Translate this page into:
Artemisia monosperma induces ROS-mediated cell death in human colorectal carcinoma cells via modulating apoptotic genes
⁎Corresponding author at: Chair for DNA Research, Department of Zoology, College of Science, King Saud University, Riyadh 11451, Saudi Arabia. masiddiqui@ksu.edu.sa (Maqsood A. Siddiqui)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background
Colorectal cancer (CRC) is the third most diagnosed cancer in the world. Artemisia monosperma (family: Asteraceae), a flowering plant species in the genus Artemisia, is widely used in traditional medicine. Plant extracts are well documented to combat against cancer diseases. Nevertheless, the outcome of A. monosperma extracts on human colorectal carcinoma cells (HCT-116) have not been investigated in details.
Objectives
Thus, herein we aimed to evaluate the cytotoxic effect of different extracts i.e. n-hexane (AM-H), chloroform (AM-C), n-butanol (AM-B), and aqueous (AM-Aq) of A. monosperma against HCT-116 cells and to identify molecular mechanism(s) involved in cytotoxicity. Methods: The HCT-116 cells were treated with 10–1000 μg/mL of extracts for 24 h.
Results
Our results displayed that all extracts significantly decreased cell viability in HCT-116 cells and changed the cellular morphology in a dose-dependent way. Among these extracts, AM-C showed higher cytotoxic activity with IC50 of 250.5 μg/mL. Further AM-C extract increased ROS production and reduced MMP level in HCT-116 cells. Moreover, AM-C extract induces cell apoptosis by the upregulation of proapoptotic related genes (p53, Bax, caspase-3, and caspase-9) and downregulation of antiapoptotic gene, Bcl-2. More significantly, these results revealed that AM-C extract enhances apoptosis in HCT-116 cells associated with ROS generation and mitochondrial dysfunction.
Conclusion
Our findings suggest that the extracts of A. monosperma can be an auspicious therapeutic agent against colorectal cancer.
Keywords
HCT-116 cells
Artemisia monosperma
Cytotoxicity
ROS
MMP
Apoptosis
1 Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer and the second leading cause of cancer associated deaths in both men and women. It occurs when cells in the rectum or colon grow uncontrolled. Globally, colorectal cancer accounts for 10% cancer incidence and 9.4% of mortality in 2020 (Xi and Xu 2021). According to American Cancer Society, the number of new colorectal cancer cases (106180 colon cancer and 44,850 rectal cancer cases), adding to a total of 151,030 new cases are estimated for 2022 (Siegel et al., 2022). Although age-standardized occurrence and death rates have significantly decreased recently in developed countries for colorectal cancer, but the incidence and death rates have increased for colorectal cancer in developing countries (Xia et al., 2022). Based on the estimate of population growth and aging, the worldwide number of new CRC cases are projected to reach 3.2 million in 2040 (Xi and Xu 2021). The rising number of CRC cases and increasing incidents amongst young population possess a substantial financial burden and an enormous public health challenge. The colon cancer growth is known to be linked with an extreme cell propagation and abnormality of cell cycle evolution and apoptosis (Mutch, 2007). The treatment of CRC includes a combination of chemotherapy, radiotherapy, and surgery. Although significant advances have been made, but novel anticancer therapeutic agent with less side effects are still needed. With relatively fewer side effects, plants have recently produced more interest in the discovery of anticancer agent (Greenwell and Rahman, 2015). Plants are rich in secondary metabolites, believed for their pharmacological and preventive properties (Eswaraiah et al., 2020; Mohamed et al., 2021). Currently, the devotion is on for commercializing the use of plants for medicinal importance with demands for anticancer agents of natural source. Plants derived anticancer agents and its phytochemical elements have been testified to affect with the molecular paths involved in the development and initiation of colon cancer (Gustin and Brenner, 2004). Numerous reports on medicinal plants used in Arabian Peninsula indicate their potential as sources for the development of anticancer agent (Ahmad et al., 2017). Artemisia monosperma (family: Asteraceae), is a flowering plant species in the genus Artemisia (El-Sherbeny et al., 2021). It is a thick leafy plant with elongated thin leaves and play important role in the ecological succession by stabilizing sand dunes (Badr et al. 2012). A. monosperma is common in desert plains and Vadis of Arabian Peninsula, and its leaves are extensively used in traditional medicine in Saudi Arabia and Egypt (Guetat et al., 2017). A. monosperma is similarly found in the desert of further Arabian countries like Kuwait, Iraq, Jordan, Lebanon, and Syria. It grows usually up to 1 m in height and is famous under the local name of “Aader” or “Salikah” in Saudi Arabia (Migahid and Hammouda, 1978). The antibacterial activity of A. monosperma has been studied by Guetat et al. (2017) against Streptococcus Agalactiae with strong inhibition. The antispasmodic effect of A. monosperma extract has been shown in a dose dependent manner in rat smooth muscles of uterus, ileum, and pulmonary artery (Abu-Niaaj et al., 2019). A. monosperma has been described to have numerous biological actions such as; antioxidant, anti-inflammatory, and antimicrobial (El Zalabani et al., 2017). The ethanolic extract of A. monosperma instigated from Israel was reported to be cytotoxic on several cancer cell lines (Solowey et al., 2014). Various types of secondary metabolites and bioactive phytochemicals have also been documented in A. monosperma plants including flavonoids, alkaloids, coumarins, acetylene, triterpenes, and sesquiterpene (Khan et al., 2012). Although several reports in the literature have been stated on the biological and cytotoxic properties of A. monosperma, but detailed reports on anticancer potential have not been studied. Therefore, this driven us to investigate the anticancer effect of A. monosperma against human colorectal carcinoma cell line (HCT-116). Firstly, we have ascertained the cytotoxic potential of different A. monosperma plant extracts i.e. n-hexane (AM-H), chloroform (AM-C), n-butanol (AM-B), and aqueous (AM-Aq) against HCT-116 cells. Secondly, the utmost effective extract AM-C was used for the identification of the mechanism(s) involved in the cell death.
