Assessing the efficacy of Thidiazuron, 6- Benzylamino purine and Kinetin in modulating flower senescence in cut spikes of Consolida ajacis (L.) Schur.
⁎Corresponding authors. inayatullahtahirku@gmail.com (Inayatullah Tahir), parvaizbot@yahoo.com (Parvaiz Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The current study assesses the efficacy of thidiazuron and cytokinins (6- benzylamino purine and kinetin) in modulating flower senescence in excised spikes of Consolida ajacis. The spikes harvested at commercial maturity were transported to laboratory and arranged into multiple sets. Each set of spikes was subjected to different treatments of thidiazuron (TDZ), benzylamino purine (BAP) and kinetin (KIN) viz., 25 µM, 50 µM, 75 µM and 100 µM. One set of spikes designated as control was kept in distilled water. Inclusion of these growth regulators significantly improved the postharvest longevity of C. ajacis cut spikes. The enhancement in display life was proportionate with upsurge in the activity of superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT). Flowers held in different test solutions of BAP, KIN and TDZ, exhibited higher values of sugars, phenols and proteins. Furthermore, these growth regulators minimized membrane leakiness by reducing lipoxygenase activity (LOX) and improved membrane stability. Among the various growth regulators tested, 50 µM TDZ was found to be highly efficacious in mitigating flower senescence in C. ajacis cut spikes as compared to KIN and BAP.
Keywords
Postharvest longevity
thidiazuron
growth regulators
sugars
catalase
membrane stability
1 Introduction
Flower senescence involves programmed death of petal tissues resulting in loss of their function. It is viewed as end stage of development characterised by wilting, withering and color fading of petal tissues (Fischer, 2012). Petal senescence is accompanied by leakage of membranes, decrease in sugars, proteins and antioxidant enzymes (Ul Haq et al., 2021). It is a key postharvest attribute affecting the marketability of cut flowers. Postharvest senescence is regulated by various factors like, humidity, temperature, sugar content, hydration status, light and phytoharmones (Reid, 2009). The optimum levels of all these factors improve postharvest longevity and delay in the onset of senescence. Moreover, understanding the key biochemical mechanisms governing flower senescence is crucial to modulate postharvest performance of cut flowers. Senescence is influenced by the interaction of various plant harmones including cytokinins, ethylene and abscisic acid. Typically, ethylene and ABA facilitate senescence, while as cytokinins retard senescence (Iqbal et al., 2017). Cytokinins retard senescence in ethylene sensitive as well as ethylene insensitive flower systems. Cytokinins postpone onset of senescence by lowering ethylene output in ethylene responsive flower systems (Van Doorn and Woltering, 2008). While as, cytokinins inhibit sensescence initiation in ethylene insensitive flowers by decreasing ABA synthesis (Trivellini et al., 2015). Cytokinins rather prevent instigation of senescence by promoting sugar accumulation and reducing memebrane leakage of petal tissues (Iqbal et al., 2017). Furthermore, cytokinins boost antioxidant capacity by augmenting activity of antioxidant enzymes and reduce lipid peroxidation by neutralizing free radicals (Xu et al., 2012). Cytokinins prevent protein degradation and thus maintain protein content of the petal tissues (Chen et al., 2018). Additionally, cytokinins enhance phenolic enrichment, which activates a defensive mechanism against postharvest senescence (Schmitzer et al., 2010). Cytokinin content and senescence has been reported to be inversely related in some studies (Hönig et al., 2018). However, ethylene levels have been found to be positively correlated with the cytokinin breakdown. Increased ethylene levels trigger cytokinin breakdown via O- glycosylation, resulting in senescence (Chang et al., 2003). Reportedly, all the three growth regulators have been found to improve postharvest life in Iris germanica L. However, phenyl urea type cytokinin (TDZ) has shown more promising effects than adenine type cytokinins (BAP and KIN) (Ahmad et al., 2018).
C. ajacis is beautiful ornamental of Ranunculaceae. It has immense potential as cut flower because of its ravishing spikes which adds gracefulness to the garden and rooms. However, increased sensitivity to ethylene triggers early senescence of its flowers. Although, application of ethylene antagonists have positively ameliorated its display life (Shahri and Tahir 2010), nevertheless, the role of cytokinins and TDZ as modulators of senescence in C. ajacis flowers is yet to be examined. Therefore, the present research aims to evaluate the role of these growth regulators in orchestrating antioxidant and biochemical mechanisms underlying senescence of C. ajacis cut spikes.
