Translate this page into:
Ascorbic acid-mediated Fe/Cu nanoparticles and their application for removal of COD and phenols from industrial wastewater
⁎Corresponding authors. sfadil@ksu.edu.sa (Syed Farooq Adil), sadia.saifpk@gmail.com (Sadia Saif)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Industrial activities release a large number of pollutants in the environment, which deteriorate environmental quality, and human health. Organic and inorganic pollutants increase the chemical oxygen demand (COD) of the receiving water bodies, one such pollutant is phenol, which is an organic compound that is toxic even at low concentrations. The high COD leads to a lower amount of dissolved oxygen (DO) which is important factor for aquatic life. These pollutants are a serious threat to human health and the environment. In this study, economic and environmentally friendly synthesis of iron copper (Fe/Cu) nanoparticles are reported, which were characterized using UV visible spectroscopy, FT-IR, XRD, TGA, and SEM. The developed particles were then employed to treat industrial wastewaters containing phenol and for reduction of chemical oxygen demand (COD). The average size reported by SEM analysis was between 140 nm and 160 nm. Batch experiments showed that a 83.33% mg/L reduction in COD and 76.91% of phenol removal was attained at pH 4, adsorbent dose of 0.5 g/100 mL, and contact time of 60 min, respectively. It was observed that the efficiency of phenol removal increased with an increase in the initial concentration of both, COD and phenols. The results demonstrated that the Fe/Cu nanoparticles are effective adsorbent material to remove phenols from industrial wastewaters as well as for the reduction of COD.
Keywords
Ascorbic acid
Nanoparticles
Fe/Cu
COD
Phenols
Wastewater
1 Introduction
Industrial development resulted in the development of a variety of toxic wastes which are released into the environment without proper treatment. As a result, these pollutants have become a major threat to the health of living organisms and the environment. Pakistan possesses a large number of industrial zones in different cities like Lahore, Karachi, Gujranwala, Faislabad, Sialkot, etc. Effluents discharged from textile, paper and pulp, steel, paint, tanning, and detergent industries contain different types of organic and inorganic pollutants that are directly discharged into surface waters bodies and in open drains from industries. These compounds cause a variety of environmental issues and human health complications (Ilyas et al., 2019; Muhammad et al., 2021; Nasir et al., 2012). A commonly used parameter to determine the quality of water and wastewater is Chemical Oxygen Demand (COD). COD is the estimation of oxygen required to oxidize the chemicals present in wastewater or the overall amount of organic compounds. COD is not only used to determine the amount of biologically active substances such as bacteria, but also biologically inactive organic matter in water (Khan and Ali, 2018; Li et al., 2018). Industries that release wastewater with a high COD include food processing industries such as breweries, followed by chemical manufacturing, dying industries, and pharmaceutical industries (Azizullah et al., 2011; Charles et al., 2016; Emongor, 2005; Nasir et al., 2012). Like, U.S. Environmental Protection Agency (EPA), the National Environmental Quality Standards, Pakistan has specified a discharge limit of 150 mg/L COD to inland surface waters (NEQS, 2005).
Another common pollutant of water from industries is phenol, which is a persistent organic compound that is formed during industrial manufacturing processes and as a byproduct of several chemical processes. Phenol is also released from wood treatment facilities and paper pulp mills as well as pharmaceutical industries where it is used in the manufacture of throat lozenges, antiseptic lotions, mouths washes and gargles (Darisimall, 2006; Galgale et al., 2014; Ge et al., 2017). Phenol and its derivatives persist in the environment for a long period and accumulate in the fatty tissues of living organisms and exhibit highly toxic effects. The largest group of phenol are chlorophenols and nitrophenols found in soil, drinking water, and wastewater. Phenol has multiple human health issues such as damage to DNA, weakening of muscles, necrotic lesions and burning in the mouth, stomach and esophagus as well as pulse fluctuations (Gosselin et al., 1984; Schweigert et al., 2001). Phenolic compounds are nerve poisoning agents and the United States environmental protection agency (USEPA) and European Union (EU) have enlisted the phenolic compounds as the pollutant of major concern. Phenols are highly toxic due to which U.S. EPA has set a discharge limit of 0.1 mg/L in inland surface waters (USEPA, 1977).
Conventional wastewater treatment methods can be utilized in the reduction of COD in wastewater, depending upon the type and characteristics of wastewater and its source (Satyawali and Balakrishnan, 2008). Phenol treatment and its removal is comparatively limited due to their poor ability to adjust to fluctuation in phenol load (Jiang et al., 2007). However, each have their limitations in terms of cost, pollutants treated, maintenance, and pollution load. Hence, there has been considerable research in the field of nanotechnology for the removal of pollutants and treatment of wastewater.
Nanoparticles possess remarkable properties, such as a high degree of functionalization and high reactivity, large surface areas, high area to mass ratios, and size-dependent properties which make them a suitable candidate for the application in wastewater treatment (Cloete, 2010) and biomedical applications (AlSalhi et al., 2020; Fakhar-e-Alam et al., 2021). Nano sorbents are a type of adsorbent material developed using nanoparticles that adsorb particles on their surface through some form of physical or chemical interaction and these can be synthesized and developed using natural and environmentally friendly techniques (Bora and Dutta, 2014). There are two types of metal-containing nanoparticles, monometallic and bimetallic nanoparticles, which include only a single metal atom, or bimetallic nanoparticles, respectively, which are made of two different metals. The bimetallic nanoparticles have gained more importance due to the two metal atoms present in their structure as it has been found that bi-metallization considerably enhances the catalytic properties of nanoparticles, which is not possible in the case of monometallic catalysts (Sharma et al., 2016). Some commonly used adsorbents mostly include oxides of various metals such as iron (Fe), silicon (Si), tungsten (W), manganese (Mn), titanium (Ti), copper (Cu), etc. Metal oxides have the advantage of being low cost and have the ability to functionalize their adsorption capacity and selectivity to treat various pollutants (Bora and Dutta, 2014). Various studies have shown the efficacy of bimetallic nanoparticles for the removal of various contaminants such as heavy metals and organic pollutants from aqueous media. Some commonly used bimetallic nanoparticles include gold-based, platinum based, iron-based, and palladium-based and nicked-based bimetallic nanoparticles (Sharma et al., 2019). Furthermore, nanosorbents based on ‘Fe’ have been studied for the removal of pollutants. ‘Fe’ has the added advantage of easy removal from aqueous media due to its magnetic properties.