2 Materials and methods
2.1 Plant material and extraction
The aerial part of A. monosperma was collected from central region of Riyadh, Saudi Arabia in month of October/November. The plant was kindly authenticated by a taxonomist at Botany Department, College of Science, King Saud University, Riyadh. The plant was shade dried and grounded into coarse powder. Nearly 500 gm of plant powder was separately soaked in n-hexane, chloroform, n-butanol, and distilled water for 3 days with frequent shaking. Then extract was collected respectively by filtration using a Whatman filter#1. The obtained extracts were dried separately on a rotary evaporator at 40 °C under reduced pressure until solvent was fully evaporated. The resultant n-hexane, chloroform, n-butanol, and distilled water extracts were named as AM-H, AM-C, AM-B, and AM-Aq, respectively. The extracts were stored at 4 °C in dark until use.
2.2 Cell culture
HCT-116, a human colorectal carcinoma cell line was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in DMEM, with 10% fetal bovine serum and 1% antibiotic/antimycotic solution in a CO2 incubator (5% CO2 and humidified atmosphere) at 37 °C.
2.3 Treatment and sample preparation
AM-Aq, AM-H, AM-B, and AM-C extracts were diluted in DMSO. Then it was suspended in DMEM cell culture medium to give a final concentrations of 1000 μg/mL. Further serial dilution was made to reach desired concentrations of respective extracts in the culture medium. The fully grown HCT-116 cells were harvested using trypsin and seeded in the microwell plates as per the experimental demand. Firstly, cytotoxic potential of AM-Aq, AM-H, AM-B, and AM-C extracts was assessed by exposing them with a range of concentrations (10, 25, 50, 100, 250, 500, and 1000 μg/mL) using MTT and NRU assays. Then, the extract exhibiting highest cytotoxic potential i.e. AM-C against HCT-116 cells was selected for further studies.
2.4 MTT assay
MTT assay was performed in accordance with the earlier study with slight modifications (Siddiqui et al., 2008). In brief, HCT-116 cells were collected, counted, and plated in a 96-well plate at a density of 1 × 104 cells/well. Then, cells were exposed to diverse concentrations of AM-Aq, AM-H, AM-B, and AM-C extracts separately for 24 h at 37 °C. After treatment, 10 μl of MTT (5 mg/mL) was added to wells and incubated for 4 h. The solution was then aspirated and 200 μl DMSO was added to each well to dissolve the formazan crystal. The plate was read at 550 nm by micro plate reader. The results were examined as the percentage cell viability in respect to the control.
2.5 NRU assay
The NRU assay was performed following the protocol defined by Siddiqui et al. (2008). After the treatment of HCT-116 at 10–1000 μg/mL of AM-Aq, AM-H, AM-B, and AM-C extracts for 24 h, the solution was removed from each well. The wells were then rapidly washed once with PBS and 100 μl of NR solution (50 μg/mL in medium) was added to wells. The plate was incubated for 3 h further to endorse dye uptake by lysosomes. Afterward, the solution was discarded from the wells and incorporated dye was extracted in 200 μl of neutral red distain solution (water: ethanol: glacial acetic acid, 49: 50: 1 (v/v/v)). The absorbance values were read at 550 nm using a microplate reader.