2 Materials and methods
2.1 Plant material
Spikes of C. ajacis were collected from the field plots at commercial maturity (Fig. 1) and carried to laboratory. The spikes were recut to size of 30 cm and placed in 100 ml flasks containing test solutions of various growth regulators viz, TDZ, BAP, and KIN. Each growth regulator comprised of four concentrations viz, 25 µM, 50 µM, 75 µM and 100 µM. The treatment effects were compared with control set of spikes. All the concentrations including control consisted of 10 replicates. The different biochemical parameters were analyzed on day 2 and day 5 of transfer of spikes to their respective test solutions. The experiment was performed under controlled conditions with an average temperature of 25 ± 2 °C, 12 h light period a day and a relative humidity (RH) of 60 ± 10 %.

- One day before anthesis stage of C. ajacis flowers used for the present study.
2.2 Postharvest longevity and flower diameter
The average postharvest longevity was calculated from day of transfer to test solutions till senescence of an average 70 % florets on each spikes. The floral diameter was measured as an average of two perpendicular distances across a flower on day 2nd and day 5th of the experiment.
2.3 Membrane stability index
Membrane stability index (MSI) was estimated by a method of Sairam (1994) as seepage of solutes from floral tissues. MSI was calculated as: MSI = [1- C1/C2] * 100
C1 indicates conductivity of floral tissues dipped in distilled water at 25 °C for 30 min, while as C2 indicates conductivity of floral tissues dipped in distilled water at 100 °C for 15 min.
2.4 Estimation of sugars, amino acids and phenols
1 g of tepal tissue was fixed from each concentration in 70 % hot ethanol. The tepal tissues were subsequently macerated and centrifuged. The quantification of α- amino acids, total sugars, reducing sugars, non -reducing sugars and total phenols was performed by taking suitable volume of aliquot from the supernatant. α-amino acids were estimated by Rosen (1957) method using glycine as standard. Swain and Hills method (1959) was used for estimation of total phenols with Gallic acid acting as standard. Reducing sugars were determined by Nelson (1944) method using glucose as standard. For calculating total sugars, invertase was used to convert non-reducing sugars to reducing sugars. The amount of non– reducing sugars as evaluated as difference between total and reducing sugars.
2.5 Protein estimation
Proteins were quantified by macerating 1g of floral tissue in 100 mM phosphate buffer (pH 7.2) containing NaCl (150 mM), EDTA (1mM), Triton-X-100 (10%), glycerol (10%), PVP (10%) and Dithiotheritol (1mM). The mixture was centifuged at 12000 g for 15 minutes at 4°C. The proteins were quantified by Lowry et al. method (1951) by taking suitable volume of aliquot from the supernatant.
2.6 Enzyme assay
2.6.1 Superoxide dismutase activity
The activity of superoxide dismutase enzyme was estimated by Dhindsa et al. method (1981). The SOD activity was expressed as units min−1 mg−1 protein.
2.6.2 Catalase activity
The activity of catalase was assayed by the method of Aebi (1984) and was expressed as μmol H2O2 red. min−1 mg−1 protein.
2.6.3 Ascorbate peroxidase activity
Ascorbate peroxidase activity was determined by a method of Chen and Asada (1989) and was expressed as μmol min−1 mg−1 protein.
2.6.4 Lipoxygenase activity
Lipoxygenase activity was determined by the method of Axelrod et al. method (1981) and the units were expressed as μmol min−1 mg−1 protein.
2.7 Experimental design and statistical analysis
Complete randomized design was followed during the experiment and the statistical analysis was performed through SPSS software. The treatments means were considered significant at (P < 0.05) according to Duncan’s test.
3 Results
3.1 Postharvest longevity and floral diameter
Individual spikes of C. ajacis lasted for about 7 days in the distilled water. Flower senescence in C. ajacis involves turgidity loss, wilting and ultimately abscission of tepals Fig. 2 (A-C). Flowers held in optimum concentration of TDZ (50 µM), BAP (50μΜ) and KIN (75 µM) showed maximum increase in longevity by 123 %, 68.4 % and 91.7 % respectively (Fig. 3A). In addition, flowers supplied with test solutions had significantly higher flower diameter as compared to control. The flower diameter increased by 25.6 %, 12.95 and 16.2 % respectively in TDZ (50 µM), BAP (50 µM) and KIN (75 µM) treated flowers. Moreover, flower diameter showed a maximum increase up to day 2 and then decreased gradually from day 2 to day 5 (Fig. 3B).
