Translate this page into:
Aptness of diverse queen cup materials for larval graft acceptance and queen bee emergence in managed honey bee (Apis mellifera) colonies
⁎Corresponding author at: The Unit of Bee Research and Honey Production, Biology Department, Faculty of Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia. khalidtalpur@hotmail.com (Khalid Ali Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Artificial queen rearing has changed the beekeeping business in contrast to natural queen replacement, as it provides a newly mated queen into a bee colony, reduces the time between eggs not being laid, and increases the production of young bees in a colony. This study was conducted to investigate the larval acceptance rate grafted in queen cups made from different materials and to find out whether the various materials used for queen cups were suitable for the acceptance of grafted larvae in cell builder colonies. The evaluated materials included fresh bee wax (T1), old bee wax (T2), uncapping bee wax (T3), pure paraffin (T4), 50% paraffin + 50% old bee wax (T5), and brown plastic queen cups (T6) as a control. Results indicated that T3 was the material that most increased the larval acceptance (5.2 ± 0.37), followed by T4 (2.4 ± 0.5), T2 (2.2 ± 0.58), T5 (1.8 ± 0.37), T1 (1.6 ± 0.4), and minimum larval acceptance was in the T6 (1.0 ± 0.4) respectively during the spring season of the year 2020–2021. Similar findings were reported of larval acceptance rate during the spring season of 2021–2022. In addition, the wax material that least affected larval acceptance was the fresh comb bee wax. However, all materials used were coupled with a more larval acceptance rate than the control treatment during both spring seasons of 2020–2021 and 2021–2022. The larval acceptance rate was statistically significant in T3 as a compared to other queen cell cup materials during the both spring seasons (p = 0.001). These findings imply that using different types of bee wax for preparing queen cell cups during larvae grafting, particularly uncapping bee wax, may stimulate and promote grafted larvae acceptance during the queen rearing process.
Keywords
Apis mellifera
Bee wax materials
Cell builder colonies
Artificial queen rearing
1 Introduction
Honey bees are highly eusocial insects that prefer to live in colonies which are made up of one female queen who conducts all reproductive functions, large numbers of female workers, and a small number of seasonal males known as drones (Winston, 1991). Honey bees are an important natural supplier of a variety of products including honey, propolis, royal jelly, bee venom, and pollen which have been used in multiple pharmaceutical and cosmetic industries (Schmidt, 1997; Ahmad et al., 2020). Besides from that, their contribution to the ecology is astounding, particularly in terms of pollination, which is critical for both food production and agricultural revenue (Abbasi et al., 2021; Naz et al., 2022). As a result, for greater output, strong beekeeping techniques, skills, and disease management are essential.
Notably, the health of the bee queen is very important for colony health and its performance (McAfee et al., 2021; Khan et al., 2022). The honey bee queen actively regulates such an extreme type of reproduction monopoly by secreting glandular pheromones, that is very appealing to workers, restrict queen rearing, and decrease worker ovary activation (Melathopoulos et al., 1996; Hoover et al., 2003; Bortolotti and Costa, 2014). Remarkably, honey bee queen rearing is the most important beekeeping practice for rapid multiplication of bee population, as well as replacing old queens every year to enhance honey production, and inserting new queens in case of sudden loss during colony manipulation, transportation and diseases (Dhaliwal et al., 2017; Khan and Ghramh, 2022). Even though queen bee rearing can be done in the presence of the bee queen in a nurse bee colony, it is more effective in queen less colonies and when emergency queen cells are not present.
Many workers have reported on the reactions of colonies to various queen raising procedures due to variances in environmental, behavioral, and biological aspects. Climate parameters such as temperature, relative humidity, and pollen supply have been described as critical determinants in regulating the acceptance and quality of artificially grown queens (Cengiz et al., 2009; Jagdale et al., 2021). The quality of newly emerged queens is quantified with different morphological characteristics such as thoracic width, wing length, and wet weight (Delaney et al., 2011; De Souza et al., 2013).