2.6 Morphological changes
Observation of morphological alterations of dead cells was performed by using phase contrast microscopy. In brief, 2 × 104 HCT-116 cells were incubated with various extracts for 24 h. The morphological variations of the apoptosis cells were observed at 20 × magnifications under phase contrast microscope (Olympus, CKX41, Japan).
2.7 Analysis of mitochondrial membrane potential (MMP)
The effect of AM-C extract on the MMP was analyzed by mitochondrial specific fluorescent dye Rhodamine-123 (Sigma Aldrich, USA) (Siddiqui et al., 2013). Rh-123 is a dye that can selectively enter into the mitochondria and shows a strong red fluorescence in healthy mitochondria with normal membrane potential. In the unhealthy cells with reduced MMP, the red fluorescence disappears. In brief, HCT-116 cells treated with diverse concentrations of AM-C for 24 h, were incubated with 10 μg/mL of Rh-123 dye for 1 h in dark. Subsequently, the Rh-123 fluorescence in cells was measured with 538 nm excitation and 480 nm emission using a fluorescence microplate reader. The Rh-123 fluorescence was also observed on fluorescence microscope.
2.8 Determination of ROS generation
The intracellular ROS production was assessed by dichlorodihydrofluorescein diacetate (DCF-DA; Sigma Aldrich, USA) assay (Siddiqui et al., 2013). Briefly, HCT-116 cells were seeded at a density of 2x104 cells/well of 48 well plate. Then, cells were treated with diverse concentrations of AM-C for 24 h. Further cells were washed and incubated with DCF-DA dye (20 μM) for 60 min in dark. The DCF fluorescence intensity was measured at 485 nm excitation and 530 emission and cell images were grabbed under fluorescence microscope.
2.9 Determination of apoptotic-pathway related genes by RT-qPCR
HCT-116 cells were seeded in a 6-well plate at a density of 1x105 cells/well and incubated overnight for cell attachment. Next, the cells were treated with 250 μg/mL of AM-C for 24 h. After 24 h incubation, treated and untreated cells were trypsinized and collected by centrifugation. The total RNA extraction was done by using Qiagen RNeasy mini kit as per the instruction provided with the kits. The quality and concentration of RNA was measured by nanodrop 800. Further, cDNA was prepared by reverse transcriptase according to the M−MLV and Oligo (dT) primer (Promega). The Roche Light Cycler® 480 analysis system was used to examine the apoptosis related genes in HCT-116 cells treated with AM-C extract. The details about primers and cycling conditions are described in our prior published work (Al-Oqail et al., 2017).
2.10 Statistical analysis
The data are expressed as mean ± S.D. The results were analyzed by one-way analysis of variance employing student t-test. A difference at p < 0.05 was considered as statistically significant. Each experiment was performed in three replicates.
3 Results
3.1 MTT assay
The cytotoxic potential of AM-Aq, AM-H, AM-B, and AM-C extracts on HCT-116 cells was measured by MTT assay. HCT-116 cells were treated with 0, 10, 25, 50, 100, 250, 500, and 1000 μg/mL for 24 h. The cytotoxic activities of the AM-Aq, AM-H, AM-B, and AM-C extracts of the plants are summarized in Fig. 1. As shown in figure, all the extracts exhibited a concentration dependent cytotoxic effect against HCT-116 cells. It is worth noting that AM-C extract exhibited higher cytotoxicity in HCT-116 cells with IC50 value of 264.8 μg/mL. The AM-B extract displayed moderate cytotoxicity with IC50 value of 714.2 μg/mL, while AM-H and AM-Aq extracts showed significantly lower cytotoxicity with IC50 value of > 1000 μg/mL against HCT-116 cells in this study.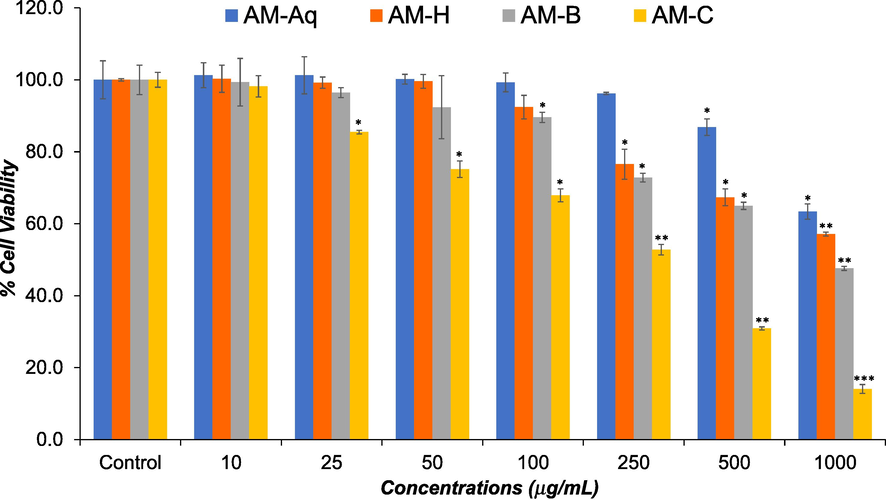
Effects of AM-Aq, AM-H, AM-B, and AM-C extracts on viability of human colon cancer cells (HCT-116). HCT-116 cells were treated with various concentrations (10 – 1000 μg/mL) of extracts for 24 h. Cell viability was measured by MTT assay. The data represent the mean and standards deviation of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 vs control.