A significant amount of work for water treatment has been reported for bimetallic nanoparticles, especially for iron and copper (Fe/Cu) bimetallic nanoparticles (O’Connell et al., 2008). Iron and copper (Fe-Cu) bimetallic nanoparticles have been reported to treat multiple pollutants from industrial wastewater such as heavy metals like chromium (Jiang et al., 2018) and arsenic (Babaee et al., 2017), nitrates (Guo et al., 2018), dyes such as malachite green (Zhang et al., 2018) from wastewater. Chitosan stabilized Fe-Cu nanoparticles were used by Jiang et al. (Jiang et al., 2018) for remediation of hexavalent chromium from wastewater and it was reported that this combination achieved 96% chromium removal efficiency from ultrapure water, 90% from river water, and 85% from tannery water. Similarly, Babaee et al. (2018) studied the removal of arsenic (III) and arsenic (V) from aqueous media by utilizing Fe-Cu nanoparticles. 21.32 mg/g sorption capacity was reported for As (V) while the maximum sorption capacity for As (III) was reported to be 19.68 mg/g.
Bimetallic nanoparticles have also been reported to treat waters contaminated with nitrates and phenols. Asgari and Ramavandi studied the removal of phenols by using pumice-supported bimetallic particles. It was reported that 97.3% removal of phenols by pumice bimetals was reported at pH 8 and a 30 min contact time (Asgari and Ramavandi, 2012). In another study, Trujillo-Reyes et al. (2010) studied the removal of indigo blue dye using bimetallic nanoparticles of Fe-Cu and composites of C-Fe-Cu alloy. It was reported that Fe-Cu nanoparticles effectively removed indigo blue dye from wastewater in 12 mins while the MCL-NP removed the dye in about 15 mins and CAC-NP removed the dye in 15 mins. Muradova et al. (2016) studied the removal of nitrate using zerovalent iron nanoparticles and iron copper bimetallic nanoparticles which were developed using the NaBH4 reduction method under ambient pressure and at room temperature. Complete nitrate removal was observed after 60 mins. In another study, Shubair et al. (2018) reported the removal of nitrates from aqueous media using zero-valent iron and bimetallic nanoparticles composed of copper-coated iron nanoparticles. It was reported that optimum removal was possible when a 10 cm layer of zero-valent iron particles and sand was used with nitrate removal efficiency at 97% while for bimetallic particles, 5 cm sand column packed with particles was used which removed 100% of the nitrate particles.
However in all the above cited studies it can be said that iron copper bimetallic nanoparticles employed were prepared using chemical approach. Since our groups has been involved in the green synthesis of various nanoparticles and their applications (Khan et al., 2020a; Khanz et al., 2014; Khan et al., 2020b; Shaik et al., 2016; Shaik et al., 2017). In continuation of our previous studies, in the current study we aimed to synthesize bimetallic nanoparticles of Fe/Cu using an environmentally benign approach and their application for the treatment of phenols and reduction of COD from industrial wastewater (Fig. 1).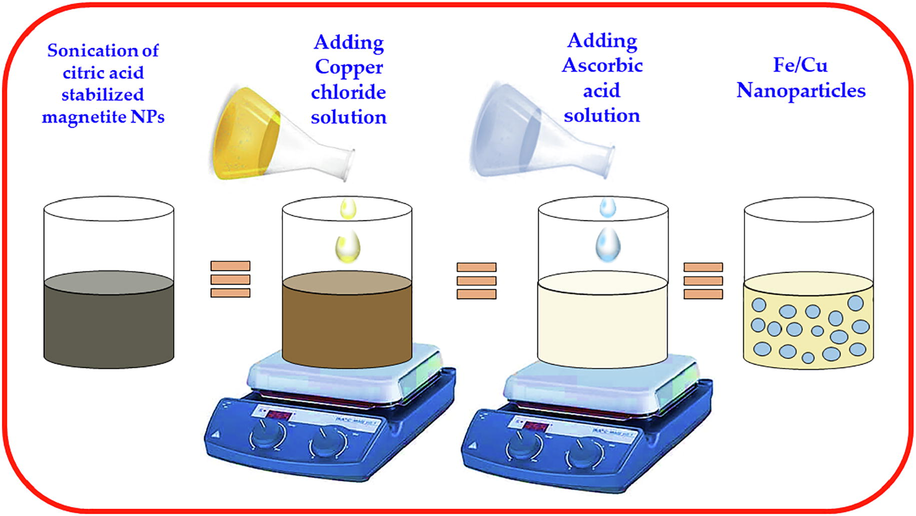
Steps of synthesis of Fe/Cu nanoparticles from citrate coated Fe3O4 nanoparticles.
2 Material and methods
2.1 Chemicals
The chemicals used in this study include iron chloride (FeCl3·6H2O), iron sulphate (FeSO4·H2O), sodium hydroxide (NaOH), copper chloride (CuCl2·7H2O), ascorbic acid (C6H8O6) and N2 purged distilled water which was formed by passing N2 gas from distilled water for 10 mins at a flow rate of 25 mL/s. This N2 water was used as a solvent for all solutions during synthesis. Wastewater was collected from the paper and pulp industry for phenol analysis and the study of COD wastewater was collected from the drain of the industrial zone of Lahore.
2.2 Synthesis of Fe/Cu nanoparticles
2.2.1 Synthesis of Fe3O4 nanoparticles
0.1 M hydrochloric acid solution was prepared in 100 mL of N2 purged water. To this solution, 0.1 M iron sulphate and 0.2 M iron chloride salts were added in an Erlenmeyer flask. Special care was taken to minimize oxygen exposure. 70 mL of 2 M NaOH was added dropwise to the solution. Dark-colored precipitates of Fe nanoparticles were developed due to precipitation. The reaction mixture was stirred for 40 mins at room temperature. These developed particles were centrifuged for 10 mins to separate the particles from the solution. The supernatant was discarded and nanoparticles were washed with ethanol: water (30:70) and centrifuged at 4000 rpm for 15 mins. The process was repeated three times after which the nanoparticles were dried in an oven at 105 ℃ for 20 mins. The nanoparticles were cooled in a desiccator, crushed, and stored in an eppendorf tube till further use.