The colony brood raising cycle is considered by a full ending of brood rearing in the late fall and a decline in colony size over the winter in temperate climates. When nectar and pollen become accessible, brood rearing and colony expansion begin (Thomas, 1985; Ahmad and Dar, 2013). During July and August, royal jelly is used to produce the most queen cells (Gene et al., 2005). At the end of March to the end of September, queen bees can be reared (Koç and Karacaoglu, 2004). Furthermore, the acceptance and emergence of queens are influenced by the raising period or season of queen development, according to this finding. Grafting using several techniques including wet grafting with royal jelly, the rate of acceptance, and other queen quality metrics were reported to vary (Büchler et al., 2013; Kamel et al., 2013).
The current research was conducted to evaluate the rate of larval graft acceptance in different queen cell cup materials for A. mellifera queen production during spring breeding season conditions in Islamabad, Pakistan.
2 Materials and methods
The present research work was carried out during the spring seasons of 2020–21 and 2021–22 conducted at Honeybee Research Institute (HBRI), NARC-PARC Islamabad under Agricultural Linkages Program (ALP) project entitled “Quality Honeybee queen production through non-traditional techniques” Natural Resources (NR-047).
2.1 Selection of cell builder and breeder colonies
The selection of cell builder colonies needed a large number of the nurse bee population, sealed and unsealed worker brood, and food stores such as pollen and honey. Six strong queens less cell builder five frame colonies were prepared before 2–3 h prior to grafting. Two to three frames of young nurse worker bees were shacked in each cell builders avoiding the queen bee. The grafting larvae were taken from the breeder colony. The breeder colony was maintained by feeding them sugar syrup, artificial supplemental diets, and the addition of sealed brood from other colonies. The Doolittle (larval grafting) technique of queen raising was used to graft in queen less cell builder colonies.
2.2 Queen cell cup materials
Different wax materials including fresh and old bee brood wax, capping wax and paraffin wax was utilized for the preparation of queen cups. From these materials, six different treatments were comprised such as fresh bee wax (T1) harvested from newly constructed honey combs (Extra Growth), old bee wax (T2) extracted from old brood combs, capping bee wax (T3) obtained from uncapping, 100 % Paraffin wax (T4), 50 % paraffin wax + 50% bee wax (T5) from old brood combs and brown plastic cups (T6) (control). The queen cups were prepared in a wax room by melting of each wax material separately a day before grafting. Each treatment will have 50 queen cups (6 × 50 = 300 queen cups) and be mounted on grafting bars randomly of 10 grafting frames. Each grafting frame contained three bars, 10 cups/grafting bar, and 30 cups /grafting frame having mixed all materials queen cell cups affixed. Larvae were grafted onto the bottom of primed fake queen cell cups fastened to the queen rearing frame with the use of a grafting needle after 24 h of hatching. Entrance of cell builder colony kept closed for 72 h and kept colonies were put under shade, however, remain ventilated to allow freely flow of oxygen for their respiration. After 72 h, cell builder colonies were monitored; accepted and unaccepted larvae were counted, and their entrances were opened. The percentage of larval acceptance was calculated by using simple percentage formula.
2.3 Recording of observations
For each treatment, the number of grafted and accepted larvae was recorded. The mature queen cells were removed at 10–11 days, after grafting prior queens’ emergence in queen nursery cages individually having 5 nurse bees with 10 g candy (1:1). The total number of queen bees that have emerged has been counted. It was measured what percentage of larval grafts were accepted and when the queen emerged.
2.4 Statistical analysis
All statistical data like larval acceptance rate were recorded using SPSS software (version 20). The difference between the groups was measured by ANOVA and post hoc Tukey test. A p-value < 0.05 was noticed as statistically significant. Graph Pad Prism (version 9.1.3) software for plotting the graphs was applied.
