Translate this page into:
Assessment of a nanobiofungicide with antimicrobial potential against mycopathogenic Fusarium species
⁎Corresponding author. syalomar@ksu.edu.sa (Suliman Yousef Alomar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
In the current study, a new potential nanobiofungicide was investigated as hybrid composite antifungals; zinc oxide nanoparticles (ZNPs) in combination with microbial hydrolytic enzymes (Chitinases).
Methods
The Chitinase enzyme was achieved from a local isolate, which was identified as Bacillus licheniformis AG-W3. The enzyme production was achieved after 72 h of incubation at pH 7.5 and temperature 45 °C. The mean diameter of the synthesized nanoparticles (38–76 nm) was established by means of scanning electron microscope (SEM). Additionally, their antifungal activity was evaluated against Fusarium species.
Results
Based on the results obtained, ZNPs amended with Chitinase at 0.1 % concentrations showed the strongest inhibitory effect (89.1 %). A synergistic effect of Chitinase (0.03U/ml) in combination with ZNPs (0.005 %) was observed up to 89.1 %. The SEM results exhibits agglomerated spherical particles due to high surface energy. The average size calculated for calcined zinc nanoparticles are (38–76 nm) and this is in good agreement with the XRD results (Eq. (1): 46 nm).
Conclusions
It was concluded that the combination of ZNPs with Chitinase enzyme exhibits enhanced antifungal activity compared to their individual effects, considered a new alternative for synthetic fungicides. The development of novel nanocomposites for sustainable management of fungal diseases can mitigate the emergence of persistent and resilient fungal diseases and the crop losses that they cause. In addition, the importance of the current study was to establishing a consortium of chitinolytic microorganisms and nanoparticles appears to bring superior outcomes in fighting fungal phytopathogens, and also a potential alternative to these chemical pesticides, residing in soil and already the part of endophytic microbiome, have minimum altering effect on ecosystem.
Keywords
Microbial Chitinases
Nanoparticles
Plant protection
Nanobiofungicide
1 Introduction
Nanoformulation of fungicides can be environmentally advantageous with respect to traditional ways of application (e.g. spray). With the more controlled delivery of the active ingredients, the evaporation and leaching of substances to the environment is reduced (de Oliveira, 2021). They also help to reduce the application dosage level (Nuruzzaman et al., 2016) and be designed improve the solubility and stability of biopesticide (Damalas & Koutroubas, 2018). Biotechnological approaches to control phytopathogens and enhanced plant health are in use in agriculture; they aim to be environment friendly without neglecting crops’ needs (Alina et al., 2015). In contrast, synthetic fungicides can have adverse effects on the ecosystem and public health due to their toxicity and uncontrolled released to the surrounding environment of the crop where they are applied (Mullin et al., 2010). Particular idiosyncrasies have arisen in innovative nano-agrochemicals due to the viable applications of nanotechnology in a myriad of agriculture sectors. Furthermore, the fungicides exposure is of various types for instance direct occupational, direct non-occupational and indirect exposure. Occupational exposure is the most unsafe one, as interrelated to broad range illnesses for instance airway obstruction and lungs diseases. The possible genetic damage initiated by occupational pesticide exposure is much more than smoking and alcohol consumption (Nascimento et al., 2022). Numerous epidemiological and molecular researches have established a close relationship between persistent pesticides exposure and increased diseases threat for instance endocrine disruptors, neurodegenerative and reproductive disorders, respiratory problems and birth deficiencies (Gea et al., 2022). Besides, teratogenic, carcinogenic and mutagenic natures of fungicides are assumed to be a causative source of cancer growth in humans (Gea et al., 2022).
The Fusarium genus contains one of the utmost complex and adaptive species the Eumycota. Fusarium oxysporum (Fo) species complex includes plant, animals and human pathogens and a diverse of non-pathogens (Gordon, 2017). Members of Fusarium species are ubiquitous soil borne pathogens of a wide range of horticultural and food crops which cause destructive vascular wilts, rots and damping off diseases (Bodah, 2017; Xia et al., 2021). Some Fusarium species are capable of producing mycotoxins, which are the most important natural contaminants of food and agricultural commodities (Khan et al., 2013; Kumar et al., 2016) and suspected to be implicated in numerous diseases among living beings (Barajas-Ramirez et al., 2021; Zou et al., 2022). Fusarium oxysporium (Fo) is an important soil inhabiting fungus known for its phylogenetic diversity, some of its strain are saprophytic or non-pathogenic. Nevertheless, pathogenic strains cause destructive vascular wilt disease and often limit the production of economically important agricultural commodities (Shahzad et al., 2017).
A major concern in agriculture is the phytopathological diseases caused by several pathogens for instance citrus black spot, speck rot, Fusariosis, rice blast and dessert/beer bananas by guignardia citricarpa, phacidiopycnis washingtonensis, Fusarium guttiforme, magnaporthe oryzae and Fusarium oxysporium f. sp., respectively that resulting in billions of dollars lost due to the spoilage and damage of crops (Carnielli-Queiroz et al., 2019; Faganello et al., 2017; Ndayihanzamaso et al., 2020; Sikdar et al., 2014). To protect crops from the damaging effects of phytopathogens, it has been estimated that more than 600 million dollars are spent on fungicides each year (González-Fernández et al., 2010). About 25 % of food crops are damaged by mycotoxins only (Patel et al., 2014). Soils are involved in suppressing plant diseases and microorganisms and are proposed to be involved in suppressiveness. Formulations with enzymes from bacterial showing anti-phytopathogenic activities could be used as an alternative source for biological control. A very promising candidate for biocontrol are microbial Chitinases (Zhang et al., 2001).