3.2 NRU assay
As shown in Fig. 2, the AM-Aq, AM-H, AM-B, and AM-C extracts also showed the dose dependent cytotoxicity in HCT-116 cells in NRU assay. The AM-C extract showed highest cytotoxic response with IC50 value of 250.5 μg/mL followed by AM-B extract with IC50 value of 737.4 μg/mL. Like MTT assay, AM-H, and AM-Aq extracts showed significantly lower cytotoxicity with IC50 value of > 1000 μg/mL.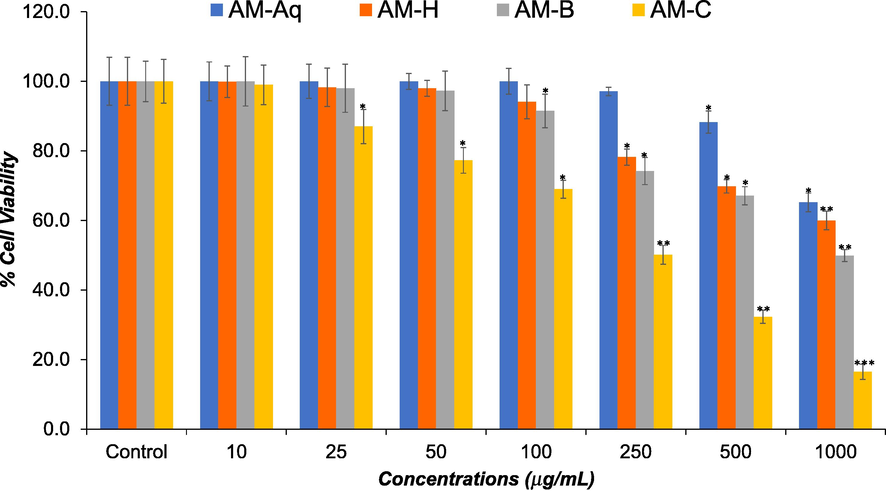
Effects of AM-Aq, AM-H, AM-B, and AM-C extracts on viability of human colon cancer cells (HCT-116). HCT-116 cells were treated with various concentrations (10 – 1000 μg/mL) of extracts for 24 h. The cell viability was assessed by NRU assay. The data represent the mean and standards deviation of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 vs control.
3.3 Morphological changes
The cytotoxic nature of extracts was investigated by morphological changes in HCT-116 cells. After treating the cells with extracts for 24 h, morphological changes in HCT-116 cells were observed in comparison to untreated control cells (Fig. 3). Microscopic observation of control cells showed that they maintained their regular morphology. However, exposure of HCT-116 cells with extracts exhibited typical apoptosis features such as shrinkage, losing contact with other cells, membrane blebbing, and rounded bodies. Based on the cytotoxicity assays, the most effective extract AM-C was used for further investigations.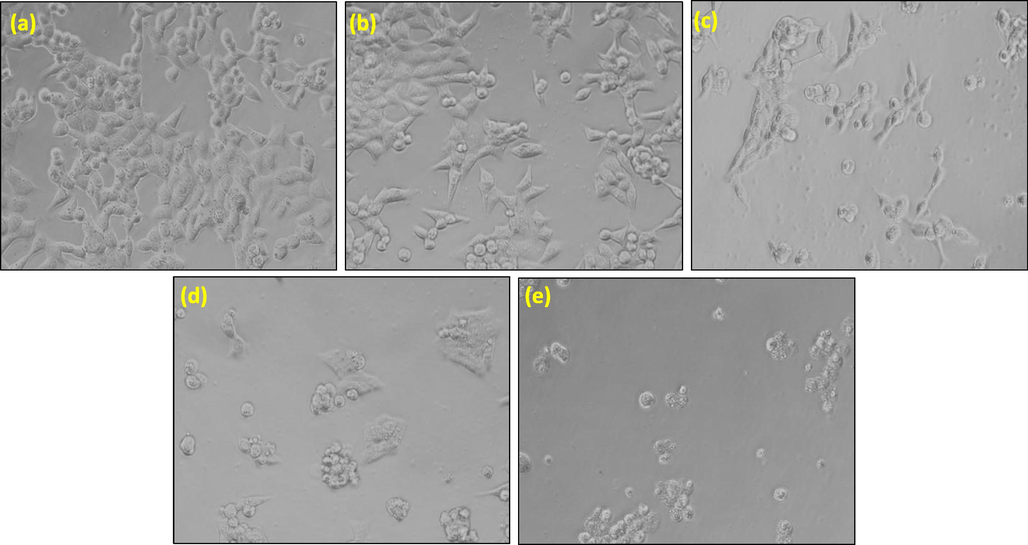
Representative morphological changes in HCT-116 cells after the exposure of 1000 μg/mL concentration of AM extract for 24 h. (a) Control, (b) AM-Aq, (c) AM-H, (d) AM-B and (e) AM-C. Cell images were grabbed at 20 × magnification.
