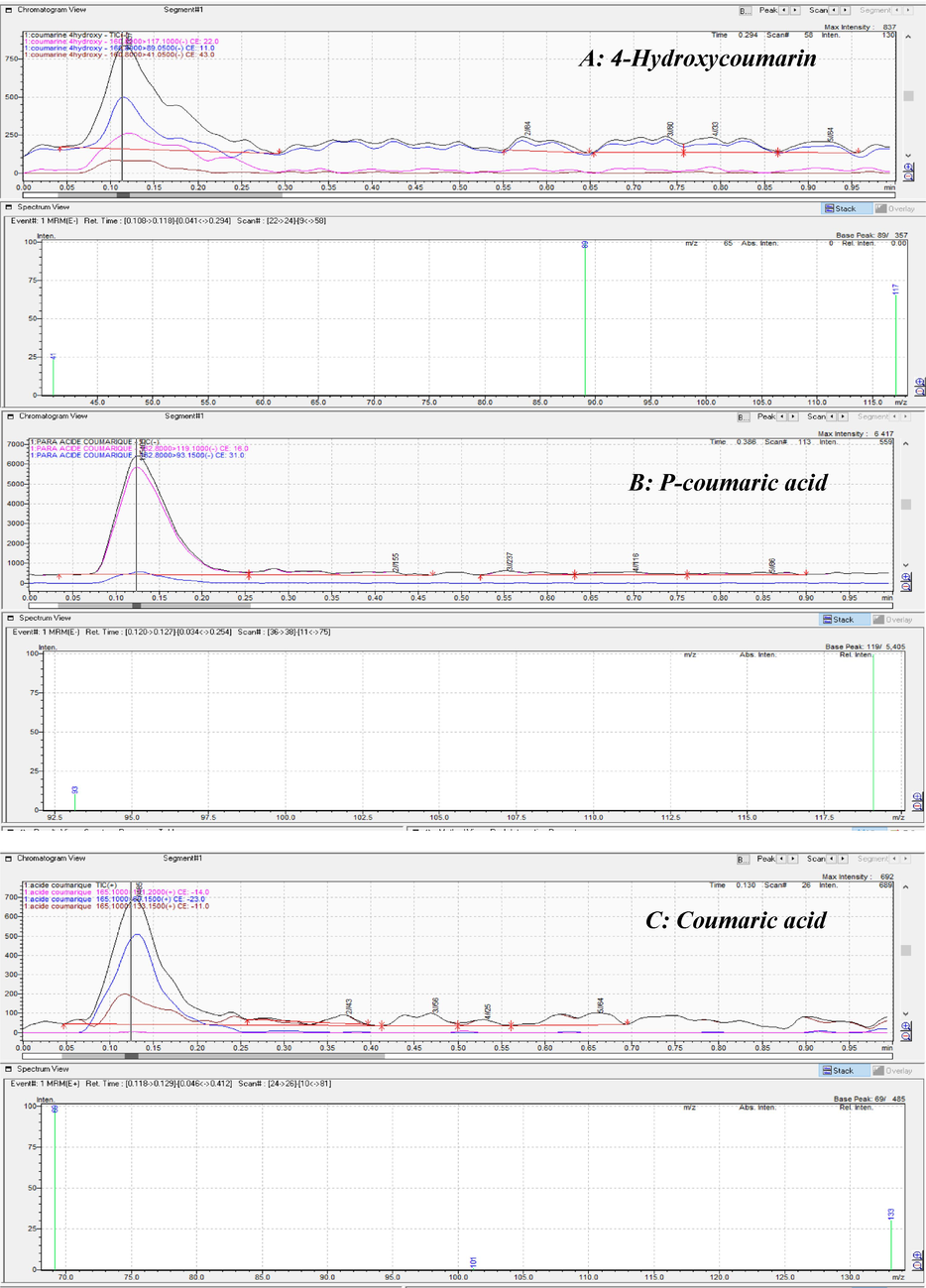
UPLC/MS chromatography profiles of coumarins detected in H. strobilaceum fraction.