With reference to Chitinases hydrolyse, are the second most abundant polysaccharides into its residues. They have been classified into Glycosyl Hydrolase (GH) family 18 and 19. The GH family-18 Chitinases have been found extensively in bacteria and its existence in rectangle area (IDGIDDYE) in the amino acid sequence that specifies its association to the glycosyl hydrolase family-18. In addition, Chitinases can be endochitinases, which cleave the chitin polymers at internal sites to generate monomers of N-acetyl-D-glucosamine, and exochitinases hydrolyze the chitin into chitotriose, chitobiose and N-acetyl-D-glucosamine (Essghaier et al., 2021).
The potential use of Chitinolytic (Chitinase) enzymes makes them an attractive candidate for a wide range of biotechnological approaches, such as recycling nitrogen and carbon through chitin hydrolyzation. The Chitinase enzyme is responsible for the degradation of chitin, which is found to be produced by wide range of organisms. Though chitin is a major constituent of fungal cell walls and invertebrate exoskeleton. Bacterial Chitinases can be generated at low cost for industrial purpose. Because bacterial Chitinase is thermostable, hydrophobic in nature, with less occurrence of thermolabile residues (Dutta et al., 2021). The use of nano-materials as novel approaches has been widely studied to enhance crop yields because they offer advantages (Servin et al., 2015). They offer increase surface area with subsequent affinity for the active substance as well as greater coverage of the surface area of the plant. In relation to pros and cons of this study, nanobiofungicide can be prepared in a simple cost effective way as biohybrid nanocide materials, a new environmentally friendly nanobiofungicide against numerous fungal pathogenic organisms. Hybrids are prepared by means of the bonding between harmless antimicrobial agents; comprising Chitinases (microbial), usually known as safe (GRAS) constituents that improve the synergistic activity. The current work will describe the preparation of a new hybrid functional nan- antimicrobial with inorganic particles, biopolymers, and to test it’s effect against the most devastating fungus: Fusarium species.
2 Materials and Methods
2.1 Culture and growth condition of fungi
Pathogenic fungi Fusarium species was obtained from Mycology Lab., Plant Pathology PMAS-UAAR. Fungal mycopathogens were maintained on potato dextrose Agar (PDA) plates and stored at 4 °C for further analysis.
2.2 Isolation and identification of Chitinolytic bacteria
Chitinolytic bacteria were isolated from agricultural land located in Punjab, Pakistan and screened on a commercial Chitinase detection agar medium (Hsu & Lockwood, 1975) where additional nutrients were added: K2PO4 (0.03 %), K2HPO4 (0.07 %), MgSO4·7H2O (0.05 %), and FeSO4·7H2O in a trace amount of 0.001 %, ZnSO4 also in a trace amount of 0.0001 % and colloidal chitin 0.5 %. The pH of the medium was maintained at 7.5. Bacteria producing maximum chitin degrading zones were selected and purified. Molecular identification of the selected strain was performed using 16S rDNA 27F and 1492R primers and sequenced commercially from Macrogen Korea. The obtained sequences were used to identify the bacteria using the Basic Local Alignment Search Tool (BLAST) in National Center for Biotechnology Information (NCBI).
2.3 Production of Chitinase enzyme
2.3.1 Chitinase enzyme assay
Chitinase assay was performed following a protocol (Babashpour et al., 2012), with minor modifications. In brief; cell-free culture supernatant (0.5 ml) was taken as a crude enzyme and mixed with the substrate colloidal chitin (0.5 ml), containing 1 % (w/v) colloidal chitin in phosphate buffer (pH 7.2). The reaction mixture was incubated for 30 min at 50 °C. The reaction was stopped by the addition of 3, 5-dinitrosalicylic acid (3,5-DNS). The absorbance of the media was measured at λ at 530 nm after boiling the reaction mixture (5 min) in order to stop the reaction.
2.3.2 Optimization of production parameters
The production of chitinase with time was investigated using chitinolytic isolate. For the detection of chitinase, saline suspension of the isolate was inoculated in agar media supplemented with 1 % colloidal chitin. The suspension was incubated at 37 °C on rotary shaker flask fermentation (150 rpm). Every 6 h, the enzymatic activity for the production of N-acetyl D-glucosamine (GlcNAc), which was the indicative of degradation of colloidal chitin, was monitored. This was done by taking 1 ml of culture medium; separating the supernatant by centrifugation (10 min at 10,000 rpm) and determining its protein content enzymatically. After the optimization of time to produce Chitinase, further optimization was carried out varying a single parameter at a time. Chitinolytic microbe was cultivated in the medium with different pH such as 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, and 9.0. The incubation temperature leading to maximum Chitinase production was investigated by culturing at 25, 30, 37, 45, 50 and 55 °C. The enzyme purification was performed at 4 °C. The enzyme was produced by the shake flask fermentation procedure using optimal conditions.
2.4 Synthesis of nanoparticles
Zinc oxide nanoparticles (ZNPs) were synthesized following a procedure published elsewhere (Kiyani et al., 2019) with slight modifications. In brief, a solution 0.1 M ZnCl2 was prepared from analytical grade salt (≥98 %, Merck KGaA, Darmstadt, Germany) in deionized water. A second solution, 0.1 M NaOH, was also prepared in deionized water using NaOH pellets (Merck KGaA, Darmstadt, Germany). The NaOH (0.1 M) was then slowly added to the ZnCl2 (0.1 M) under vigorous stirring. The reaction was carried out at room temperature (25 °C) and the pH of the reaction was examined up to 12. At pH 12, precipitate (ZNPs) was observed, and it was then filtered and washed thrice with distilled water. The neutralized filtered precipitate was then heated at 300 °C overnight in an oven. The powder was finally ground using pestle and mortar and then stored for further processes.