3.4 Effect of AM-C on MMP level
The reduction in the MMP level designates mitochondrial dysfunction in cells. Our results showed that exposure of AM-C to HCT-116 at 250–1000 μg/mL reduced the intensity of red fluorescence dose dependently (Fig. 4a). Our quantitative examination also displayed that AM-C reduce the MMP level by 13%, 39%, and 63% at 250, 500, and 1000 μg/mL, respectively (Fig. 4b).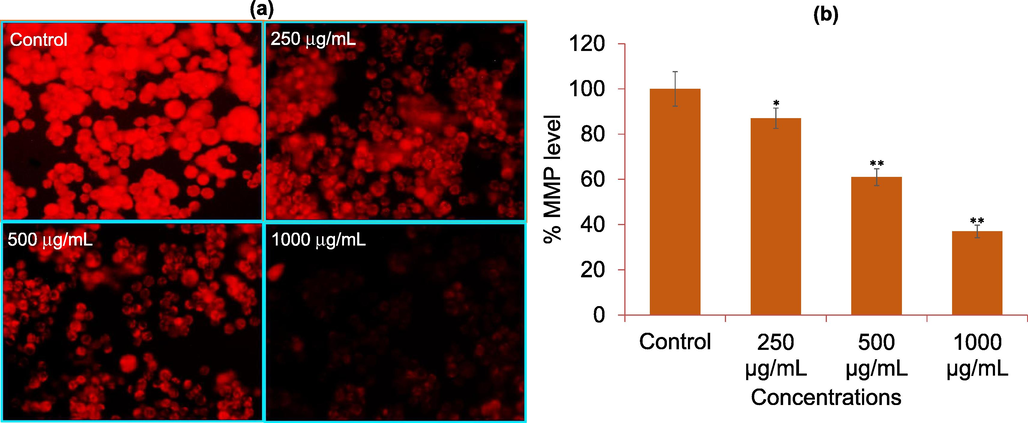
Mitochondrial membrane potential (MMP) analysis by Rhodamine123 dye in HCT-116 cells. (a) Rhodamine-123 fluorescence intensity in cells treated with 250, 500, and 1000 μg/mL of AM-C for 24 h. (b) The graph is illustrating the percent loss of mitochondrial membrane potential as compare to control group. The data are expressed as mean ± S.D. from three independent experiments. *p < 0.05 and **p < 0.01 vs control.
3.5 Effect of AM-C on ROS level
As shown in Fig. 5a, DCF fluorescence observed by fluorescence microscopic images displayed a substantial increase in ROS level at all selected concentrations compared to control. The fluorescence strength of DCF was also quantified by fluorescence reader and was found to be increased by 132%, 174%, and 216% in HCT-116 cells treated with 250, 500, and 1000 μg/mL of AM-C, respectively (Fig. 5a).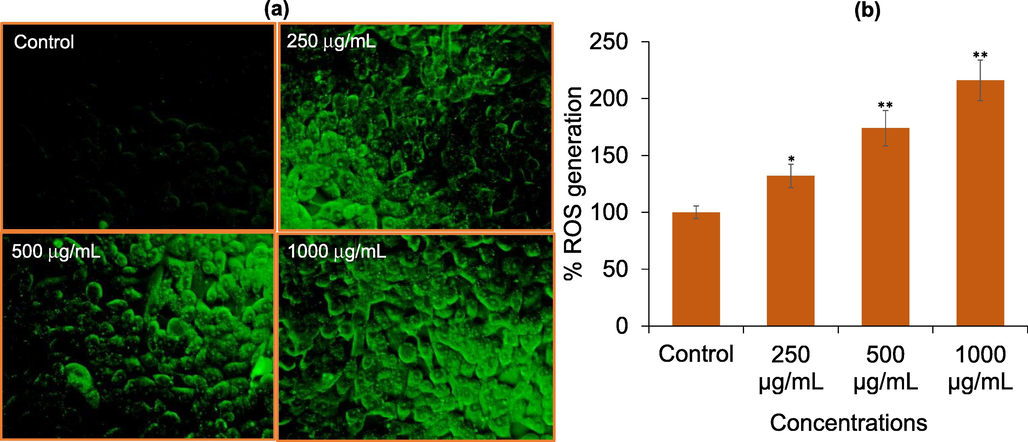
Reactive oxygen species (ROS) generation analysis by DCF-DA in HCT-116 cells. (a) DCF fluorescence intensity in cells treated with 250, 500, and 1000 μg/mL of AM-C for 24 h. (b) The graph is illustrating the induction in ROS generation in HCT-116 cells as compare to control group. The data are expressed as mean ± S.D. from three independent experiments. *p < 0.05 and **p < 0.01 vs control.