2.2.2 Citrate coating on Fe3O4 nanoparticles
Approximately 600 mg of Fe3O4 nanoparticles were suspended in 50 mL of N2 water. The pH of this solution was reduced to 6.8 using a dilute citric acid solution. The reaction was constantly stirred for 80℃ for 2–3 h. Upon cooling to room temperature, citric acid stabilized magnetite nanoparticles were formed.
2.2.3 Synthesis of Fe/Cu nanoparticles
The above solution, containing 600 mg of citrate coated Fe3O4 nanoparticles were sonicated for 45 mins. In this solution, 0.0176 M Copper Chloride solution was added dropwise, the solution was stirred for 10 mins and the temperature was maintained between 60 and 70℃. After 10 mins, 0.027 M Ascorbic Acid solution was added dropwise and allowed to stir for 20 mins. The solution changed to white color due to the formation of white-colored Fe/Cu nanoparticles. The particles were centrifuged at 4000 rpm for 20 mins and washed with ethanol three times. The particles were then oven-dried at 75 ℃ for 15 mins and cooled in a desiccator after which they were crushed and stored in an eppendorf till further use.
2.3 Characterization of synthesized nanoparticles
UV–visible analysis of as-synthesized Fe/Cu nanoparticles was carried out using lambda 35 instrument, Perkin Elmer, Waltham, MA, USA. Fourier transform infrared (FTIR) spectra of Fe/Cu nanoparticles were recorded on a 1000 FTIR instrument, Perkin-Elmer, Waltham, MA, USA. The formation of the as-prepared Fe/Cu nanoparticles and their crystallinity was confirmed by using the powder diffraction technique (D2 phaser, Bruker, Germany). The morphology of the as-synthesized Fe/Cu nanoparticles was investigated using Scanning electron microscopy (JED-2200, JEOL, Japan). Thermogravimetric analysis was carried out using Perkin-Elmer Thermogravimetric Analyzer 7, Waltham, MA, USA.
2.4 Reduction of COD and phenol removal experiments
Adsorption experiments were carried out to determine the ability of the as-synthesized particles to reduce COD and removal of phenols from industrial wastewaters. Batch experiments were conducted using different dosages of adsorbent (0.05 g, 0.1 g, 0.25 g, 0.5 g, and 1 g) and contact time (30 mins, 60 mins, 90 mins, and 120 mins) and pH (4, 6, 8, and 10). To study the effect of initial concentration different dilutions of wastewater with COD values i.e. 693.3, 1067.2, 2668, 3465 mg/L, and for phenol samples with different dilution 1.98 mg/L, 2.86 mg/L, 4.6 mg/L, and 6.5 mg/L were used. Phenols in wastewater were analyzed using photometric analysis while the reduction in COD, using strong oxidizing agents and a COD meter.
3 Results and discussion
Bimetallic nanoparticles are the combination of two different metals and exhibit enhanced properties due to the coupling of different materials. As mentioned earlier, the properties and catalytic phenomenon of bimetallic nanoparticles can be changed by varying concentrations of any constituent element (Kulkarni and Kaware, 2013; Michałowicz and Duda, 2007; Swiatkowska-Warkocka, 2021). Synthesis of bimetallic nanoparticles from metal salts can be done either (i) coreduction or (ii) successive reduction of two metal salts.
Successive reduction is usually carried out to fabricate core–shell structured bimetallic nanoparticles. The formation of core–shell structure also depends upon the metal salts used and the reducing/stabilizing agent used in the preparation (Nadagouda and Varma, 2007). In this attempted study first, citrate-coated Fe3O4 nanoparticles were synthesized and added to a second salt solution i.e., copper chloride to which ascorbic acid was added. Addition to second metal salt solution and subsequent reduction with excess stabilizing ascorbic acid the formation of the core–shell structure is anticipated.
3.1 Characterization of Fe/Cu nanoparticles
3.1.1 UV–Visible and FT-IR analysis
UV–Visible spectrometry analysis was carried out for the as-synthesized metallic particles. The spectrum shows three main peaks for the adsorbent material at 355 nm, and 525 nm as shown in Fig. 2(a). These peaks lie in the visible region of the spectrum and results are in confirmation with other studies (Logpriya et al., 2018; Umer et al., 2014). FT-IR analysis for Fe/Cu nanoparticles is given in Fig. 2(b). The effectiveness of the capping of ascorbic acid on the surface of the Fe/Cu nanoparticles is confirmed by FT-IR analysis. The multiple peaks were recorded in the range of 4000–400 cm−1 and can be seen in Fig. 2(b). The absorption peaks at 3501.5 and 3356.2 cm−1 correspond to different –OH groups and the peak at 1600.5 cm−1 stretching vibrations of C–C double bond. The peak at 920.6 cm−1 was due to the stretching of the C–O bonding group. The peaks at 880.4 cm−1 617.22 cm−1, 551.64 cm−1, 536.21 cm−1 were assigned to C–H and the peak at 551 cm−1 was due to the Fe–O bond. Significant variance in the peak positions of several bands of the ascorbic acid gives direct evidence for the covalent bonding of the biomolecule on the surface of the Fe/Cu nanoparticles.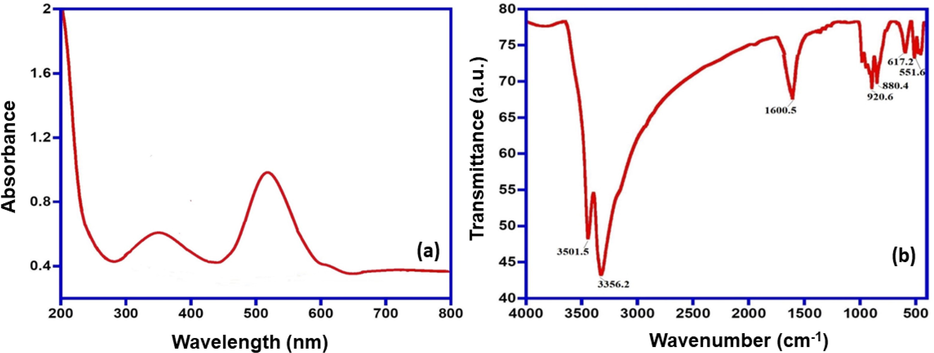
(a) UV–Visible spectrum of Fe/Cu nanoparticles and (b) FT-IR spectrum of Fe/Cu nanoparticles.