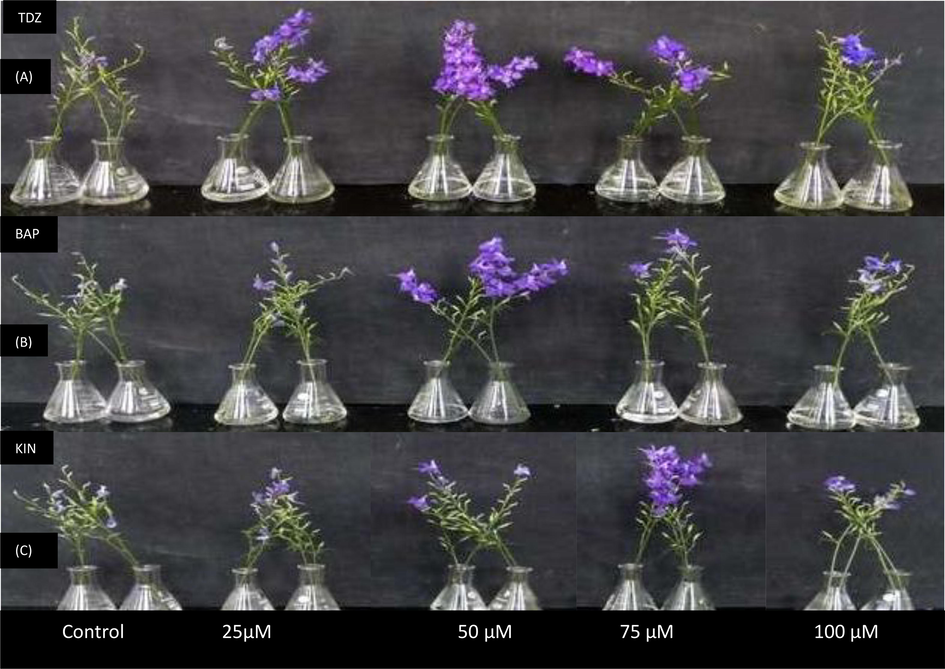
- (A-C). Photograph depicting efficacy of TDZ, BAP and KIN in accentuating postharvest longevity of C. ajacis cut spikes on day 13, day 11 and day 12 of transfer to respective test solutions.
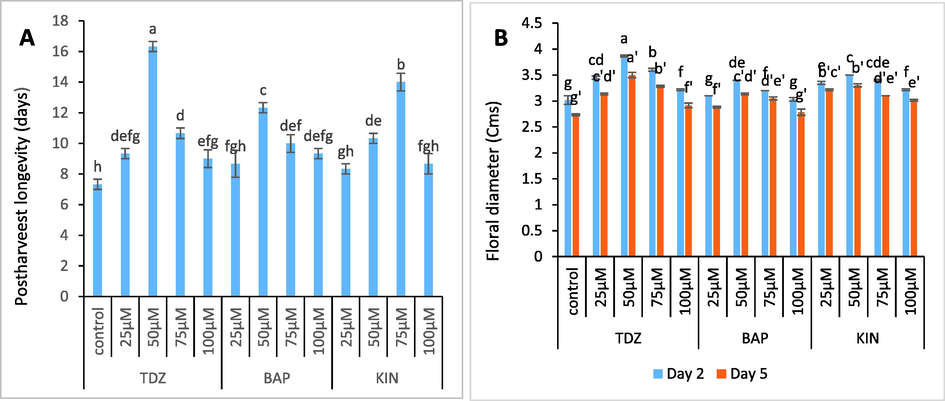
- (A-B). Graphs showing the effect of TDZ, KIN and BAP treatments on postharvest longevity and floral dimensions of C. ajacis flowers. Each value is a mean of 3 replicates with standard error S.E. indicated by bars. Treatments indicated by different superscripts differ significantly according to Duncan’s test at p ≤ 0.05.
3.2 Membrane stability index (MSI)
In the present investigation flowers held in test solutions of TDZ, BAP and KIN exhibited higher MSI values compared to the control. Decrease in MSI values in control treatment relative to treated samples suggests loss of membrane integrity with advancement in flower development, which eventually ends up with disorganization of subcellular organelles. Among the selected growth regulators, maximum increase in MSI values was recorded in 50 µM TDZ (191 %) treated samples as compared to75µM KIN (143 %) and 50 µM BAP (160 %). MSI values exbhited a decline towards the later stages of experiment. (Fig. 4).
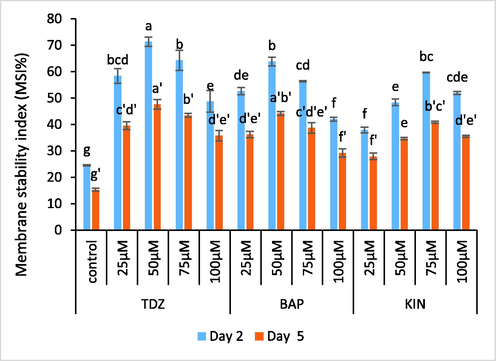
- Graphs showing the effect of TDZ, KIN and BAP treatments on MSI of C. ajacis flowers. Each value is a mean of 3 replicates with S.E. indicated by bars. Treatments indicated by different superscripts differ significantly according to Duncan’s test at p ≤ 0.05.