3.6 Effect of AM-C on apoptosis marker genes
The effect of AM-C on the mRNA expression of apoptosis genes are given in Fig. 6. As estimated by RT-qPCR, the HCT-116 cells treated with AM-C resulted in the significant (p < 0.01) upregulation of p53 (2.9-fold), Bax (2.6-fold), caspase-3 (2.1-fold), and caspase-9 (2.3-fold) genes. However, AM-C at 250 μg/mL concentration resulted in the significant downregulation of Bcl-2 (0.45-fold) gene.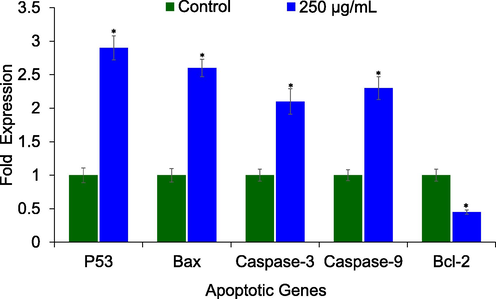
AM-C extract regulated the mRNA expression of apoptotic genes. Human colon cancer cells (HCT-116) were treated with 250 μg/mL of AM-C extract for 24 h. The data represent the mean and standards deviation of three independent experiments. *p < 0.01 vs control.
4 Discussion
Because of the variations in the lifestyle, such as the lack of physical activities, the use of alcohol and smoking, little consumption of fruits and vegetables, high body mass index, and exposure of harmful chemical elements, the occurrence of colorectal cancer has been growing regularly (Papadimitriou et al. 2021). Though initial detection and regular examination has decreased the mortality rate, but still colorectal cancer claims a plenty of lives worldwide every year (Blackmore et al. 2020). Hence, there is an imperative need for identifying novel anticancer therapeutic agent with less side effects that can help in the treatment of colorectal cancer. Plants and their extracts have recently produced more attention in the detection of anticancer agents for colorectal cancer due to their non-toxic nature (Boulaaba et al., 2013; Greenwell and Rahman, 2015). Hence, in this study, we discovered the anticancer efficacy of various plant extracts such as aqueous (AM-Aq), n-hexane (AM-H), n-butanol (AM-B), and chloroform (AM-C) extracts of A. monosperma plant against human colorectal cancer cell line (HCT-116). Preliminary screening of cytotoxic potential of these extracts was done on HCT-116 cells. The cytotoxicity of these extracts was performed by MTT and NRU assays. These MTT and NRU cytotoxicity assays with cultured cells are extensively used for cytotoxicity assessment of drugs and plant extracts (Farshori et al., 2022). We found that all the extracts exhibited a concentration dependent cytotoxic potential against HCT-116 cells, however AM-C extract exhibited higher cytotoxicity followed by AM-B that displayed moderate cytotoxicity, and AM-H and AM-Aq extracts which showed significantly lower cytotoxicity against HCT-116 cells. These findings are in agreement with the previous studies that have revealed the cytotoxic potential of A. monosperma against various cancer cell lines in vitro (Solowey et al., 2014). A number of chemical constituents of A. monosperma from Saudi Arabia and its cytotoxic potential have been reported (Khan et al., 2012). The compounds found in A. monosperma have also been shown to exhibit cytotoxicity against numerous human cancer cell lines such as pancreatic (MIA PaCa-2), larynx epidermoid carcinoma (HEp-2), lung carcinoma (A-549), and colon carcinoma (HT-29) (Whelan and Ryan, 2004). It is also reported that chloroform extract of plants has better cytotoxic effects than other solvent against cancer cell lines (Jafarian et al., 2014). The cytotoxic nature of extracts was also assessed by morphological changes in HCT-116 cells. As shown in Fig. 3, the cells treated with extracts showed typical common structures of apoptosis cells such as shrinkage, losing contact with other cells, membrane blebbing, and rounded bodies. These characteristics the typical form of morphological changes in cells are commonly used for determination and identification of apoptotic cell death (Syed Abdul Rahman et al., 2013). Based on the cytotoxicity results, most effective extract i.e. AM-C was chosen for further studies on the potential molecular mechanisms of action. We have investigated the mechanism of oxidative stress in HCT-116 cells exposed to AM-C. The role of ROS is well documented in the development of oxidative stress. As found in this study, ROS level was dose dependently increased in HCT-116 cells exposed to AM-C at diverse concentrations. These results indicate the role of ROS generation in HCT-116 cell death induced by AM-C. Cancer cells hold