3.1.2 SEM analysis
The surface morphology of the as-synthesized Fe/Cu nanoparticles was investigated using SEM analysis (Fig. 3). It was observed that the particles were agglomerated and exists as a clusters of particles. However, the clusters are found to be made up of spherical particles pon further magnification. There are many factors such as surface charge, surface stability and ionic strength of the solution that can influence the particle size. Adsorbing other charged ions and coating polymers on the surface of nanoparticles can suppress agglomeration and provide stability to nanoparticle dispersions. Moreover, probe and bath sonication can be utilized in dispersing nanoparticles agglomerates to reduce their size (Jiang et al., 2009). The SEM analysis showed that particles size ranges <100 nm and ranges between 140 and 160 nm.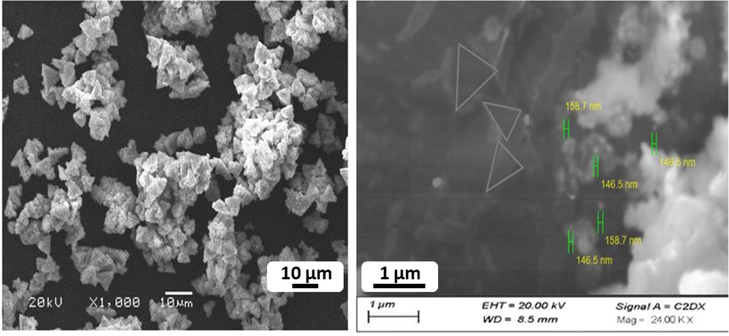
SEM analysis of Fe/Cu nanoparticles at different scale bars.
3.1.3 XRD and TGA analysis
The diffraction pattern of the as-synthesized Fe/Cu nanoparticles was examined via XRD analysis (Fig. 4(a)). The XRD patterns displayed mixed-phase reflections, the reflections marked with (↓) representative of crystalline Fe/Cu nanoparticles (FeCu4) [ICSD 03-065-7002].(Ch et al., 2019; Minic and Maričic, 2010) Moreover, there are several other additional reflections were observed as shown in Fig. 4(a). The reflections marked with (⁎) could be attributed to the presence of Fe3O4 [ICSD 03-065-7002] which appears to the major product in the compound formed, while the ones marked with (▾) could be attributed to the presence of CuO (Zhang et al., 2014). The diffraction patterns marked with (•) and (■) could be attributed to the existence of different phases of copper ferrite (CuFe2O4) (JCPDS card 77–0010) (Gomes et al., 2006; Othman et al., 2019). The results obtained by XRD analysis clearly indicate the existence of mixed phases of the as-synthesized nanocomposite. The TGA analysis of Fe/Cu nanoparticles revealed a two-step phases thermal degradation pattern for Fe/Cu nanoparticles (Fig. 4(b)). The first degradation step displays, at 50 to 200 °C was noticed that might be from the removal of moisture content. At around 250 to 550 °C, the second degradation is noticed, which might be associated to the decomposition of light volatile compounds of the ascorbic acid existing on the surface of the Fe/Cu nanoparticles as a capping agent. These results endorses the significant role of ascorbic acid in reducing, and capping/stabilizing the as synthesized Fe/Cu nanoparticles. Hence it can be concluded that the as-snthesized Fe/Cu nanoparticles is stable upto 550 °C.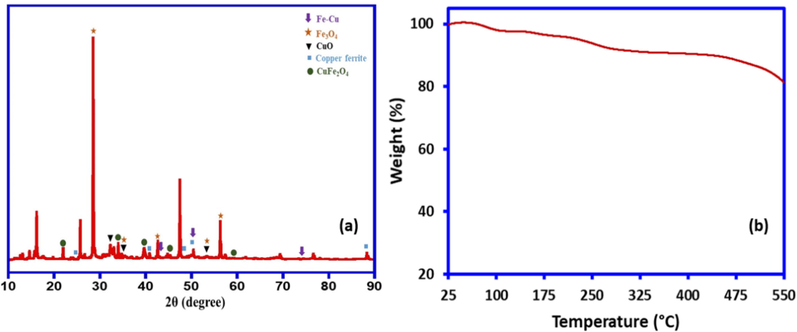
(a) XRD analysis of Fe/Cu nanoparticles and (b) TGA analysis of Fe/Cu nanoparticles.
3.2 COD reduction studies
3.2.1 Effect of adsorbent dose and time
In case of Chemical Oxygen Demand (COD) reduction experiments, it was observed that the percentage reduction of COD increased with an increase in adsorbent dose as well with increasing contact time. The reduction of COD efficiency increased from 15.38% to 60.07% for 0.05 g/L of adsorbent with an increase of time from 30 min to 120 min as shown in Fig. 5. At absorbent dose of 1 g/L removal a 54.2% reduction of COD was achieved after time 30 min and this further increased after 120 mins i.e. 89.1%. The increase in the adsorbent dose results in increases in the reduction of COD due to the increase in the availability of adsorption sites for the adsorbates. Furthermore, an increase in the reduction of COD was observed when contact time was increased to 60 mins. The reduction efficiency was observed to be maximum and in a state of equilibrium at contact time of 90 mins for all selected doses of Fe/Cu nanoparticles as shown in Fig. 5. The rate of reduction of COD was not considerably high after 90 mins of contat time as there was no steep rise in the reduction percentage was observed. This reduction trend with contact time can be explained by the interaction of adsorbate and adsorbent. Once the uptake of pollutants causing spike in COD values by available sites on the adsorbent has reached it maximum value, the reduction efficiency tends to reduce as the equilibrium stage has been achieved. It means that the active sites on the nanoparticles which take part in the adsorption process have become saturated.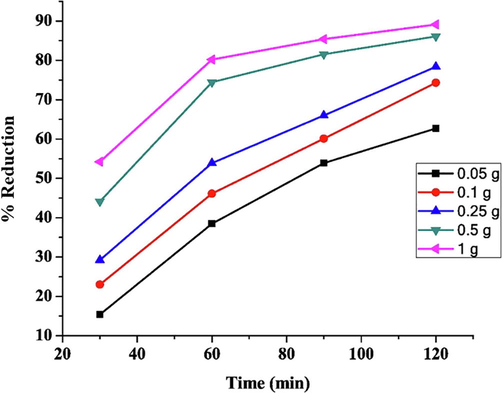
The percentage reduction of COD with respect to adsorbent dose and contact time.