The analytical approach was used to evaluate the EtOAc fraction of H. strobilaceum coumarin derivatives. The MS spectra revealed the molecular weights of the discovered substances (Fig. 2). P-coumaric acid exhibited the largest molecular mass in both positive and negative ionization modes, followed by coumaric acid and 4-hydroxycoumarin. Fig. 3 depicts the chemical structures of the identified coumarins, as well as their associated MS spectra, to aid comprehension. The molecular weights of the compounds displayed in the spectra are as follows:
-
4-Hydroxycoumarin: Molecular weight: [162.14 g/mol], MS spectrum shown in Fig. 2A.
-
P-coumaric acid: Molecular weight: [164.0473 g/mol], MS spectrum shown in Fig. 2B.
-
Coumaric acid: Molecular weight: [164.0473 g/mol], MS spectrum shown in Fig. 2C.
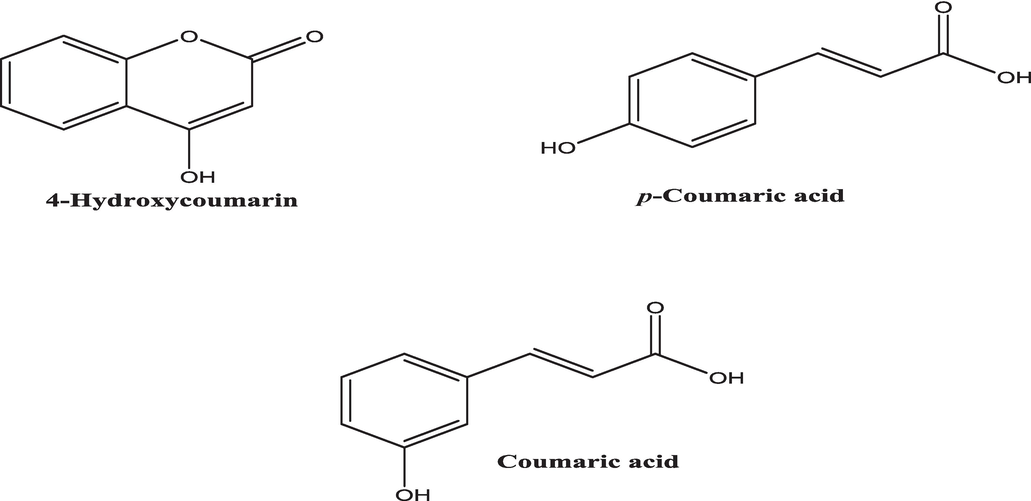
- Chemical structures of the detected coumarins in H. strobilaceum fraction.
The correlation between the chemical structures and their MS spectra is crucial for understanding the molecular characteristics and behaviors of these molecules. By clearly indicating the molecular weights in the spectra, they provide a comprehensive analysis that enhances the reproducibility and reliability of our findings.