high ROS level because of its higher metabolic rate and have been found to promote antitumorigenic signaling by initiating oxidative stress induced cancer cell death (Arfin et al., 2021). The raised level of ROS is important to cancer cell to proliferate and grow, but at same time overproduction of ROS could lead cancer cells to the oxidative stress and apoptosis (Qian et al., 2019). It has also been described that administration of plant extracts could produce excess level of ROS that leads to oxidative stress and apoptotic cancer cell death (Al-Oqail, 2021). The results showed that AM-C could promote ROS accumulation in HCT-116 cells, and indicated that AM-C induced oxidative stress and mitochondrial dysfunction during the apoptosis of HCT-116 cells. Our data displayed that AM-C could reduce the MMP level in HCT-116 cells and depolarize the mitochondrial membranes. The decrease level of MMP is one of the features of early apoptosis. The mitochondrial membrane depolarization leads to proapoptotic factors and activation of protease in mitochondria that induces apoptosis (Chen et al., 2022). Mitochondria is one of the most protuberant sources of ROS within the cells which contribute to oxidative stress (Starkov, 2008). It is documented that excessive ROS level extinguish mitochondrial membrane, causing the release of cytochrome c from mitochondria and induces the apoptosis in intrinsic apoptosis pathway (Moloney and Cotter, 2018). Apoptosis is known as program cell death and various pathways started by apoptosis leads to the cell death. The cytochrome c forms an apoptotic complex with apoptotic proteins that resulted in the activation of caspase-9 followed by the induction of effector molecules such as caspase-3 and −7 (Susin et al., 1999). Moreover, ROS has been proven to regulate the instigation of both antiapoptotic (BCl-2) and proapoptotic (Bax) family, which play a significant role in regulating the intrinsic apoptosis pathway (Qian et al., 2019). In order to confirm the mechanism of AM-C induced cytotoxicity in HCT-116 cells, the mRNA expression of apoptosis related genes was studied by quantitative real time PCR. Our findings exhibited that HCT-116 cells exposed to AM-C induces apoptosis by upregulating the mRNA expression of pro-apoptotic marker genes (p53, Bax, caspase-3, and caspase-9) and downregulating the mRNA expression of anti-apoptotic gene, Bcl-2. These results are consistent with the earlier reports exhibiting plant extracts and its bioactive components have the potential to induce apoptosis in colorectal cancer cells (Wongkaewkhiaw et al., 2022). The results from present investigation provided sufficient evidence that the extracts of A. monosperma-induced apoptosis in colorectal cancer cell death via ROS generation.
5 Conclusion
The current study concludes that the A. monosperma extracts exhibited cytotoxicity against human colorectal carcinoma cell line. The cytotoxic effect was found in a dose-dependent way. Compared to other extracts, AM-C extract exhibited higher cytotoxicity against HCT-116 cells. Furthermore, AM-C induced ROS generation that has led to apoptosis in HCT-116 cells. Moreover, the AM-C induced apoptosis by mitochondria dependent pathway with the loss of mitochondrial membrane permeability. The results found in present study provided reliable evidence that A. monosperma extract has the capability to combat colorectal cancer diseases and it can be an auspicious therapeutic agent for the management of CRC.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research (IFKSURC-1-3203).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The pharmacological properties of Artemisia monosperma (Del.) FASEB J.. 2019;33(S1):672.
- [Google Scholar]
- Role of traditional Islamic and Arabic plants in cancer therapy. J. Tradit. Complement. Med.. 2017;7(2):195-204.
- [Google Scholar]
- Anticancer efficacies of Krameria lappacea extracts against human breast cancer cell line (MCF-7): role of oxidative stress and ROS generation. Saudi Pharm. J.. 2021;29(3):244-251.
- [Google Scholar]
- Nigella sativa seed oil suppresses cell proliferation and induces ROS dependent mitochondrial apoptosis through p53 pathway in hepatocellular carcinoma cells. South Afr. J. Bot.. 2017;112:70-78.