Similarly, removal efficiencies were reported by a study in which Fe/Cu were prepared by coating copper on the surface of zero-valent iron nanoparticles (Yuan et al., 2014). It was reported that 59.8% removal of COD was achieved after 5 mins contact of Fe/Cu nanoparticles at dose of 40 g/L. While in the same study zero-valent iron nanoparticles showed a very low removal efficiency of 13.4% after 5 mins of treatment. Thus, the study proved that the surface coating of copper on iron improved the removal efficiency of the nanoparticles. In another study, copper was doped on ZnO nanoparticles which were then embedded on carbon nanotubes (CNT) having a multi-wall structure using a facile nontoxic sol method (Ahmad et al., 2014). Cu doped ZnO CNT was used to treat textile wastewater under irradiation. It was observed that the removal was as high as 98.2% for the Cu coped ZnO CNTs, which was attributed to high charge separation of CNTs that enhanced the composites ability to remove pollutants from wastewater along with an increase in surface area. In another study, Chang et al. reported the use of cuprous oxide (Cu2O) nanoparticles supported on Al2O3 for the reduction of COD from industrial wastewaters (Chang et al., 2009). A 71.8% and 93.5% reduction of COD was reportedly observed, when the Cu2O/Al2O3 prepared by two different synthetic approaches were employed.
3.2.2 Effect of initial concentration and pH
The effect of initial concentration of COD on percentage reduction efficiency of Fe/Cu nanoparticles was also studied using 0.5 g adsorbent, while the contact time was 60 min. It was observed that percentage reduction decreased with the increase in initial COD values as in Fig. 6(a). A 92% removal was observed at initial COD of 694 mg/L, however this value decreased to 78% when the initial concentration of COD was 3465 mg/L. The decrease in COD redcution with the increase in initial COD values shows that the active sites of adsorbent particles are consumed at a higher rate when the initail of COD value was high. Furthermore, the influence of hydrogen ion concentration on the reduction of COD was also studied, which is one of the most important parameters in adsorptive removal of pollutants as it influences other processes including metal speciation in wastewater (Ida and Eva, 2021). Reduction of COD by nanoparticles was carried out at pH ranging between 4–10. The contact time was set at 60 mins and the nanoparticle dose used was 0.5 g. It was observed that the percentage reduction of COD decreases with an increase in pH i.e. the best removal of 83.33% was observed at pH 4 while the reduction efficiency decreased to 36.64% when the pH was increased to 10 (Fig. 6(b)). The reason for this decrease in the removal efficiency can be attributed to strong electrostatic forces of attraction.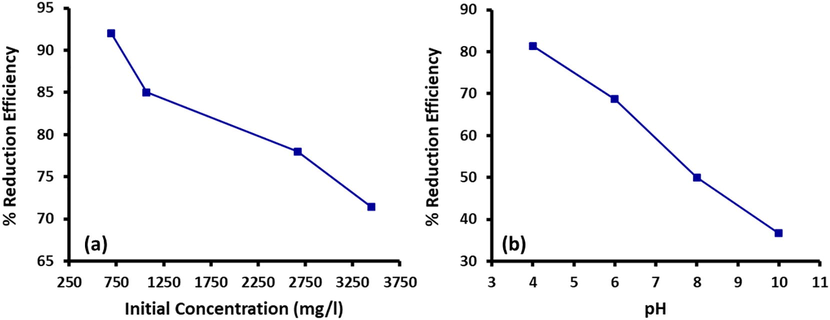
(a) Initial COD concentration vs. percentage reduction efficicncey and (b) pH vs. percentage reduction efficiecny of COD (Dose = 0.5 g, Time = 60 mins).
The adsorption sites on nanoparticles include oxides of Fe, Cu and nanoparticles of Fe/Cu as observed in the FTIR and XRD analysis. Fe/Cu nanoparticles can have a net positive charge which will attract all negative pollutant ions from the solution for adsorption. However this net positive charge can be further increased at a low pH due to hydrogen ions, which resulted in good removal of COD. At higher pH values, there is an excess of hydroxide ions which causes a reduction in the COD values. The results obtained in this study are in accordance with the study reported by Ahmad et al. wherein a maximum reduction of COD of 94.59% was at pH 4 (Ahmad et al., 2013).
3.3 Removal of phenols by batch experiments
3.3.1 Effect of adsorbent dose and time
The effect of amount of adsorbent particles on the removal of phenol was studied with respect to the time duration for which the nanoparticles came in contact with the wastewater at room temperature with neutral pH of wastewater (Fig. 7(a)). Adsorption of phenol was observed for 4 different doses of Fe/Cu nanoparticles i.e. 0.05 g, 0.1 g, 0.25 g, 0.5 g and 1 g, while time interval was 30 mins, 60 mins, 90 mins and 120 mins as seen in Fig. 7(a). The concentration of phenol in wastewater was maintained at 3.8 mg/L (pH = 5.2 ± 0.2).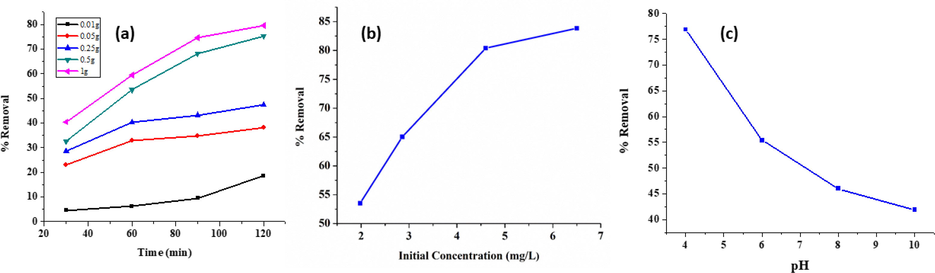
(a) Percentage removal of phenols with respect to adsorbent dose and contact time (b) effect of initial concentration of phenol on percentage removal (Dose = 0.5 g, Time = 60 mins) and (c) Effect of pH on percentage removal.
At dose 0.01 g only 4.6 % removal was achieved after 30 min and this removal was increased to 14.6% even after 120 min. The removal efficiency for 1 g nanoparticles, showed 40.5% removal after 30 mins, 59.5% removal after 60 mins, 74.67% removal after 90 mins and 79.5% removal at 120 mins of contact time. Best results were observed for 1 g adsorbent material, which was the maximum for this study, showed maximum removal efficiency. The increase in the adsorbent dose results in the increase in the removal efficiencies can be attributed to the presence of large number of adsorption sites at large doses, a trend similar to that observed for COD.