3.3 Efficacy of H. strobilaceum fraction on the black rats
The current study showed that the EtOAc fraction of this halophyte has an important effect on black rats, with the fourth dose resulting in the maximum number of deaths (Fig. 4). None of the control rats died, implying that the expected effective dose of 300 mg.kg−1 for three days was sufficient to cause 100 % mortality in black rats. Statistical analysis revealed a very highly significant difference (p = 0.0041) between doses in the EtOAc fraction.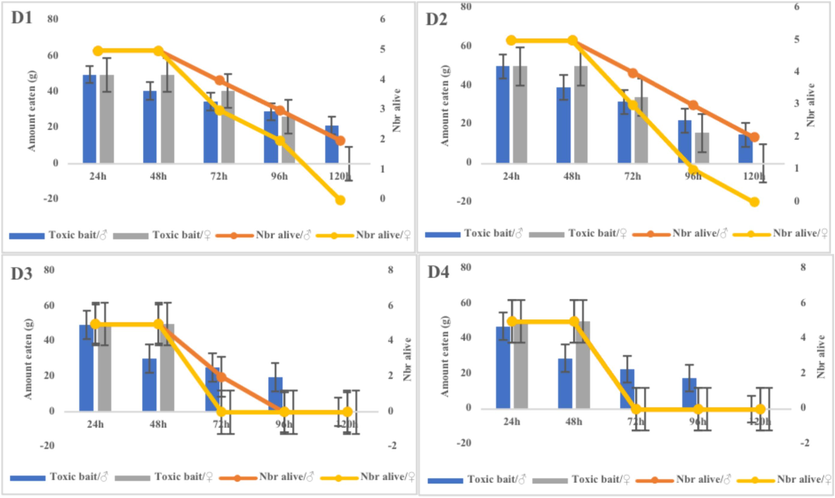
Efficacy of EtOAc fraction of H. strobilaceum in black rats.
Overall and after 48 h of treatment, all rats examined showed lethargy, anorexia, and unconsciousness, followed by sudden death after 72 h of treatment. Females were mainly impacted by these treatments, and after the first dose, no mortality was observed for 72 h, with females always dying before males (Fig. 4). On the other hand, the third and fourth doses showed that females had a higher all-cause mortality after 72 h than males (Fig. 4). At the end of the experiment, an autopsy of all the dead individuals allowed us to observe that their internal organs were all bleeding (Fig. 5).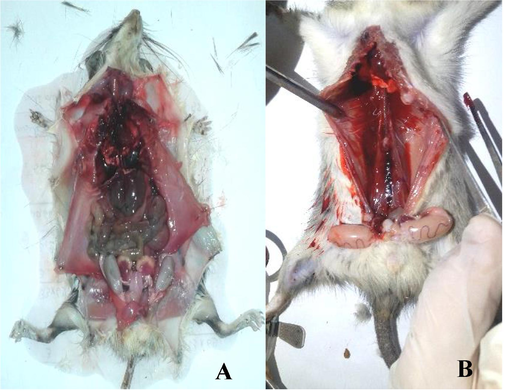
Effect of H. strobilaceum fraction on blood coagulation of R. rattus (A: control, B: treated rat (bleeding internal organs).
To establish the effect of the EtOAc fraction on internal organs, an anticoagulant factor analysis (PT and PTT) was performed. The analysis of intrinsic and extrinsic pathway factors (PT and aPTT) revealed that this plant's anticoagulant activity increased with concentration. The fourth dose had the highest values (PT=144.0 ± 0.62″ and aPTT=125.3 ± 0.80″), indicating that the aPTT increased with the PT (Fig. 6). There was a correlation between these two factors (Fig. 6). This indicated that increasing or reducing the PT value influenced the aPTT. The Probit method indicates that the EtOAc fraction had a lethal dose of 146.4 mg.kg−1. Similarly, the lethal time (LT50) indicated that 50 % of the black rat population could be eradicated in 59.37 h.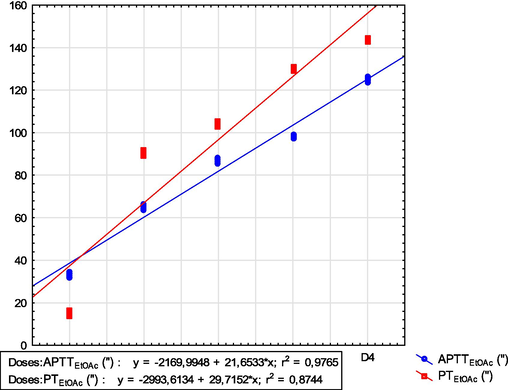
Correlation between PT and aPTT of EtOAc fraction of H. strobilaceum.