- [Google Scholar]
- Genetic diversity of Artemisia populations in central and north Saudi Arabia based on morphological variation and RAPD polymorphism. Plant Syst. Evol.. 2012;298(5):871-886.
- [Google Scholar]
- Barriers and facilitators to colorectal cancer diagnosis in New Zealand: a qualitative study. BMC Fam. Pract.. 2020;21:206.
- [Google Scholar]
- Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G2/M cell cycle arrest. Cytotechnology.. 2013;65:927-936.
- [Google Scholar]
- Extracts of Knoxia roxburghii (Spreng.) MA Rau induce apoptosis in human MCF-7 breast cancer cells via mitochondrial pathways. Molecules.. 2022;27(19):6435.
- [Google Scholar]
- Chemical profile and biological activities of essential oil of aerial parts of Artemisia monosperma Del. growing in Libya. Pharmacogn. J.. 2017;9(4):578-586.
- [Google Scholar]
- Structural and chemical adaptations of Artemisia monosperma Delile and Limbarda crithmoides (L.) Dumort. in response to arid coastal environments along the mediterranean coast of Egypt. Plants. 2021;10(3):481.
- [Google Scholar]
- GC–MS analysis for compound identification in leaf extract of Lumnitzera racemosa and evaluation of its in vitro anticancer effect against MCF7 and HeLa cell lines. J. King Saud Univ. Sci.. 2020;32(1):780-783.
- [Google Scholar]
- HPTLC estimation and anticancer potential of Aloe perryi petroleum ether extract (APPeE): A mechanistic study on human breast cancer cells (MDA-MB-231) J. King Saud Univ. Sci.. 2022;34(4):101968
- [Google Scholar]
- Medicinal plants: their use in anticancer treatment. Int. J. Pharm. Sci. Res.. 2015;6(10):4103-4112.
- [Google Scholar]
- The genus Artemisia L. in the northern region of Saudi Arabia: essential oil variability and antibacterial activities. Nat. Prod. Res.. 2017;31(5):598-603.
- [Google Scholar]
- Chemoprevention of colon cancer: current status and future prospects. Cancer Metastasis Rev.. 2004;21:323-348.
- [Google Scholar]
- Cytotoxicity of different extracts of arial parts of Ziziphus spina-christi on Hela and MDA-MB-468 tumor cells. Adv. Biomed. Res.. 2014;3:38.
- [Google Scholar]
- Determination of chemical constituents of leaf and stem essential oils of Artemisia monosperma from central Saudi Arabia. Nat. Prod. Commun.. 2012;7(8):1079-1082.
- [Google Scholar]
- Flora of Saudi Arabia. Riyadh: Riyadh University Publication; 1978. p. :837.
- Date palm (Phoenix dactylifera L.) secondary metabolites: Bioactivity and pharmaceutical potential. Phytomedicine. 2021;2021:483-531.
- [Google Scholar]
- Molecular profiling and risk stratification of adenocarcinoma of the colon. J. Surg. Oncol.. 2007;96:693-703.
- [Google Scholar]
- An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun.. 2021;12:4579.
- [Google Scholar]
- Targeting reactive oxygen species in cancer via Chinese herbal medicine. Oxid. Med. Cell. Longev.. 2019;2019:9240426.
- [Google Scholar]
- Influence of cytotoxic doses of 4-hydroxynonenal on selected neurotransmitter receptors in PC-12 cells. Toxicol. In Vitro. 2008;22(7):1681-1688.
- [Google Scholar]
- Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells. Mol. Cell. Biochem.. 2013;384(1):59-69.
- [Google Scholar]
- Evaluating medicinal plants for anticancer activity. Sci. World J.. 2014;2014:721402
- [Google Scholar]
- The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci.. 2008;1147(1):37-52.
- [Google Scholar]
- Molecular characterization of mitochondrial apoptosis-inducing factor. Nature.. 1999;397(6718):441-446.
- [Google Scholar]
- In Vitro morphological assessment of apoptosis induced by antiproliferative constituents from the rhizomes of Curcuma zedoaria. Evid. Based Complement. Alternat. Med.. 2013;2013:257108
- [Google Scholar]
- Effects of the polyacetylene capillin on human tumour cell lines. Anticancer Res.. 2004;24(4):2281-2286.
- [Google Scholar]
- Induction of apoptosis in human colorectal cancer cells by nanovesicles from fingerroot (Boesenbergia rotunda (L.) Mansf.) PloS One.. 2022;17(4):e0266044.
- [Google Scholar]
- Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol.. 2021;14(10):101174
- [Google Scholar]
- Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J.. 2022;135(5):584-590.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102763.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