2.4.1 Characterization of nanoparticle
The nature of ZNPs was determined commercially using powder X-ray diffraction (XRD) and the size and morphology of the NPs were observed with SEM and energy dispersive X-ray spectroscopy (EDS). The Debye-Scherrer equation (Eq. (1) was applied to measure the average size of the nanoparticles.
2.4.2 Effect of nanoparticles on Chitinase activity
Potential denaturation effect of Chitinase in presence of ZNPs was assessed by incubating Chitinase with the investigated nanoparticles (0.001 %–0.1 % concentrations) and measuring its inhibitory effects.
2.4.3 In-vitro assay of antifungal potential
The antifungal efficacy of the nanoformulation including natural active substances (Chitinase at 0.015 IU/ml and 0.03 IU/ml) was investigated against Fusarium species. using the poisoned medium technique (Kanwal et al., 2010) with different concentrations of the fungi (0.001 %, 0.005 %, 0.01 %, 0.02 %, 0.06 % and 0.1 % respectively). Non amended medium (without nanoformulations) served as control. An (8 mm) disc of actively growing a week-old culture was placed at the center of each of the plates amended with nanoparticle medium (nanoformulation) as well as plates with non-amended medium. The mycelial growth of the mycopathogens was examined at 2 days’ interval after inoculation. The inhibitory percentage of mycelial growth over control was recorded and calculated using the formula in Eq. (2):
2.4.4 Synergistic efficacy of nano-biofungicide
A potential synergistic effect of the nanoparticles on the Chitinase enzyme was quantified. In brief; the percentage of mycelial inhibition of fungus was measured after the application of Chitinase enzyme and the ZNPs separately. The percentage inhibition of mycelial growth obtained using the hybrid nano-bio fungicide (NPs + Chitinase: 0.015 IU/ml & 0.03 IU/ml) was also measured. To calculate the synergistic effect, the individual treatment of nanoparticles was subtracted from the hybrid nano-bio fungicide mycelial growth % inhibition.
2.5 Statistical analysis
Statistical analysis was carried out in triplicates; results were shown as means ± standard errors of the mean. p < 0.05 was considered statistically significant. Microsoft excel software was applied to construct the figures in the current study.
3 Results
3.1 Selection and identification of Chitinolytic bacteria
The bacterial isolates able to hydrolyse chitin were identified by them producing haloes (due to chitin hydrolysis over 5 mm) in agar culture plates. These were selected and subjected to enzyme production. In particular, the isolates from the strain AG-W3 were finally confirmed at species level, using 16S rDNA universal primers, and their sequence was obtained. The sequence analysis revealed that the isolate with Chitinolytic properties was Bacillus licheniformis and their sequence was obtained to submit at NCBI and get an accession number (Bacillus licheniformis strain AG-W3; MG662175).
3.2 Optimization parameter of Chitinase production
The effect of incubation time on Chitinase production is shown in Fig. 1. Its maximum production took place between 48 and 72 h of incubation. Thus, the incubation period of 72 h was chosen to test the effect of different parameters on the enzyme production. After the incubation, cells were harvested; cell-free supernatant was taken and assessed for Chitinase enzyme activity. The initial pH of the production media influences the availability of the metabolic ions because microorganisms are quite sensitive to proton concentrations in the media. Thus, Chitinolytic microbe was cultivated in the medium with different pH (5.5–9.0). The finding revealed that pH of the medium actively affected the growth and activity of the microbes (Fig. 2). pH 7.5 led to the maximum enzymatic activity. The incubation temperature for Chitinase production was investigated by culturing at different temperatures for instance 25 °C, 30 °C, 37 °C, 45 °C, 50 °C and 55 °C to achieve maximum Chitinase. The influence of temperature on Chitinase production exhibited maximum Chitinase activity at 45 °C. The obtained results have been depicted in Fig. 3. The extra-cellular Chitinases were produced by Bacillus licheniformis AG-W3, during different growth phases.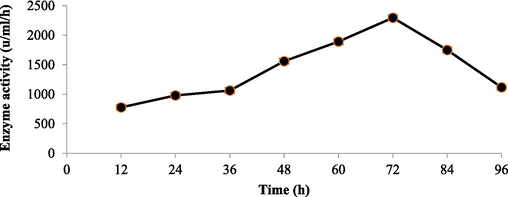
Screening of Chitinase activity in the supernatant of bacterial isolates with incubatio time (Bacillus licheniformis AG-W3).
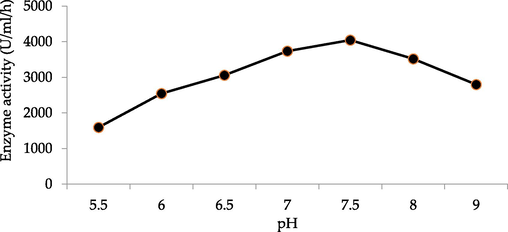
Effect of pH effects on selected hyperchitinase-producing strain (Bacillus licheniformis AG-W3).
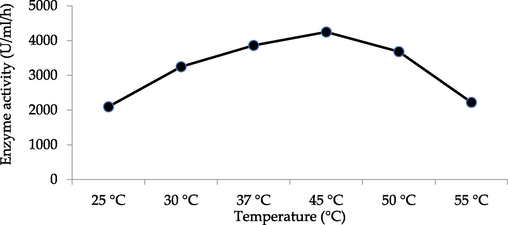
Comparative study of selected hyperchitinase producing strain (Bacillus licheniformis AG-W3) in different temperatures.