3.4 Histological section
The liver is the primary tissue target for rodenticide persistence since all anticoagulants work by inhibiting the synthesis of blood clotting factors. The obtained PT and aPTT analysis results were then validated by histological sections. While the sections of the liver mentioned below showed cellular change, significant necrosis, and cellular infiltration, inflammation of the portal vein space was associated with peliosis in the blood vessels, and the hepatocytes increased in size and their cytoplasm was cleared, as viewed during the destruction process (Fig. 7). Furthermore, all treated rats kidneys showed vascular blockage with an inflammatory filtrate (Fig. 8).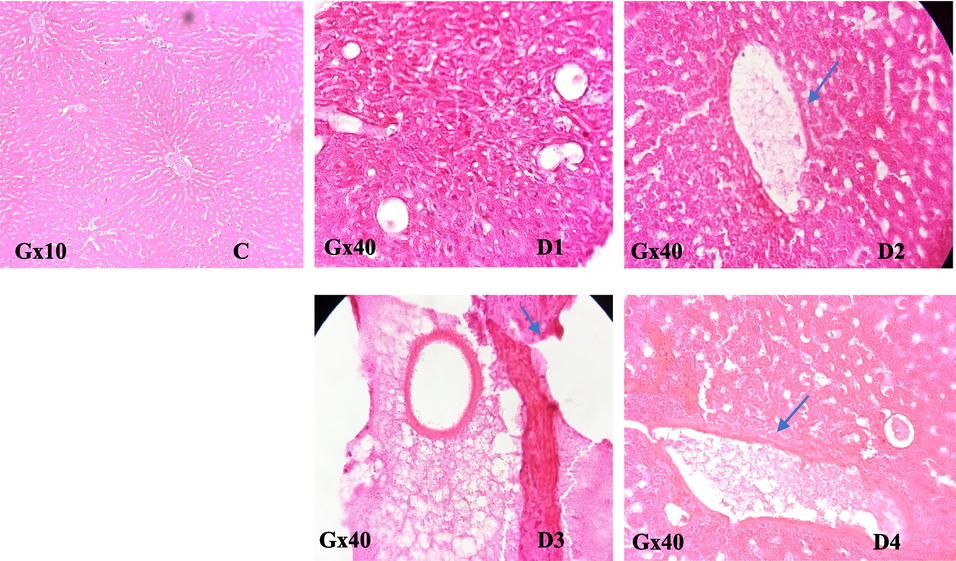
Effect of EtOAc fraction of H. strobilaceum on the liver of treated black rats compared to control.
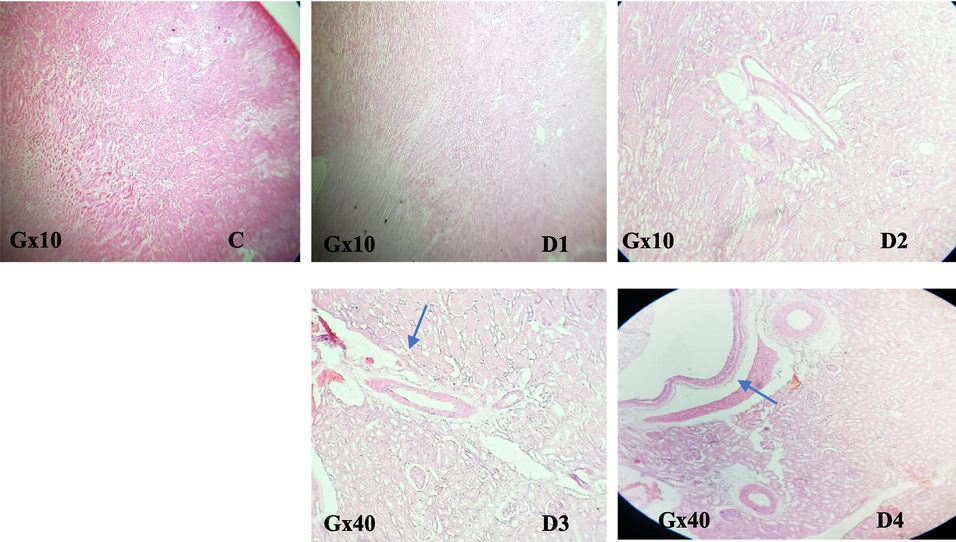
Effect of EtOAc fraction of H. strobilaceum on the kidney of treated black rats compared to control.