3.3 Soluble proteins and α- amino acids
The higher protein concentration was recorded in spikes held in test solutions of BAP, KIN and TDZ. Meanwhile, α-amino acid concentration was found lower in the spikes held in these test solutions. Therefore, α- amino acids were found to be maximum in spikes held in distilled water. The percent increase in protein concentration of floral tissues held in TDZ (50 µM), BAP (50 µM) and KIN (75 µM) was found to be 74.28 %, 25.7 % and 37.14 % respectively. Likewise, the concentration of α- amino acids for these optimum concentrations was found to be 60.7 %, 51.7 % and 53.57 %. The effectiveness of these growth regulators decreased towards end stages of flower development resulting decrease in the soluble proteins with corresponding increase in α- amino acids Fig. 5 (A-B).
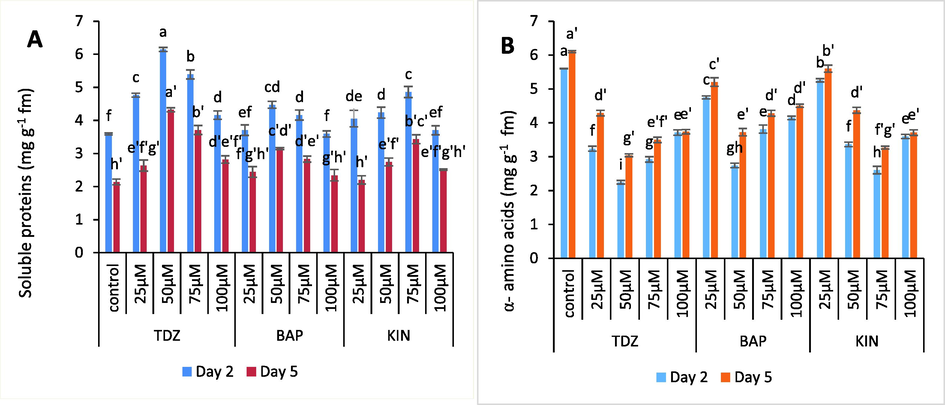
- (A-B). Graphs showing the effect of TDZ, KIN and BAP treatments on protein and α-amino acid content of C. ajacis flowers. Each value is a mean of 3 replicates with S.E. indicated by bars. Treatments indicated by different superscripts differ significantly according to Duncan’s test at p ≤ 0.05.
3.4 Phenols and sugars
As compared to untreated flowers, higher phenolic enrichment occurred in tepal tissues held in BAP, KIN and TDZ solutions, with maximum in TDZ treated samples. The phenolic concentration of tepal tissues held in TDZ (75 µM), BAP (50 µM) and KIN (50 µM) increased by 253 %, 115.3 % and 215 % respectively (Fig. 6A). Likewise, the tepal tissues held in 50 µM TDZ, 50 µM BAP and 75 µM KIN maintained higher sugar content as compared to control treatment Fig. 6 (B-D). The sugar concentration for these optimum treatments was found to be 66.2 %, 34.9 % and 60.2 % respectively. Moreover, TDZ treated tepal tissues retained more sugar content as compared to BAP and KIN. The concentration of both sugars and phenols declined towards end of experiment.