3.3 Characterization of nanoparticles
SEM analysis of ZNPs shows the spherical shape of the particles but due to the high surface energy and being in dry form they are aggregated (Fig. 4A). The diameter of the particles calculated with XRD (Eq. (1) was 46 and it agrees with the direct observations in SEM (38–76 nm). The elemental composition of the nanoparticles was observed by EDS (Fig. 4B). The zinc, oxygen with traces of copper amounts have been presented in Table 1. Analysis of ZNPs by XRD showed the diffraction pattern produced from peak intensities, related to size and shape. The XRD 2 theta degrees signals were at positions; 31.66, 34.35, 36.19, 56.45, and 62.73 of the spectra respectively (Fig. 5), the d-spacing from this pattern confirm the formation of ZnO nanoparticles.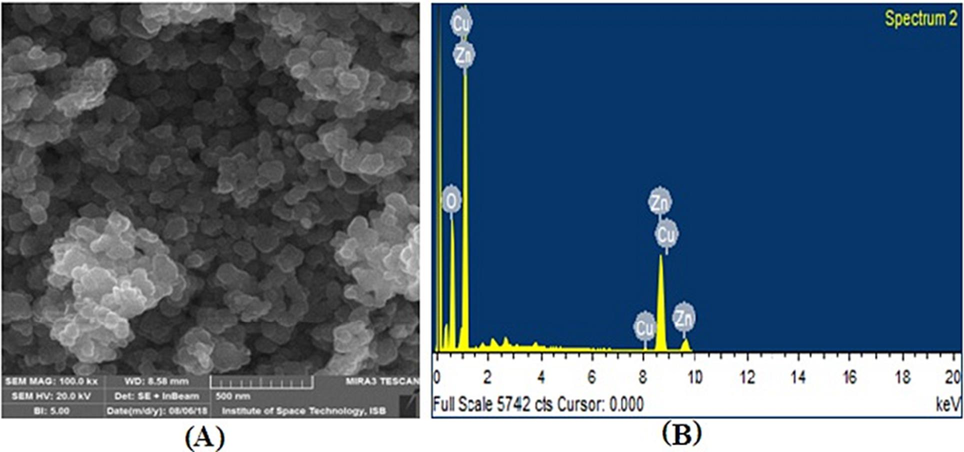
(A) SEM image of zinc oxide nanoparticles (B) EDX spectrum of a representative ZNPs.
Element
(%)
Atomic (%)
Zinc
71.85
38.97
Oxygen
27.33
60.57
Copper
0.82
0.46
Total
100
100
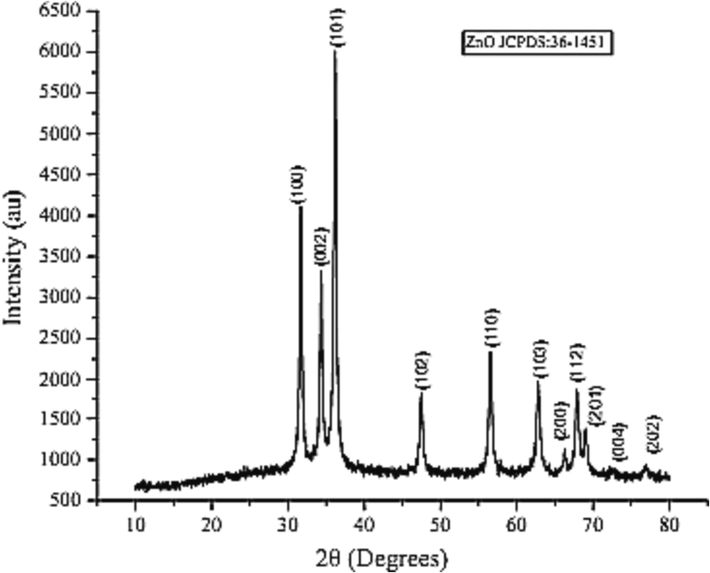
X-ray powder diffractogram of the prepared ZNPs.
3.4 Inhibitory effect of NPs on Chitinase activity
The purified enzyme was pre-incubated with the ZNPs in phosphate buffer with a concentration ranging from 0.001 to 0.1 % (mass of ZNPs /mass media) for 1 h without substrate. The residual activity of the purified enzyme was estimated by adding colloidal chitin as substrate with standard assay protocol. In presence of 0.001 % ZNPs Chitinase showed ̴ 100 % residual activity, followed by a gradual decline as the concentration of nanoparticles increased. At concentrations <0.001 % ZNPs, it was observed that nanoparticles have no significant inhibitory effect on Chitinase activity. The residual activity of Chitinase was high and moderate in the presence of ZNPs with concentrations 0.001 %-0.01 %. Nonetheless moderate inhibition effect (∼9%) was observed at 0.1 % ZNP concentration as shown in Fig. 6.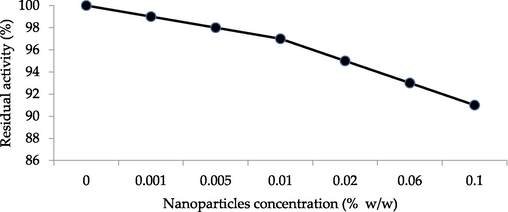
Inhibitory effect of nanoparticles on Chitinase residual activity.