4 Discussion
As the first report of H. strobilaceum as a rodenticide, the current experiment enabled us to detect three distinct coumarins using UPLC-ESI/MS-MS chromatography (coumaric acid, p-coumaric acid, and 4-hydroxycoumarine). The rodenticide tests revealed that the fourth dose caused the highest number of deaths (100 % after three days of treatment). In contrast, after 48 h of therapy, all rats evaluated displayed lethargy, anorexia, and unconsciousness, followed by sudden death at 72 h. The LD50 was 146.4 mg.kg−1, with a LT50 of 59.37 h. At the end of the experiment, the autopsies on all deceased individuals allowed us to observe the bleeding of their internal organs. Our findings on the composition of this plant were comparable to those reported by AbdelRazek et al. (2020) and Gheraissa et al. (2023). Furthermore, various research has demonstrated the phenolic compounds richness of this halophyte, with Handoussa et al. (2019) showing that the ethyl acetate extract of the aerial part of H. strobilaceum has a phenolic content of 29.42 mg GAE/g DW. Furthermore, Gheraissa et al. (2023) reported that the hydro-methanolic extract of this plant has 24.97 ± 0.09 µg GAE/g DW as polyphenol content, and 12.17 ± 0.16 and 5.43 ± 0.06 µg QE/g DW as total flavonoid and flavanols content, respectively. On the other hand, coumarins are benzopyrone molecules that belong to the flavonoid group of phytochemicals, with over 1300 discovered in plants, bacteria, and fungi (WHO, 2020; Tsivileva et al., 2022). Coumarins have distinct antiviral, antimicrobial, antioxidant, anti-inflammatory, antiadipogenic, cytotoxic, apoptosis, antiproliferative, cytotoxic, antituberculous, and anticoagulant properties (Umashankar et al., 2015; Bouhaoui et al., 2021). Coumarins and their derivatives have grown in importance in synthesis and production because of their diverse pharmacological properties, and they have later been employed in rodenticide formulations. As bait avoidance or aversion, rodents, particularly rats (Rattus spp.), are smart enough to link the emergence of uncomfortable health symptoms with the new food source. Consequently, anticoagulant rodenticides have a long history of effective use because of their beneficial properties, which include a significant delay between the lethal dose and the onset of symptoms after ingestion (Howard et al., 2020). The current study demonstrated the interesting potential of this halophyte to inhibit Vit-K of the tested rodents due to its anticoagulant-killing activity, with all-cause mortality observed at 72 h at the highest doses. Bates (2016) validated our finding, stating that anticoagulant toxicoses develop progressively, causing anorexia, hemorrhage, shock, unconsciousness, and eventually death. Furthermore, Aleksandrov et al. (2024) highlighted that anticoagulant rodenticides (ARs) compete with vitamin K (VK), which is essential for the synthesis of blood clotting factors in the liver, resulting in blood coagulation inhibition and, in many cases, animal mortality due to hemorrhage. Moreover, PT and aPTT analysis confirmed the influence of the ethyl acetate fraction of H. strobilaceum over the control, whereas blood coagulation increased with treatment concentration. Additionally, the prolongation of PT and aPTT implied inhibition of both the extrinsic and intrinsic coagulation pathways. All anticoagulant rodenticides work in the same way, the active anticoagulant stops the epoxide reductase enzyme from being active when a rodent eats the bait, which stops activated vitamin K from being recycled (Silverman, 1980). Nevertheless, reducing the biological availability of vitamin K prevents these vital components from being synthesized, which in turn prevents the coagulation process. Given that prothrombin is essential to both the intrinsic and extrinsic coagulation cascades, this suggests that the chemical is a highly effective anticoagulant medication (Boron and Boulpaep, 2016). Thus, the presence of coumarin derivatives in this plant, i.e. 4-hydroxycoumarin, p-coumaric acid, and coumaric acid, explains the anticoagulant activity reported in the rats tested. Furthermore, coumarin derivatives are known for their anticoagulant properties (inhibiting vitamin K), as are 4-hydroxycoumarin and its derivatives, which are commonly employed in anticoagulant rodenticides and antithrombotic medicines). These anticoagulants, which target vitamin K 2,3-epoxide reductase in liver microsomes, are antagonists of vitamin K (Kasperkiewicz et al., 2020; Ramsis et al., 2023). This explains the severe damage observed in the black rats' internal organs such as the liver and kidney, as well as the sudden death of treated rats, given that the liver is vital in maintaining the balance between thrombosis and hemorrhage. Moreover, a high PT indicates significant liver diseases or cirrhosis. It could also mean that there is a higher danger of internal bleeding. In addition, Flores-Morales et al. (2023) demonstrated that coumarins are quickly absorbed, dispersed throughout the body, and found in the liver and kidneys in high concentrations.
5 Conclusions
The actual experiment and the acquired data revealed that the EtOAc fraction of H. strobilaceum had strong anticoagulant activity. These findings were supported by the extension of clotting times (extrinsic and intrinsic pathways) and substantial damage to internal organs such as the liver and kidney of black rats. Consequently, H. strobilaceum emerges as a significant plant for protecting native fauna against invasive rodents, making it particularly useful in environmental and stored product programs aimed at eradicating rodent pests. Future studies should include detailed mechanistic investigations to understand the specific biochemical pathways involved in comprehensive toxicological assessments to ensure the safety of non-target species, large-scale field trials to assess practical effectiveness, and comparative studies with other anticoagulant plants and commercial rodenticides. Additionally, isolating and characterizing individual active compounds within the EtOAc fraction, as well as investigating the long-term impact on rat populations and resistance development will help to understand H. strobilaceum's potential as an eco-friendly rodent control agent.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Randa Mlik: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Salim Meddour: Writing – review & editing, Validation, Methodology, Data curation. Nour Elhouda Mekhadmi: Writing – review & editing, Methodology. Abdellah Henni: Writing – review & editing, Methodology, Formal analysis. Walid Boussebaa: Methodology, Formal analysis, Data curation. Asma Abid: Writing – review & editing, Methodology, Formal analysis. Amar Eddoud: Writing – review & editing, Validation, Methodology, Formal analysis. Makhlouf Sekour: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Formal analysis, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of changing culture media on metabolites of endophytic fungi from Halocnemum strobilaceum. Arch. Pharm. Sci. Ain Shams Univ.. 2020;4:135-144.
- [Google Scholar]
- Toxicology of chemical biocides: anticoagulant rodenticides – beyond hemostasis disturbance. Comparative Biochem. Physiol. Part C: Toxicol. Pharmacol.. 2024;277:109841
- [CrossRef] [Google Scholar]
- Annual and stationary variation of Black Rat Rattus rattus (Linnaeus, 1758) damage on date palm inflorescences in Southeastern Algeria. World J. Environ. Biosci.. 2018;7:102-107.
- [Google Scholar]
- Biological invasions in international seaports: a case study of exotic rodents in Cotonou. Urban Ecosystems. 2023;26:1051-1065.
- [CrossRef] [Google Scholar]
- Anticoagulant toxicoses develop progressively, causing anorexia, hemorrhage, shock, unconsciousness, and eventually death. Companion Animal.. 2016;21(8):466-471.