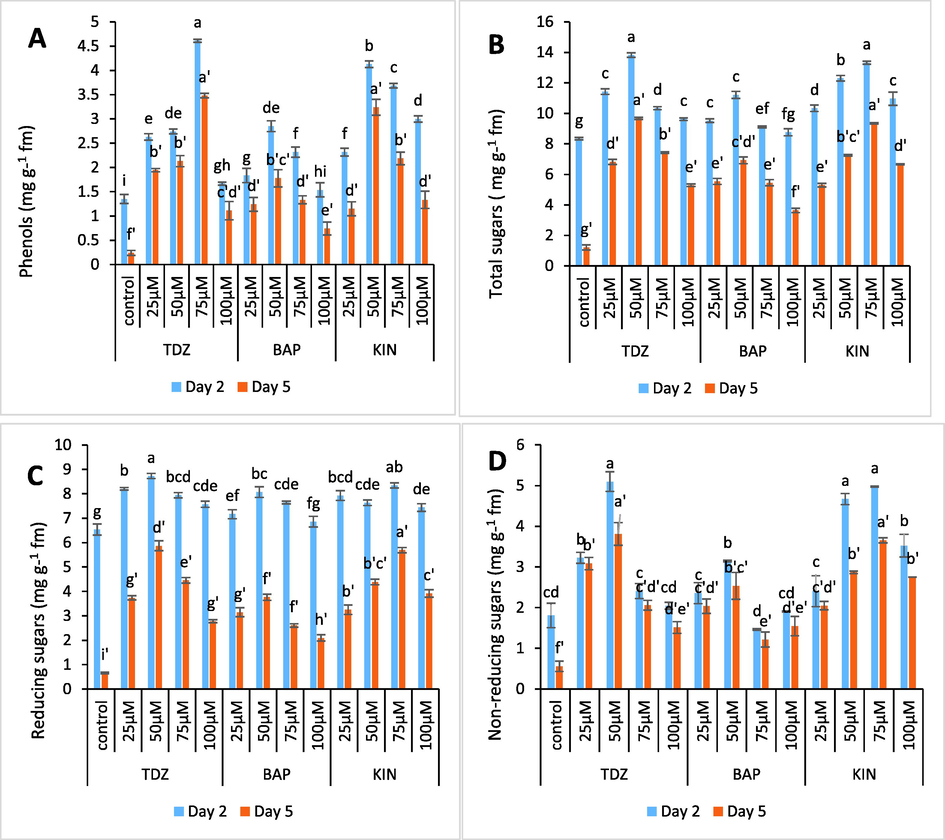
- (A-D) Graphs showing the effect of TDZ, KIN and BAP treatments on phenolic and sugar content of C. ajacis flowers. Each value is a mean of 3 replicates with S.E. indicated by bars. Treatments indicated by different superscripts differ significantly according to Duncan’s test at p < 0.05.
3.5 Activity of antioxidant and Lipoxygenase enzyme
Application of all the three growth regulators (BAP, KIN and TDZ) caused a notable amplification in SOD, APX and CAT activity in comparison to control. The peak in activity of SOD and CAT was recorded at, 50 µM TDZ, 50 µM BAP and 75 µM KIN Fig. 7 (A-B). The CAT activity for these three treatments increased by 364.8 %, 265.9 % and 350 % respectively, meanwhile, the APX activity peaked at 50 µM TDZ, 75 µM BAP and 75 µM KIN (Fig. 7C). The percent increase in APX activity for these treatments include, 271.2 %, 157.5 % and 243.9 %. Conversely, spikes placed in these test solutions exbhited low LOX activity in tepal tissues as compared to untreated spikes. LOX activity significantly reduced by 31.5 % in 50 µM BAP, 54.3 % in 50 µM TDZ and 40.3 % in 75 µM KIN treatments (Fig. 7D). However, antioxidant enzyme activity subsequently reduced towards later phase of experiment, with parallel rise in LOX activity.
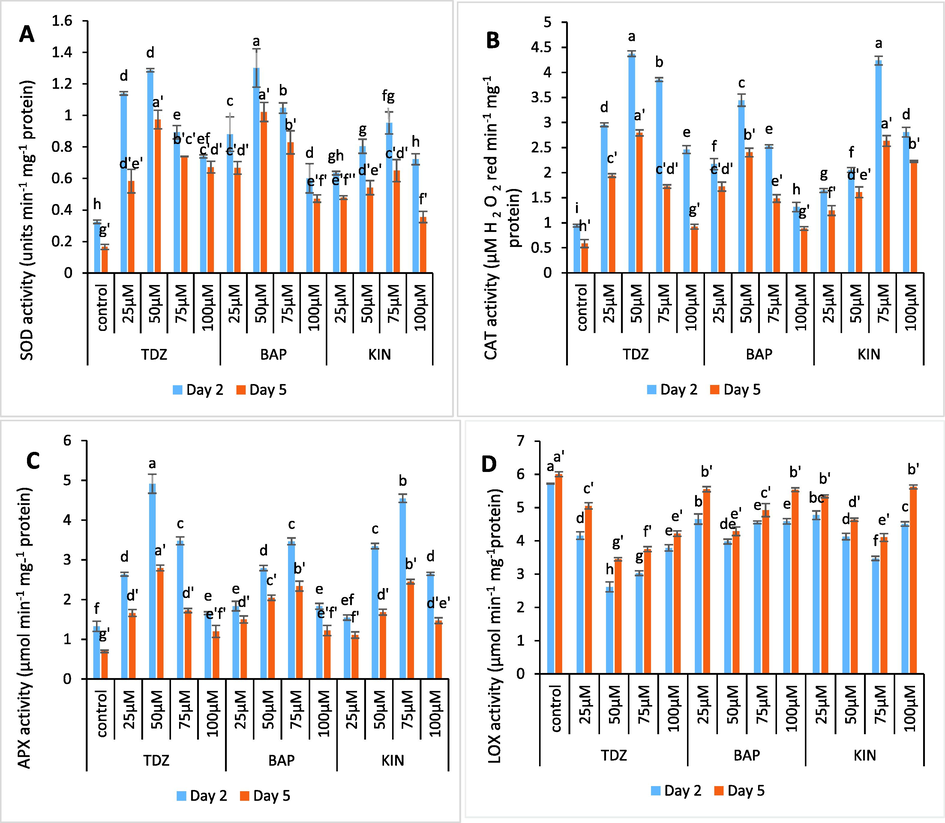
- (A-D). Graphs showing the effect of TDZ, KIN and BAP treatments on activity of SOD, CAT, APX and LOX enzyme in C. ajacis flowers. Each value is a mean of 3 replicates with S.E. indicated by bars. Treatments indicated by different superscripts differ significantly according to Duncan’s test at p ≤ 0.05.