3.5 In vitro antifungal bioassay
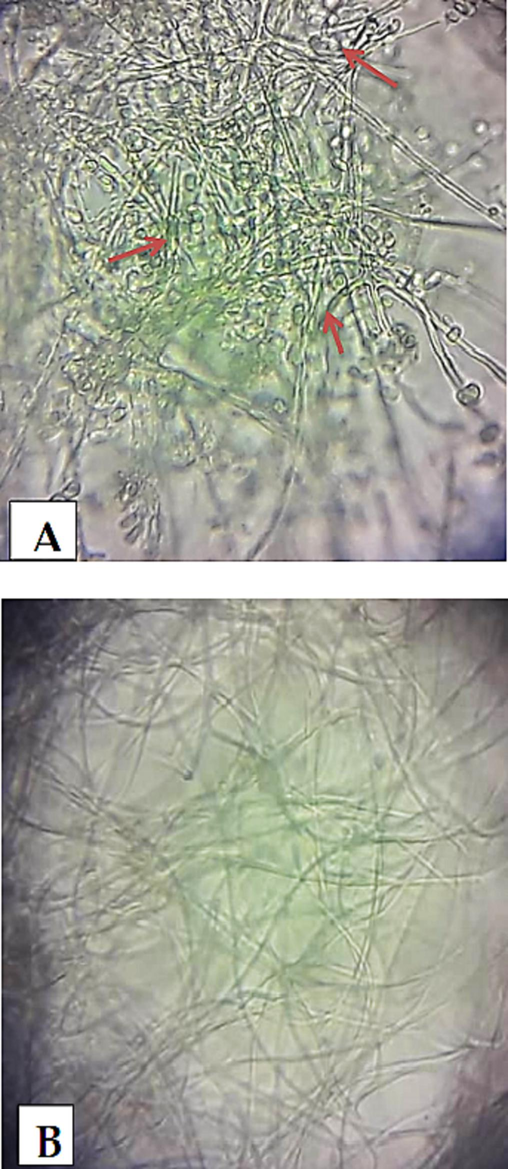
During the in vitro bioassay, it was observed that mycelial growth of Fusarium species was comprehensively controlled by 0.06 and 0.1 % concentrations of ZNPs keeping the enzyme (Chitinase) concentration either at 0.015 or 0.03 IU/ml. Mycelial growth inhibition was observed in Fusarium species exhibiting a dose dependency effect (Table 2). The maximum inhibitory effects (89.5 %) were recorded at 0.1 % ZNPs concentration with 0.03 IU/ml of Chitinase, while the minimum inhibitory effect was observed at 0.001 % concentration. ZNPs concentrations 0.001–0.01 % showed a gradual increase in growth inhibition. Thickening and rupturing of hyphal cell wall along with constriction of hyphal diameter were observed. Sporulation and low hyphal growth were also noticed with a compound microscope (Fig. 7aA & B). Rupture of the fungal cell wall was observed in SEM. (Fig. 7bC).
NPs (%)
Mycelial growth inhibition%
0
0.015 IU/ml Chitinase
0.03 IU/ml Chitinase
0
0
5.0
10.0
0.001
34.8 ± 2.2
45.8 ± 2.2
62.5 ± 2.1
0.005
48.5 ± 3.7
62.3 ± 1.7
76.8 ± 1.1
0.01
56.0 ± 0.8
72.2 ± 3.1
84.0 ± 1.8
0.02
61.3 ± 0.2
79.6 ± 0.2
87.3 ± 0.2
0.06
65.4 ± 0.1
81.1 ± 1.0
87.5 ± 0.4
0.1
69.3 ± 0.7
83.1 ± 3.2
89.1 ± 1.4
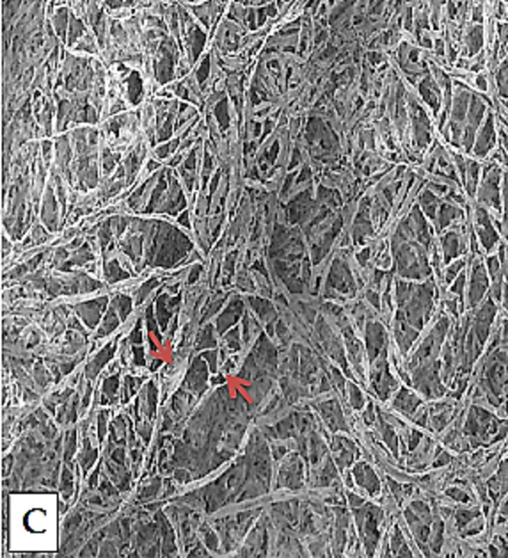
Antifungal bioassay presenting rupture of the fungal cell wall designated with arrows (Electron microscopic studies).
3.6 Synergistic effect of Chitinase & inorganic nanoparticles
The effect of the metal nanoparticles with Chitinase, constituting the nanobiofungicide, is reported in Table 3. It was observed that at low concentration of nanoparticles the synergistic effect gradually rises with a maximum of 18.3 % inhibition (in the case of 0.005 % ZNPs and 0.03 IU/ ml Chitinase). At moderate concentration the synergistic effect retains, as the concentration of ZNPs further increases. At 0.06 % ZNPs, the inhibitory effect shows a gradual decline although the significance of the differences is yet to be established with replicate analysis. The results in Table 2 also revealed that Chitinase enzyme concentration also plays an important role in synergistic mycelial growth inhibition, as shown from the results, lower synergistic effect at lower enzyme concentration (0.015 IU/ml) while gradual increased synergistic effect with higher concentration (0.03 IU/ml).
NPs (%)
Inhibition using 0.015 IU/ml (%)
Inhibition using 0.03 IU/ml (%)
0
5.0
10.0
0.001
6.0
17.7
0.005
8.8
18.3
0.01
11.2
18.0
0.02
13.6
16.0
0.06
10.7
12.1
0.1
9.8
10.2
4 Discussion
In the current study, the Chitinolytic organisms were isolated from water and soil samples of diverse habitats, such as agricultural fields, springs, fish markets, and domestic samples. Though Chitinolytic microbes are ubiquitous in distribution, nevertheless, different studies showed their isolation from particular sources such as cotton fields (Jha et al., 2016), from potato fields (Servin et al., 2015), freshwater ponds for shrimp (Hsu and Lockwood, 1975), degraded stalk of mushrooms (Kiyani et al., 2019)coastal soil enriched with crab shells (Kanwal et al., 2010), lily plant (Jha et al., 2016), from marine environment Marine bacterial Chitinase as sources of energy, eco-friendly agent, and industrial biocatalyst (Jahromi & Barzkar, 2018), red palm weavil’s gut (Khiyami & Masmali, 2008) and intestine of the South American sea lion (Konagaya et al., 2006).