The influence of time on phenol removal was studied by Luo et al. (2015). Fe-Cu bimetallic nanoparticles were attached on ZSM-5 zeolite and were used to remove phenol from wastewater. It was observed that the optimum contact time was 40 mins for Fe-Cu nanoparticles suspended on mesoporous ZSM-5 zeolite which gave approximately 95% phenol removal with 0.02 mg nanoparticles. Similar removal trend was observed for phenol removal in the current study where increase in contact time resulted in increase in removal efficiency as it provided a greater contact with adsorption sites, till an equilibrium was reached and the increase in removal efficiency gradually became insignificant.
3.3.2 Effect of initial concentration and pH
The effect of initial concentration of phenol on percentage removal are shown in Fig. 7(b). Percentage removal was observed to increase with the increase in phenol concentration. The removal increased from 53.53% at 1.98 mg/L initial concentration to 83.84% at 6.5 mg/L initial concentration. Phenol adsorption has also been reported to increase with increase in initial concentration, when treated with bentonite. Increase in the concentration of phenol increase the mass transfer driving force between the adsorbent and adsorbate, which increases the rate at which phenol transfers from the solution to the particle surface (Banat et al., 2000). The pH values selected were 4, 6, 8, and 10 which were adjusted using NaOH and HCl (initial conc. of phenol was 1.48 mg/L) and the result obtained are shown in Fig. 7(c). It was observed that the percentage removal of phenol decreases with an increase in pH with the best removal of 76.69% observed at pH 4 while the removal efficiency decreased to 41.89 % at pH 10. Phenol is a weak acid, and at lower pH values it can be easily removed. However at higher pH, the adsorption process is influenced due to the presence of repulsive forces which increase due to the presence of hydroxide ions in the solution. As a result, at higher pH values, phenol removal efficiency decreases. Similar removal phenomena has been reported by Halhouli et al. (1997) which studied the adsorption of phenol using activated carbon. In another study, TiO2 catalyst was doped with Cu/Fe supported on zeolite and used to study the removal of a phenol compound diclofenac. It was reported that the highest removal efficiency was at pH 4 with the percentage removal being as high as 97.8% (Castañeda-Juárez et al., 2019).
3.4 Adsorption isotherms
The adsorption isotherm can be used to investigate the relationship between the amount of adsorbate and adsorbent in the aqueous phase at equilibrium state.
According to Langmuir model “monolayer sorption occurs on the solid surface with identical homogeneous sites” (Langmuir, 1918). It also suggests that no further adsorption takes place once the active sites are covered with adsorbate.
The saturated monolayer isotherm are calculated by the following equation:
qe = mg of adsorbate per g of adsorbent at equilibrium (equilibrium uptake)
qm = mg of solute adsorbed per g of adsorbent
KL = Langmuir constant (liter of adsorbent per mg of adsorbate L/mg)
Ce = equilibrium concentration of adsorbate in solution (mg/L).
The adsorption data of COD reduction by Fe/Cu NPs were fitted according to the linear form of the Langmuir adsorption (R2 = 0.9507) (Fig. 8(a)).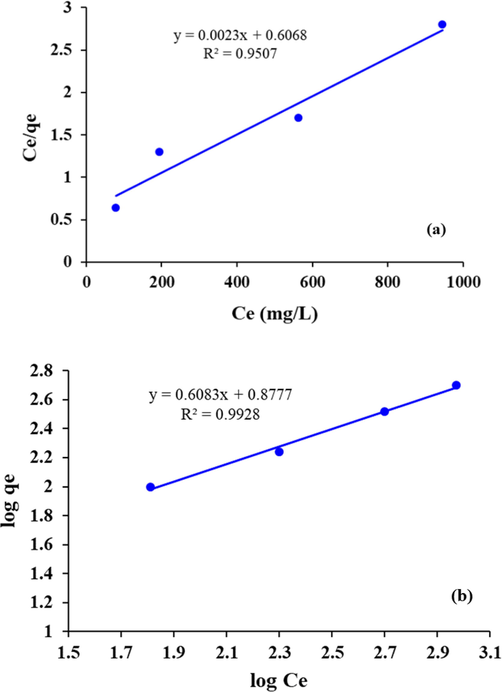
(a) Langmuir and (b) Freundlich model for COD reduction by Fe/Cu NPs.
Freundlich isotherm is an empirical model assuming that the distribution of the heat on the adsorbent surface is nonuniform, namely heterogeneous adsorption (Freundlich and Heller, 1939). The equation is stated as follows:
The Freundlich isotherm model relates to adsorption on heterogeneous surfaces with the interaction between the adsorbed molecules. The well-known linear expression for the Freundlich model is given as:
qe = mg of adsorbate per g of adsorbent at equilibrium (mg/g)
Kf = Freundlich constant
1/n = Heterogeneity factor which is related to adsorption intensity (n is always greater than one)
Ce = concentration of adsorbate in solution (mg/L)
The values of n and Kf obtained from the slope and intercept of the plot of log qe against log Ce and values are given in Table 1.
COD
Phenol
Langmuir isotherm
qm (mg/g)
434.1
−0.95
KL (L/mg)
0.003
−0.090
R2
0.950
0.981
Freundlich isotherm
n
1.31
0.86
Kf (mg/g)
20.3
4.2
R2
0.992
0.995
The adsorption data of COD reduction by Fe/Cu NPs were fitted according to the linear form of the Freundlich adsorption (R2 = 0.992) (Fig. 8(b)).