4 Discussion
Based on the findings, our study justifies TDZ and cytokinins as potential postharvest treatments for improving the marketability of C. ajacis cut spikes. TDZ and cytokinins treatments caused a profound increase in postharvest longevity in comparison to control. Our results indicate that Cytokinin mediated enhancement in postharvest longevity may be due to higher respirable substrates, improved hydraulic conductivity and membrane stability. These findings are quite consistent with the study conducted in Chrysanthemum where BAP and TDZ treatments prolonged vase life by maintaining respirable substrates, improved membrane stability and water uptake in florets (Kaur and Singh, 2015). Application of cytokinins is also known to prolong postharvest longevity of various flowers like, Anemone (Moneruzzaman et al., 2010). Besides longevity, other postharvest attributes like floral diameter also exhibited increase due to cytokinin and TDZ treatments. Expansion in floral dimensions may be linked to sugar enrichment in tepal tissues, which in turn increases the turgidity of floral tissues through endosmosis, thus leading to increase in flower diameter (Reid, 2003). Increase in flower diameter by cytokinin application has been observed in Dendranthema grandiflorium (EL, 2018).
Enhanced LOX activity is regarded as characteristic feature of senescent plant tissues as observed in petal tissues of Chrysanthemum (Khokhar and Mukherjee, 2013). Lipoxygenase affects membrane fluidity by degrading membrane lipids and increases leakiness of the cell membrane (Khokhar and Mukherjee, 2013). However, tepal tissues of C. ajacis treated with cytokinins exhibited lower LOX activity as compared to untreated samples. Consequently, decrease in LOX activity causes a corresponding increase in MSI value in cytokinin treated flowers and improve membrane stability. The increased membrane stability can be also correlated with the inhibition of protease leakage from the vacuoles thereby retaining optimum content of phospholipids, proteins and thiols crucial for membrane integrity (Dek et al., 2017). Decreased LOX activity by cytokinin application has been reported in Iris germanica. (Ahmad et al., 2018). Our observations are also consistent with the findings reported in Brocelli florets where cytokinin treatment reduced malondialdehyde (MDA) content by preventing lipid peroxidation (Xu et al., 2012;).
Proteins serve as crucial metabolites in the regulation of postharvest senescence. Sensecent tissues are characterised by lower concentration of soluble proteins. Meanwhile, the concentration of proteins was found to be higher in BAP, KIN and TDZ treated tepal tissues. Accumulation of proteins in tepal tissues can be linked with inhibition of protease activity by cytokinins and TDZ. Upsurge in protease activity has shown to trigger protein breakdown in Lilium longiflorum (Battelli et al., 2011). Hydrolyzed proteins are mobilized as α- amino acids from petals to growing organs during senescence (Jones, 2013). However, studies conducted in Lilium candidum and Raphanus sativus reported a decrease in protease activity by exogenous application of cytokinins and TDZ (Khokhar and Mukherjee, 2011; Sharafi et al., 2013). Moreover, a decreased content of α- amino acids was found in cytokinin treated tissues as compared to untreated ones. This may be due to curtailed protein breakdown by cytokinins, decreasing quantity of α- amino acids (Criado et al., 2007). Thus, implicating cytokinins as potent inhibitors of proteases, arresting protein breakdown and improving flower longevity.