The profile of enzyme production of the isolates during the present research was assessed for five days, by cultivating in a minimum salt medium along with colloidal chitin, incubated under shaking conditions. During this study, the maximum enzyme production was observed on 3rd day of incubation. A rise in Chitinase production was obtained during the exponential growth phase to the stationary growth phase. Previous investigation results reflect that the incubation time is governed by cultural characteristics. Such type of investigation reported that enzyme production depends on the growth rate of microbial culture (Chakrabortty et al., 2012). As observed from the level of protein, it was evident that Chitinase production was indeed correlated to the growth of the microbial culture in the selected medium. Previous reports demonstrated that a rise in Chitinase production by Streptomyces hygroscopicus is shown from the exponential growth phase to the stationary growth phase (Priya et al., 2011). Similar results were obtained from Bacillus circulans (n°4.1) on the 4th day (Wiwat et al., 1999). Maximum Chitinase production from Streptomyces spp. NK1057 was been reported after the 5th day of incubation (Nawani & Kapadnis, 2004) and Beauveria bassiana (Suresh & Chandrasekaran, 1998).
The pH of the medium affects the growth and activity of the microorganisms. The results of an earlier investigation found that Bacillus subtalis produce maximum Chitinases at a pH 7–8. Previous studies reflect the hypothesis that most bacterial species produce Chitinase at neutral pH or slightly alkaline such as certain Bacillus strains and Pseudomonas aeruginosa K-187, these produced maximum Chitinase at pH 7 (Ghorbel-Bellaaj et al., 2012). Previous studies suggested that A. xylosoxydans (Vaidya et al., 2001), Micrococcus sp. AG84 (Kuddus & Ahmad, 2013), Serratia marcescens XJ-01 (J.-L. Xia et al., 2011) and Aeromonas spp. JKI (Al-Ahmadi et al., 2008), produced maximum Chitinases under alkaline conditions. B. pabuli K1 produced maximum Chitinase at pH 8 (Frändberg & Schnürer, 1994). B. laterosporus produces Chitinase at pH 8 (Shanmugaiah et al., 2008).
During this investigation, the influence of temperature on Chitinase production exhibited maximum Chitinase activity at 45 °C. Temperature also influenced protein denaturation, cell growth, and enzyme inhibition, thus it plays a significant role in biological processes (Chakrabortty et al., 2012). B. licheniformis show maximum Chitinase activity at 50 °C (Toharisman et al., 2005) whereas Tsujibo et al. have reported the maximum Chitinase activity at 50 °C by Streptomyces thermoviolaceous OPC-520 (Tsujibo et al., 1993), which is same as that B. Licheniformis. Strain JS. Bacillus p. BG-11 produces Chitinases at 50 °C (Bhushan, 2000). On the other hand, Chitinase productions from Serratia marcescenses at 30 °C were reported by Kannan and co-workers (Kannan et al., 2010). It is unclear why some mesophiles have evolved thermostable enzymes, but the such strategy may enable energy conservation through a decreased need for enzyme synthesis due to increased enzymatic stability (Yeoman et al., 2010). Thermostable enzymes can be obtained from thermophile as well as mesophilic organisms. Thermostable enzymes used by industry are still produced from mesophiles and commercial enzymes thermophiles are still scarce (Coolbear et al., 1992).
Chitinase with alkaline pH is considered to have major potential in the biological control of insect pests. Chitinases with better stability and activity in these conditions can be used in synergism with other biocontrol agents. Although alkaline Chitinases are also considered useful in the management of Chitinous wastes, generated by seafood manufacturing industries (Nawani & Kapadnis, 2003). Metals ions may result in enhancing the enzyme activity by acting as a binding between enzyme and substrate, combing with both, thus holding the substrate at the active site of the enzyme. The effect of metal ions may also be due to the participation of sulfhydryl groups in the active site of the enzyme (Tsujibo et al., 1993).
During the present study, the application of isolated Chitinase enzyme as an antifungal agent showed the results of approximately10% mycelial growth inhibition. While ZNPs application with different concentrations revealed a maximum fungal mycelial growth inhibition of 69.3 % by using the ZNPs concentration (0.1 %). The results of ZNPs used as an antimicrobial agent during earlier studied, revealed a significant inhibitory effect on the growth of B. cinerea by disturbing cell features and causing mat deformity while showing the inhibitory effect on P. expansum conidiophore and conidia development (He et al., 2011). ZNPs are far less toxic to plants and plant beneficial soil micro-organisms, could be better than AgNPs in N mediated plant safety against fungal pathogens (Dimkpa et al., 2013).
The ZNPs along with the Chitinase enzyme (0.03 IU/ml) exhibited a maximum of 89.9 % mycelial growth inhibition, which was recorded during this project. Nanoformulations can advance prevention of pest defense, stability of pesticides in the field, and be benign to both plants and animals, while killing the pests with new modes of action. This formulation might be prepared with low cost (Athanassiou et al., 2018; Benelli et al., 2016).