The plot of Ce/qe versus Ce for phenol (Fig. 9(a)) was found to be linear with a negative slope, indicating that the adsorption behavior of the tested adsorbent does not follow the Langmuir isotherm. A linear plot (Fig. 9(b)) was obtained in the case of Freundlich isotherm. Based on R2 value (0.995) the linear form of the Freundlich isotherm appears to produce a reasonable and applicable model on the ongoing adsorption process for phenol. Different constant values are given in Table 1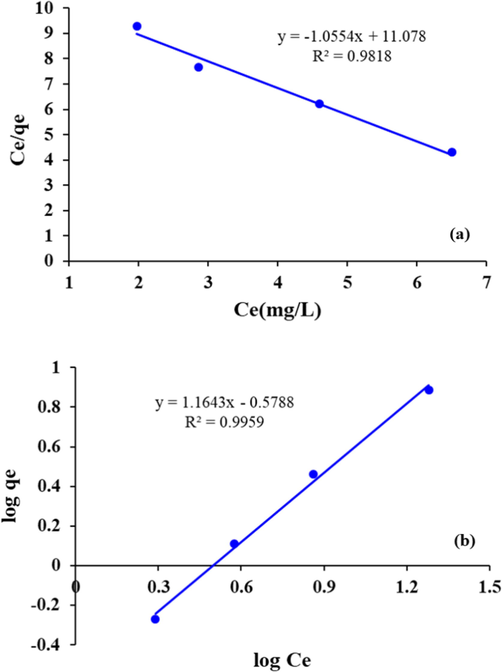
(a) Langmuir and (b) Freundlich model for Phenol removal by Fe/Cu NPs.
The n value indicates the degree of nonlinearity between solution concentration and adsorption as follows: If n = 1, then adsorption is linear;
if n < 1 then adsorption is a chemical process
if n > 1, then adsorption is a physical process. In this study the n value for phenol was found to be 0.864, Table 1.
4 Conclusions
In this present study, iron and copper precursor salts were used to synthesize Fe/Cu nanoparticles using ascorbic acid in a two-step synthesis approach. The as-synthesized Fe/Cu nanoparticles were characterized by different characterization techniques such as UV–visible spectroscopy, X-ray powder diffraction, FTIR, thermal gravimetric analysis, and scanning electron microscopy. The average size of the as-synthesized nanoparticles was between 140 nm and 160 nm. The Fe/Cu nanoparticles were then successfully used to treat industrial wastewaters possessing high chemical oxygen demand (COD) values and containing phenols, the results obtained revealed that the as-synthesized Fe/Cu nanoparticles possess a 83.33% and 76.91% efficiency for COD reduction and phenol removal, respectively. The optimum pH was found to be 4, while the adsorbent dose is 1 g, and contact time of 60 min, correspondingly. It was also noticed that the initial concentration of phenol and initial COD values played an vital role on the efficiency of Fe/Cu nanoparticles. Hence it can be concluded that the Fe/Cu nanoparticles are effective adsorbent material for the removal of phenol and reduction of COD from industrial wastewaters. Further studies into the optimization of the Fe/Cu molar ratio for further enhanced activity is warranted.
Acknowledgements
The authors would like to acknowledge the Researchers supporting project number (RSP-2021/222), King Saud University, Riyadh, Saudi Arabia and laboratory facilities of environmental sciences department of Kinnarid College for Women and PCSIR Labs, Lahore Pakistan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Enhancing visible light responsive photocatalytic activity by decorating Mn-doped ZnO nanoparticles on graphene. Ceram. Int.. 2014;40(7):10085-10097.
- [Google Scholar]
- Enhancement in visible light-responsive photocatalytic activity by embedding Cu-doped ZnO nanoparticles on multi-walled carbon nanotubes. Appl. Surf. Sci.. 2013;285:702-712.
- [Google Scholar]
- Synthesis of NiO nanoparticles and their evaluation for photodynamic therapy against HeLa cancer cells. J. King Saud Univ. Sci.. 2020;32(2):1395-1402.
- [Google Scholar]
- Asgari, G., Ramavandi, B., 2012. Study of phenol adsorption from wastewater using pumice modified by Mg/Cu bimetallic particles.
- Water pollution in Pakistan and its impact on public health—a review. Environ. Int.. 2011;37(2):479-497.
- [Google Scholar]
- Stabilization of Fe/Cu nanoparticles by starch and efficiency of arsenic adsorption from aqueous solutions. Environ. Earth Sci.. 2017;76:1-12.
- [Google Scholar]
- Removal of arsenic (III) and arsenic (V) from aqueous solutions through adsorption by Fe/Cu nanoparticles. J. Chem. Technol. Biotechnol.. 2018;93(1):63-71.
- [Google Scholar]
- Applications of nanotechnology in wastewater treatment—a review. J. Nanosci. Nanotechnol.. 2014;14(1):613-626.
- [Google Scholar]
- Synthesis of TiO2 catalysts doped with Cu, Fe, and Fe/Cu supported on clinoptilolite zeolite by an electrochemical-thermal method for the degradation of diclofenac by heterogeneous photocatalysis. J. Photochem. Photobiol., A. 2019;380:111834.
- [Google Scholar]
- Essential metal-based nanoparticles (copper/iron NPs) as potent nematicidal agents against Meloidogyne spp. J. Nanotechnol. Res.. 2019;1:44-58.
- [Google Scholar]
- Preparation of Al2O3-supported nano-Cu2O catalysts for the oxidative treatment of industrial wastewater. Russ. J. Phys. Chem. A. 2009;83(13):2308-2312.
- [Google Scholar]
- Pollutant removal from industrial discharge water using individual and combined effects of adsorption and ion-exchange processes: Chemical abatement. J. Saudi Chem. Soc.. 2016;20(2):185-194.
- [Google Scholar]
- Nanotechnology in water treatment applications. Caister Academic Press; 2010.
- Darisimall, 2006. Sore throat lozenges and sprays.
- Pollution indicators in Gaborone industrial effluent. J. Appl. Sci. (Pakistan) 2005
- [Google Scholar]
- Assessment of green and chemically synthesized copper oxide nanoparticles against hepatocellular carcinoma. J. King Saud Univ. Sci.. 2021;33(8):101669.
- [Google Scholar]
- The adsorption of cis-and trans-azobenzene. J. Am. Chem. Soc.. 1939;61(8):2228-2230.
- [Google Scholar]
- Treatment of wastewater containing high concentration of phenol and total dissolved solids in moving bed biofilm reactor. IJIRSET. 2014;3:10924-10930.
- [Google Scholar]
- The toxic effects of chlorophenols and associated mechanisms in fish. Aquat. Toxicol.. 2017;184:78-93.
- [Google Scholar]
- Cation distribution in copper ferrite nanoparticles of ferrofluids: A synchrotron XRD and EXAFS investigation. J. Magn. Magn. Mater.. 2006;300(1):e213-e216.
- [Google Scholar]
- Clinical toxicology of commercial products. Williams & Wilkins Baltimore. 1984;1085
- [Google Scholar]
- Chemical reduction of nitrate using nanoscale bimetallic iron/copper particles. Polish J. Environ. Stud.. 2018;27(5):2023-2028.