Inclusion of cytokinins and TDZ led to increase in phenolic content of floral tissues as compared to untreated ones. This may be linked to cytokinin mediated expression of phenolic synthesis enzymes like Phenyl ammonia lyase, Chalcone synthase and Chalcone isomerase (Deikman and Hammer, 1995). Phenolic enrichment was found to occur in floral tissues of Iris germanica treated with BAP, KIN and TDZ (Ahmad et al., 2018). Decrease in phenolic content induces senescence as noticed in Iris japonica and Iris versicolor (Ahmad and Tahir, 2017). Phenolic enrichment stimulates anti- ROS mechanisms to offset the stress related mutilation (Cavaiuolo et al., 2013). Higher phenolic content enables the plant to overcome endogenous disturbances including biotic and abiotic stress (Trivellini et al., 2015) Cytokinin induced phenolic enrichment suggests their explicit role in activation of antioxidant systems to prevent oxidation of membrane lipids and proteins during senescence. Besides phenols, carbohydrates content was also found higher in cytokinin and TDZ supplied flowers relative to control. Carbohydrate enrichment in petal tissues may be correlated with increased vase life of ornamentals like Eustoma (Chang et al., 2003). In addition, cytokinins enable mobilization of nutrients by stimulating cell wall invertase (Werner et al., 2008) and thus promote transfer of carbohydrates and other solutes from leaves to flowers (Chathuri and Sarananda, 2011). Reportedly, cytokinins and TDZ intensify sink activity, to prevent back flow of sugars from petal tissues to ovary during remobilization of nutrients (Ahmad et al., 2018). In conclusion, cytokinin mediated sugar accumulation specifies their unequivocal role in sugar metabolism and photosynthesis.
Treatment of C. ajacis flowers with different cytokinins showed a considerable upturn in the activity of SOD, APX and CAT enzymes in comparison to untreated ones. Upturn in antioxidant enzymes due to cytokinins has been implicated in ROS scavenging in Nicotiana (Tahir et al., 2018). Rise in ROS concentration is a well-known feature of senescent plant tissues as reported in Dianthus caryophyllus and Hemarocallus fulva (Rogers and Munné-Bosch, 2016). Cytokinin treatment therefore intensified the activity of antioxidant enzymes to offset the deleterious effects of ROS. Upregulation of antioxidant system shields the plant cells from oxidative stress through detoxification of superoxide ion (o2–) to water and oxygen (Xu et al., 2012).
5 Conclusion
Based on findings, the present study proposes considerable role of cytokinins and TDZ in accentuating postharvest performance of C. ajacis cut spikes. The inclusion of these growth regulators improved postharvest performance by modulating various biochemical pathways like, upregulation of antioxidant system, sustaining membrane integrity and maintaining higher content of respirable substrates especially sugars and proteins. Our study authenticates TDZ as best postharvest treatment among the selected growth regulators to maximize postharvest longevity of C. ajacis cut spikes for their efficient marketability. Although the current study provides key insights into physio-biochemical mechanisms underlying senescence, however elucidating molecular interaction of cytokinins with other phytohormones particularly ethylene may offer a promising strategy to modulate flower senescence and unveil novel techniques to maintain quality of these fascinating climacteric flowers.
6 Ethics approval
N/A.
7 Consent to participate
All authors consent to participate in this manuscript.
8 Consent for publication
All authors consent to publish this manuscript in Journal of King Saud University-Science.
9 Availability of data and material
Data will be available on request to corresponding or first author.
10 Code availability
Not Applicable.
CRediT authorship contribution statement
Aehsan ul Haq: Conceptualization, Formal analysis, Writing – original draft. Sumira Farooq: Methodology, Visualization. Mohammad Lateef Lone: Software, Supervision. Foziya Altaf: Validation. Shazia Parveen: Validation. Inayatullah Tahir: Conceptualization, Investigation, Writing – original draft. Ajaz Ahmad: Writing – review & editing. Prashant Kaushik: Writing – review & editing. Parvaiz Ahmad: Data curation, Writing - review & editing.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/350), King Saud University, Riyadh, Saudi Arabia
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Regulatory role of phenols in flower development and senescence in the genus Iris. Indian J. Plant Physiol.. 2017;22(1):135-140.
- [Google Scholar]
- Adenine type and diphenyl urea derived cytokinins improve the postharvest performance of Iris germanica L. cut scapes. Physiol. Mol. Biol. Plants. 2018;24(6):1127-1137.
- [Google Scholar]
- [53] Lipoxygenase from soybeans: EC 1.13.11.12 Linoleate:oxygen oxidoreductase. In: Methods in Enzymology. Academic Press; 1981. p. :441-451.
- [Google Scholar]
- Changes in ultrastructure, protease and caspase-like activities during flower senescence in Lilium longiflorum. Plant Sci.. 2011;180(5):716-725.
- [Google Scholar]
- The antioxidants changes in ornamental flowers during development and senescence. Antioxidants. 2013;2:132-155.
- [Google Scholar]
- Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol.. 2003;132:2174-2183.
- [Google Scholar]
- Effect of 6-BAP and sucrose pulsing on vase life of lotus (Nelumbo nucifera) Tropical Agricultural Research. 2011;22
- [Google Scholar]
- Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol.. 1989;30:987-998.