ZNPs along with Chitinase enzyme exhibit a maximum 89.5 % mycelial growth inhibition. Previous work demonstrated that ZNPs inhibited the growth of B. cinerea by disturbing cell features and causing mate deformity ultimately resulting in the death of fungal hyphae (He et al., 2011; Nuruzzaman et al., 2016). Similar results were obtained by using a 0.1 % concentration of NCPs, against A. alternate, M. phaseolina and R. solani, that showed 89.5 %, 63.0 %, and 60.1 % growth inhibition, respectively (Saharan et al., 2013). Copper-chitosan nanoparticles inhibited mycelial growth and scolorotia formation in R. solani and S. rolfsii (Rubina et al., 2017). Zataria multiflora (Essential Oil) encapsulated in chitosan nanoparticles with a controlled release reduced the occurrence of B. cinerea (Mohammadi et al., 2015). The study therefore confirms the fungicidal nature of the nanoformulations and the potential uses of these nanoformulations as an alternative to chemical fungicides for management of mycopathogenic Fusarium species. However, further studies should be conducted to evaluate the efficacy under field conditions.
5 Conclusions
This work has developed zinc oxide nanoparticles in combination with a natural biologically active substance (Chitinase) as a hybrid composite against phytopathogens. This is a new alternative of formulating pesticides. In this case, an inhibition of up to 89.1 % mycelial growth on mycopathogenic fungi and 18.3 % inhibition of Fusarium species were achieved. Future work should address the impact of such formulation in the ecosystem, and study its time-controlled released and stability of the effect and changes in the composite with temperature and solar irradiation. There is a need to discover eco-friendly hybrid nanofungicides as an alternative to conventional fungicides. New green nanobiofungicide can be produced with a suitable synergistic approach, to protect the soil and surrounding water, control their dispersion and targeted toxicity, to protect crops and enhance their yield. For future recommendation, it is significant to examine the synergistic influence of one of these mixtures in terms of antimicrobial growth, lowering pesticide utilization and delaying development of resistance. Required extensive research on larger scale to establish potential ecofriendly nano-based products.
Acknowledgements
The authors would like to thank the Researchers Supporting Project Number (RSP2023R35), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Optimization of medium and cultivation conditions for chitinase production by the newly isolated: Aeromonas sp. Biotechnology. 2008;7(2):266-272.
- [Google Scholar]
- Biodiversity of Bacillus subtilis group and beneficial traits of Bacillus species useful in plant protection. Romanian Biotechnol. Lett.. 2015;20(5):10737-10750.
- [Google Scholar]
- Nanoparticles for pest control: current status and future perspectives. J. Pest. Sci.. 2018;91:1-15.
- [Google Scholar]
- Characterization of a chitinase (Chit62) from Serratia marcescens B4A and its efficacy as a bioshield against plant fungal pathogens. Biochem. Genet.. 2012;50:722-735.
- [Google Scholar]
- Mycotoxins in foods, from the field to the plate: a review. Int. Food Res. J.. 2021;28(2)
- [Google Scholar]
- Mosquito vectors and the spread of cancer: an overlooked connection? Parasitol. Res.. 2016;115:2131-2137.
- [Google Scholar]
- Production and characterization of a thermostable chitinase from a new alkalophilic Bacillus sp. BG-11. J. Appl. Microbiol.. 2000;88(5):800-808.
- [Google Scholar]
- Root rot diseases in plants: a review of common causal agents and management strategies. Agric. Res. Technol. Open Access J. 2017;5:555661
- [Google Scholar]
- A rapid and reliable method for molecular detection of Fusarium guttiforme, the etiological agent of pineapple fusariosis. Braz. Arch. Biol. Technol.. 2019;62
- [Google Scholar]
- Optimization of process parameters for chitinase production by a marine isolate of Serratia marcescens. Int. J. Pharm. Biol. Sci.. 2012;2(2):8-20.
- [Google Scholar]
- The enzymes from extreme thermophiles: bacterial sources, thermostabilities and industrial relevance. Enzymes Products Bacteria Fungi Plant Cells 1992:57-98.
- [Google Scholar]
- Current status and recent developments in biopesticide use. Agriculture. 2018;8(1):13.
- [Google Scholar]
- Nano-biopesticides: present concepts and future perspectives in integrated pest management. Adv. Nano-Fertilizers Nano-Pesticides Agric. 2021:1-27.
- [Google Scholar]
- Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals. 2013;26:913-924.
- [Google Scholar]
- In silico characterization of bacterial chitinase: illuminating its relationship with archaeal and eukaryotic cousins. J. Genet. Eng. Biotechnol.. 2021;19:1-11.
- [Google Scholar]
- Potentialities and characterization of an antifungal chitinase produced by a halotolerant Bacillus licheniformis. Curr. Microbiol.. 2021;78:513-521.
- [Google Scholar]
- Molecular diagnosis of Guignardia citricarpa in asymptomatic sweet orange tissue. Rev. Bras. Frutic.. 2017;39
- [Google Scholar]
- Chitinolytic properties of Bacillus pabuli K1. J. Appl. Bacteriol.. 1994;76(4):361-367.
- [Google Scholar]
- Assessment of five pesticides as endocrine-disrupting chemicals: effects on estrogen receptors and aromatase. Int. J. Environ. Res. Public Health. 2022;19(4):1959.
- [Google Scholar]
- Chitin extraction from shrimp shell waste using Bacillus bacteria. Int. J. Biol. Macromol.. 2012;51(5):1196-1201.
- [Google Scholar]
- Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol.. 2017;55:23-39.
- [Google Scholar]
- Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res.. 2011;166(3):207-215.
- [Google Scholar]
- Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl. Microbiol.. 1975;29(3):422-426.
- [Google Scholar]
- Marine bacterial chitinase as sources of energy, eco-friendly agent, and industrial biocatalyst. Int. J. Biol. Macromol.. 2018;120:2147-2154.