- [CrossRef] [Google Scholar]
- Medical Physiology. Philadelphia: Elsevier; 2016.
- Synthesis and biological properties of coumarin derivatives. A review. Chemistry Select.. 2021;6:5848-5870.
- [Google Scholar]
- Comparative assessment on rodent impacts and cultural perceptions of ecologically based rodent management in 3 Afro-Malagasy farming regions. Integrative Zool.. 2020;15:578-594.
- [CrossRef] [Google Scholar]
- Prolifération de rongeurs dans les milieux urbains et agricoles d’Afrique subsaharienne. Le côté obscur des rodenticides chimiques de synthèse. Environnement, Risques & Sante.. 2023;22:205-211.
- [CrossRef] [Google Scholar]
- Increased rat-borne zoonotic disease hazard in greener urban areas. Science of the Total Environment.. 2023;896(20):165069
- [CrossRef] [Google Scholar]
- Endepols, S., Buckle, A., Eason, C., Pelz, H.J., Meyer, A., Berny, P., Baert, K., Prescott, C., 2015. Guidelines on anticoagulant rodenticide resistance management. Rodenticide Resistance Action Committee (RRAC)/Crop Life International 32 pp.
- Therapeutic effects of coumarins with different substitution patterns. Molecules. 2023;28:2413.
- [CrossRef] [Google Scholar]
- Rodent borne zoonoses: a brief review. Pharma Innovation J.. 2021;10(8):721-725.
- [CrossRef] [Google Scholar]
- Gheraissa, N., Chemsa, A.E., Cherrada, N., Erol, E., Elsharkawy, E.R., Ghemam-Amara, D., Zeghoud, S., Rebiai, A., Messaoudi, M., Sawicka, B., et al., 2023. Biochemical Profile and In Vitro Therapeutic Properties of Two Euhalophytes, Halocnemum strobilaceum Pall. and Suaeda fruticosa (L.) Forske., Grown in the Sabkha Ecosystem in the Algerian Sahara. Molecules. 28, 3580. Doi: 10.3390/molecules28083580.
- UPLC–ESI-PDA–MSn profiling of phenolics involved in biological activities of the medicinal plant Halocnemum strobilaceum (Pall.) Iran. J. Pharm. Res.. 2019;18:422-429.
- [Google Scholar]
- Socio-ecological drivers of vertebrate biodiversity and human-animal interfaces across an urban landscape. Global Change Biol.. 2021;27:781-792.
- [CrossRef] [Google Scholar]
- Howard, A., Hamilton, D., Musgrave, I., Jolley, J., 2020. Anticoagulant rodenticide excretion in rats following median lethal dose (LD50) administration. South Australian Research and Development Institute, Final Report. 33p.
- Using long-term monitoring of red fox populations to assess changes in rodent control practices. J. Appl. Ecol.. 2013;50(6):1406-1414.
- [CrossRef] [Google Scholar]
- Jarzębowska, G., 2023. Species Cleansing: The Rhetoric of Rat Control in the People’s Republic of Poland 1945–1956. Cambridge University.
- Jurišić, A., Ć upina, A.I., Kavran, M., Potkonjak, A., Ivanovíc, I., Bjelić-Cˇ abrilo, O., Meseldžija, M., Dudíc, M., Poljakovíc-Pajnik, L., Vasíc, V., 2022. Surveillance Strategies of Rodents in Agroecosystems, Forestry and Urban Environments. Sustainability. 14, 9233. Doi: 10.3390/su14159233.
- Antagonists of Vitamin K-popular coumarin drugs and new synthetic and natural coumarin derivatives. Molecules. 2020;25:1465.
- [CrossRef] [Google Scholar]
- Evaluation of anticoagulant rodenticide sensitivity by examining in vivo and in vitro responses in avian species, focusing on raptors. Environ. Pollut.. 2024;341:122837
- [CrossRef] [Google Scholar]
- Significance of major international seaports in the distribution of murine typhus in Taiwan. PLoS Negl. Trop. Dis.. 2017;11:e0005430.