- [Google Scholar]
- Effects of temperature and inorganic salts on the adsorption of phenol from multicomponent systems onto a decolorizing carbon. Sep. Sci. Technol.. 1997;32(18):3027-3036.
- [Google Scholar]
- Ida, S., Eva, T., 2021. Removal of Heavy Metals during Primary Treatment of Municipal Wastewater and Possibilities of Enhanced Removal: A Review. Water 13, 1121.
- Environmental and health impacts of industrial wastewater effluents in Pakistan: a review. Rev. Environ. Health. 2019;34(2):171-186.
- [Google Scholar]
- Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total Environ.. 2018;644:1181-1189.
- [Google Scholar]
- Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res.. 2009;11(1):77-89.
- [Google Scholar]
- Biodegradation of phenol at high initial concentration by Alcaligenes faecalis. J. Hazard. Mater.. 2007;147(1-2):672-676.
- [Google Scholar]
- Synthesis of Au, Ag, and Au–Ag bimetallic nanoparticles using Pulicaria undulata extract and their catalytic activity for the reduction of 4-nitrophenol. Nanomaterials. 2020;10(9):1885.
- [Google Scholar]
- Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalton Trans.. 2014;43(24):9026-9031.
- [Google Scholar]
- Enhanced antimicrobial activity of biofunctionalized zirconia nanoparticles. ACS Omega. 2020;5(4):1987-1996.
- [Google Scholar]
- Review on research for removal of phenol from wastewater. Int. J. Sci. Res. Publ.. 2013;3:1-5.
- [Google Scholar]
- The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc.. 1918;40(9):1361-1403.
- [Google Scholar]
- Analytical approaches for determining chemical oxygen demand in water bodies: a review. Crit. Rev. Anal. Chem.. 2018;48(1):47-65.
- [Google Scholar]
- Preparation and characterization of ascorbic acid-mediated chitosan–copper oxide nanocomposite for anti-microbial, sporicidal and biofilm-inhibitory activity. J. Nanostruct. Chem.. 2018;8(3):301-309.
- [Google Scholar]
- A facile strategy for enhancing FeCu bimetallic promotion for catalytic phenol oxidation. Catal. Sci. Technol.. 2015;5(6):3159-3165.
- [Google Scholar]
- Phase transformations and electrical and magnetic properties of Fe73. 5Cu1Nb3Si15. 5B7 amorphous alloy. J. Optoelectron. Adv. Mater.. 2010;12:233.
- [Google Scholar]
- Assessment of industrial wastewater for potentially toxic elements, human health (dermal) risks, and pollution sources: A case study of Gadoon Amazai industrial estate, Swabi, Pakistan. J. Hazard. Mater.. 2021;419:126450.
- [CrossRef] [Google Scholar]
- Nitrates removal by bimetallic nanoparticles in water. Chem. Eng. Trans.. 2016;47:205-210.
- [Google Scholar]
- A greener synthesis of core (Fe, Cu)-shell (Au, Pt, Pd, and Ag) nanocrystals using aqueous vitamin C. Cryst. Growth Des.. 2007;7(12):2582-2587.
- [Google Scholar]
- Industrial waste water management in district Gujranwala of Pakistan-current status and future suggestions. Pak. J. Agri. Sci. 2012;49:79-85.
- [Google Scholar]
- NEQS, National Environmental Quality Standards for Municipal and Liquid Industrial Effluents. 2005.
- Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol.. 2008;99(15):6709-6724.
- [Google Scholar]
- Catalytic properties of phosphate-coated CuFe2O4 nanoparticles for phenol degradation. J. Nanomater.. 2019;2019:1-8.
- [Google Scholar]
- Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: a review. J. Environ. Manage.. 2008;86(3):481-497.
- [Google Scholar]
- Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals: minireview. Environ. Microbiol.. 2001;3(2):81-91.
- [Google Scholar]
- “Miswak” based green synthesis of silver nanoparticles: evaluation and comparison of their microbicidal activities with the chemical synthesis. Molecules. 2016;21(11):1478.
- [Google Scholar]
- Green synthesis and characterization of palladium nanoparticles using Origanum vulgare L. extract and their catalytic activity. Molecules. 2017;22(1):165.
- [Google Scholar]
- Polyacrylamide@ Zr (IV) vanadophosphate nanocomposite: ion exchange properties, antibacterial activity, and photocatalytic behavior. J. Ind. Eng. Chem.. 2016;33:201-208.
- [Google Scholar]
- Novel development of nanoparticles to bimetallic nanoparticles and their composites: a review. J. King Saud Univ. Sci.. 2019;31(2):257-269.
- [Google Scholar]
- Multilayer system of nanoscale zero valent iron and Nano-Fe/Cu particles for nitrate removal in porous media. Sep. Purif. Technol.. 2018;193:242-254.
- [Google Scholar]
- Bimetal CuFe nanoparticles—synthesis, properties, and applications. Appl. Sci.. 2021;11(5):1978.
- [Google Scholar]
- Removal of indigo blue in aqueous solution using Fe/Cu nanoparticles and C/Fe–Cu nanoalloy composites. Water Air Soil Pollut.. 2010;207(1-4):307-317.
- [Google Scholar]
- A green method for the synthesis of copper nanoparticles using L-ascorbic acid. Matéria (Rio de janeiro). 2014;19(3):197-203.
- [Google Scholar]
- USEPA, 1977. Sampling and Analysis Procedure for Screening of Industrial Effluents for Priority Pollutants. Environment Monitoring and Support Laboratory, Cincinnati, OH, USA.
- Removal of high-concentration CI acid orange 7 from aqueous solution by zerovalent iron/copper (Fe/Cu) bimetallic particles. Ind. Eng. Chem. Res.. 2014;53(7):2605-2613.
- [Google Scholar]
- Green and size-specific synthesis of stable Fe–Cu oxides as earth-abundant adsorbents for malachite green removal. ACS Sustainable Chem. Eng.. 2018;6(7):9229-9236.
- [Google Scholar]
- Synthesis of a Fe 3 O 4–CuO@ meso-SiO 2 nanostructure as a magnetically recyclable and efficient catalyst for styrene epoxidation. Catal. Sci. Technol.. 2014;4(9):3082-3089.
- [Google Scholar]