- [Google Scholar]
- Characterization of extracellular chitinase produced from Streptomyces rubiginosus isolated from rhizosphere of Gossypium sp. Cogent Food Agric.. 2016;2(1):1198225
- [Google Scholar]
- Optimization of chitinase production from Serratia marcescens-A classical approach. Biological Segment. 2010;1(1):1-23.
- [Google Scholar]
- Antifungal activity of flavonoids isolated from mango (Mangifera indica L.) leaves. Nat. Prod. Res.. 2010;24(20):1907-1914.
- [Google Scholar]
- Analysis of aflatoxins in nonalcoholic beer using liquid–liquid extraction and ultraperformance LC-MS/MS. J. Sep. Sci.. 2013;36(3):572-577.
- [Google Scholar]
- Characteristics of thermostable chitinase enzymes of Bacillus licheniformis isolated from Red Palm Weavil Gut. Aus J Basic Appl Sci. 2008;2(4):943-948.
- [Google Scholar]
- Antioxidant and anti-gout effects of orally administered zinc oxide nanoparticles in gouty mice. J. Trace Elem. Med Biol.. 2019;56:169-177.
- [Google Scholar]
- Purification and characterization of chitinases from Clostridium sp. E-16 isolated from the intestinal tract of the South American sea lion (Otaria flavescens) Lett. Appl. Microbiol.. 2006;43(2):187-193.
- [CrossRef] [Google Scholar]
- Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J. Genet. Eng. Biotechnol.. 2013;11(1):39-46.
- [Google Scholar]
- Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT-Food Sci. Technol.. 2016;69:522-528.
- [Google Scholar]
- Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol.. 2015;28:73-80.
- [Google Scholar]
- High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One. 2010;5(3):e9754.
- [Google Scholar]
- Farmers exposed to pesticides have almost five times more DNA damage: a meta-analysis study. Environ. Sci. Pollut. Res.. 2022;29(1):805-816.
- [Google Scholar]
- Nawani, N., Kapadnis, B., 2003. Chitin degrading potential of bacteria from extreme and moderate environment.
- Production dynamics and characterization of chitinolytic system of Streptomyces sp. NK1057, a well equipped chitin degrader. World J. Microbiol. Biotechnol.. 2004;20:487-494.
- [Google Scholar]
- The development of a multiplex PCR assay for the detection of Fusarium oxysporum f. sp. cubense lineage VI strains in East and Central Africa. Eur. J. Plant Pathol.. 2020;158:495-509.
- [Google Scholar]
- Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J. Agric. Food Chem.. 2016;64(7):1447-1483.
- [Google Scholar]
- Agronanotechnology for plant fungal disease management: a review. Int. J. Curr. Microbiol. App. Sci.. 2014;3(10):71-84.
- [Google Scholar]
- Production of chitinase by Streptomyces hygroscopicus VMCH2 by optimisation of cultural conditions. Int. J. Pharm. Bio. Sci. 2011;2(2)
- [Google Scholar]
- Synthesis and characterization of chitosan–copper nanocomposites and their fungicidal activity against two sclerotia-forming plant pathogenic fungi. J. Nanostruct. Chem.. 2017;7:249-258.
- [Google Scholar]
- Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol.. 2013;62:677-683.
- [Google Scholar]
- A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res.. 2015;17:1-21.
- [Google Scholar]
- Plant growth-promoting endophytic bacteria versus pathogenic infections: an example of Bacillus amyloliquefaciens RWL-1 and Fusarium oxysporum f. sp. lycopersici in tomato. PeerJ. 2017;5:e3107.
- [Google Scholar]
- Optimization of cultural conditions for production of chitinase by Bacillus laterosporous MML2270 isolated from rice rhizosphere soil. Afr. J. Biotechnol.. 2008;7(15)
- [Google Scholar]
- Development of PCR assays for diagnosis and detection of the pathogens Phacidiopycnis washingtonensis and Sphaeropsis pyriputrescens in apple fruit. Plant Dis.. 2014;98(2):241-246.
- [Google Scholar]
- Utilization of prawn waste for chitinase production by the marine fungus Beauveria bassiana by solid state fermentation. World J. Microbiol. Biotechnol.. 1998;14:655-660.
- [Google Scholar]
- Purification and characterization of a thermostable chitinase from Bacillus licheniformis Mb-2. World J. Microbiol. Biotechnol.. 2005;21:733-738.
- [Google Scholar]
- Cloning, sequence, and expression of a chitinase gene from a marine bacterium, Altermonas sp. strain O-7. J. Bacteriol.. 1993;175(1):176-181.
- [Google Scholar]
- Production of chitinase and its optimization from a novel isolate Alcaligenes xylosoxydans: potential in antifungal biocontrol. World J. Microbiol. Biotechnol.. 2001;17:691-696.
- [Google Scholar]
- Purification and characterization of chitinase from Bacillus circulans No. 4.1. Curr. Microbiol.. 1999;39:134-140.
- [Google Scholar]
- G-Quadruplex-Probing CRISPR-Cas12 assay for label-free analysis of foodborne pathogens and their colonization in vivo. ACS Sensors. 2021;6(9):3295-3302.
- [Google Scholar]
- Production of Chitinase and its Optimization from a Novel Isolate Serratia marcescens XJ-01. Indian J. Microbiol.. 2011;51:301-306.
- [Google Scholar]
- Thermostable enzymes as biocatalysts in the biofuel industry. Adv. Appl. Microbiol.. 2010;70:1-55.
- [Google Scholar]
- Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology. 2001;91(2):204-211.
- [Google Scholar]
- Hydrophilic co-assembly of wheat gluten proteins and wheat bran cellulose improving the bioavailability of curcumin. Food Chem.. 2022;397:133807
- [Google Scholar]