- [Google Scholar]
- Ectoparasites of the common gundi (Ctenodactylus gundi Rothmann) from the Aures Region, Algeria. Annals Parasitol.. 2022;68(3):519-529.
- [Google Scholar]
- Mercer, M.A., Davis, J.L., Riviere, J.E., Baynes, R.E., Tell, L.A., Jaberi-Douraki, M., Maunsell, F.P., Lin, Z., 2022. Mechanisms of toxicity and residue considerations of rodenticide exposure in food Animals-a FARAD perspective. J. Am. Vet. Med. Assoc. 260 (5), Doi: 10.2460/javma.21.08.0364.
- Mlik, R., Meddour, S., Souttou, K., Sekour, M., 2017. Importance et caractérisation du rat noir Rattus rattus L. dans les milieux phoenicicoles de la région de Touggourt. Workshop national sur la Biodiversité et Agriculture durable en régions arides et semi-arides, Biskra 23 Mai 2017.
- Evaluation and Characterization of Black Rat (Rattus rattus) Population in the Phoenicultural Environments of Algeria. World J. Environ. Biosci.. 2018;3:99-107.
- [Google Scholar]
- First report of ectoparasites from black rats (Rattus rattus Linnaeus, 1758) in oasis regions from Algeria. Notulae Scientia Biologicae.. 2022;14(1):11013.
- [CrossRef] [Google Scholar]
- First data on bacterial, fungal and parasitic infections of black rats (Rattus rattus) from the palm groves of the algerian Sahara. J. Microbiol. Biotech. Food Sci.. 2024;13(5):e10186.
- [Google Scholar]
- Chemical control of rodents and its impact on rodent infestations during subsequent cropping season. Int. J. Pest Manage.. 2020;69(2):140-149.
- [CrossRef] [Google Scholar]
- Biomarkers Potency to Monitor Non-target Fauna Poisoning by Anticoagulant Rodenticides. Front. Vet. Sci.. 2020;7:616276
- [CrossRef] [Google Scholar]
- Risk of maritime introduction of plague from Madagascar to Mayotte. Acta Tropica.. 2018;187:140-143.
- [CrossRef] [Google Scholar]
- Synthetic coumarin derivatives with anticoagulation and antiplatelet aggregation inhibitory effects. Medicinal Chemistry Research.. 2023;32:2269-2278.
- [CrossRef] [Google Scholar]
- A model for a molecular mechanism of anticoagulant activity of 3-substituted 4-hydroxycoumarins. Journal of the American Chemical Society.. 1980;102:5421-5423.
- [CrossRef] [Google Scholar]
- Natural Biological Products from Plants as Rodenticides. In: Singh J., Yadav A.N., eds. Natural Bioactive Products in Sustainable Agriculture. Springer; 2020. p. :235-257.
- [Google Scholar]
- Scurrying seafarers: shipboard rats, plague, and the land/sea border. J. Global History. 2023;18:108-130.
- [CrossRef] [Google Scholar]
- Coumarins as fungal metabolites with potential medicinal properties. Antibiotics. 2022;11:1156.
- [CrossRef] [Google Scholar]
- Isolation, purification and in vitro cytotoxicity activities of coumarin isolated from endophytic fungi, Alternaria species of Crotalaria pallida. Indo. Am. J. Pharm. Res.. 2015;5:926-936.
- [Google Scholar]
- Evaluating the susceptibility of invasive black rats (Rattus rattus) and house mice (Mus musculus) to brodifacoum as a prelude to rodent eradication on Lord Howe Island. Biol. Invasions.. 2019;21:833-845.
- [CrossRef] [Google Scholar]
- World Health Organization, 2020. Cancer statistics reports. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 12 Mai 2021.