- [Google Scholar]
- Proteomic and biochemical changes during senescence of Phalaenopsis ‘Red Dragon’petals. Int. J. Mol. Sci.. 2018;19:1317.
- [Google Scholar]
- Plant growth regulators and induction of leaf senescence in nitrogen-deprived wheat plants. J. Plant Growth Regul.. 2007;26(4):301-307.
- [Google Scholar]
- Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol.. 1995;108(1):47-57.
- [Google Scholar]
- Upregulation of Phosphatidylinositol 3-Kinase (PI3K) Enhances Ethylene Biosynthesis and Accelerates Flower Senescence in Transgenic Nicotiana tabacum L. Int. J. Mol. Sci.. 2017;18(7):1533.
- [Google Scholar]
- Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot.. 1981;32(1):93-101.
- [Google Scholar]
- EL, M., 2018. Effect of kinetin and GA3 treatments on growth and flowering of Dendranthema grandiflorium cv. Art Queen plants. Middle East J, 7, 801-815.
- Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci.. 2018;19:4045.
- [Google Scholar]
- Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front. Plant Sci.. 2017;8:475.
- [Google Scholar]
- Mineral nutrient remobilization during corolla senescence in ethylene-sensitive and-insensitive flowers. AoB Plants. 2013;5(0):plt023-plt.
- [Google Scholar]
- Influence of growth regulators on physiology and senescene of cut stems of chrysanthemum (Chrysanthemum morifolium Ramat) var. thai ching queen. Int. J. Allied Pract. Res. Rev.. 2015;2:31-41.
- [Google Scholar]
- Regulation of lipoxygenase and superoxide dismutase activities and longevity of chrysanthemum cut flowers by ethanol and methanol. Indian J. Hortic.. 2013;70:76-81.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Effect of Sucrose and Kinetin on the Quality and Vase Life of'Bougainvillea glabra'var. Elizabeth Angus Bracts at Different Temperatures. Aust. J. Crop Sci.. 2010;4:474-479.
- [Google Scholar]
- A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem.. 1944;153(2):375-380.
- [Google Scholar]
- Reid, M.S., 2003. Flower development: from bud to bloom. In: VIII International Symposium on Postharvest Physiology of Ornamental Plants 669. pp: 105-110.
- Production and scavenging of reactive oxygen species and redox signaling during leaf and flower senescence: similar but different. Plant Physiol.. 2016;171(3):1560-1568.
- [Google Scholar]
- A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys.. 1957;67(1):10-15.
- [Google Scholar]
- Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol.. 1994;32
- [Google Scholar]
- Color and phenolic content changes during flower development in groundcover rose. J. Am. Soc. Hortic. Sci.. 2010;135:195-202.
- [Google Scholar]
- Role of kinetin and a morphactin in leaf disc senescence of Raphanus sativus L. under low light. Physiol. Mol. Biol. Plants. 2011;17(3):247-253.
- [Google Scholar]
- Comparative effect of ethylene antagonists: silver thiosulphate (STS) and amino-oxy acetic acid (AOA) on postharvest performance of cut spikes of Consolida ajacis cv Violet Blue. Int. J. Agric. Food Sci. Technol.. 2010;1:103-113.
- [Google Scholar]
- Sharafi, Y., Kaviani, M., Mortazavi, S.N., Talebi, S.F., 2013. Investigating enzyme activity and protein changes in postharvest (Lilium candidum cv. Navona) cut flower. International Journal of Agronomy and Plant Production.
- The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric.. 1959;10:63-68.
- [Google Scholar]
- Gibberellin and cytokinins modulate flower senescence and longevity in Nicotiana plumbaginifolia. In: XXX International Horticultural Congress IHC2018: International Symposium on Ornamental Horticulture and XI International 1263. 2018. p. :469-476.
- [Google Scholar]
- Effect of cytokinins on delaying petunia flower senescence: a transcriptome study approach. Plant Mol. Biol.. 2015;87:169-180.
- [Google Scholar]
- Polyamines accentuate vase life by augmenting antioxidant system in cut spikes of Consolida ajacis (L.) Schur. Ornamental Horticulture. 2021;27:495-504.
- [Google Scholar]
- Physiology and molecular biology of petal senescence. J. Exp. Bot.. 2008;59:453-480.
- [Google Scholar]
- Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot.. 2008;59:2659-2672.
- [Google Scholar]
- 6-Benzylaminopurine delays senescence and enhances health-promoting compounds of harvested broccoli. J. Agric. Food. Chem.. 2012;60:234-240.
- [Google Scholar]