3 Results
3.1 Queen cell larval acceptance rate during the spring season of 2020–21
The larval acceptance rate in artificial queen cell cups revealed that the mean larval acceptance rate was recorded statistically highest (5.2 ± 0.37) in T3 as compared to T4 (2.4 ± 0.5), and T2 (2.2 ± 0.58). In addition, the mean larval acceptance rate was 1.8 ± 0.37 in T5 and 1.6 ± 0.4 in T1, respectively as shown in Fig. 1. The least larval acceptance rate was 1.0 ± 0.4 in. T6.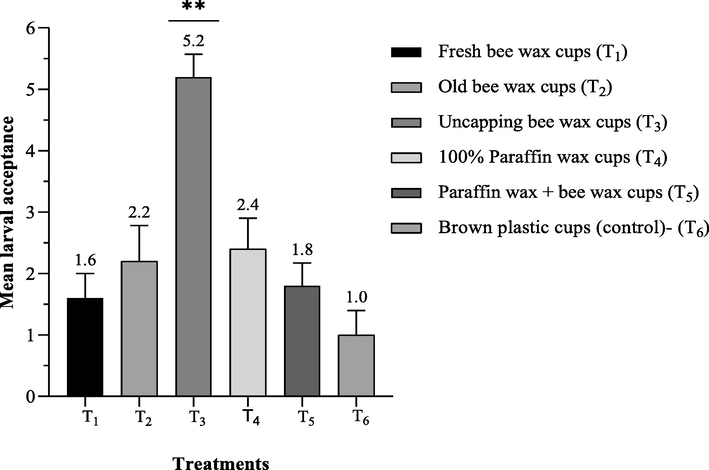
Mean the number of accepted larvae 72 h after being grafted into different wax materials queen cups representing as (Mean ± SE) during 2020–2021 queen raising seasons.
3.2 Queen cell larval acceptance rate during the spring season of 2021–22
A similar trend was recorded during the 2021–22 queen raising season, where the statistically highest mean larval acceptance (3.4 ± 0.74) was recorded in T3 followed by T4 (2.6 ± 0.24), T1 (1.6 ± 0.4), T2 (1.6 ± 0.24), and T5 (1.0 ± 0.3), respectively as shown the mean acceptance of larvae after 72 h of grafting (see Fig. 2). While minimum mean accepted larvae were noticed in the control (0.8 ± 0.2).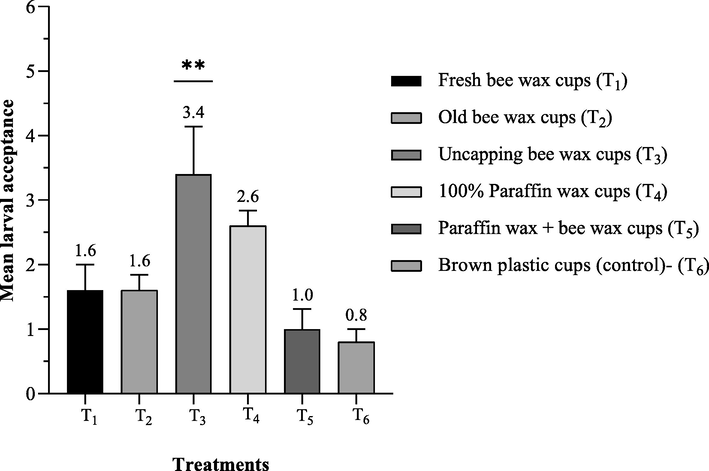
Mean number of accepted larvae 72 h after being grafted into different wax materials queen cups representing as (Mean ± SE) during 2021–2022 queen raising seasons.
3.3 Comparison of larval acceptance rate between 2020–21 and 2021–22 spring seasons
The comparison of graft larvae acceptance rate between the 2020–21 and 2021–22 spring seasons is mentioned in Table 1. The larval acceptance rate was significantly higher in T3 as a comparison to other queen cell cup materials during the spring season of 2020–21 (p < 0.001). Similarly, the larvae accepted by nurse bees were significantly more in T3 than other queen cell cup materials during spring season 2021–22 (p < 0.001). Note. Values in columns followed by different letters are significantly different from others. α = 0.05, P ≤ 0.0000 2020. α = 0.05, P ≤ 0.0010 2021.
Queen Cup Materials
Larval Acceptance (Mean ± SE)
2020–21
Larval Acceptance (Mean ± SE)
2021–22
Extra Growth bee wax cups
1.6 ± 0.4 b
1.6 ± 0.4 a
Old brood bee wax cups
2.2 ± 0.58 b
1.6 ± 0.24 ab
Uncapping bee wax cups
5.2 ± 0.37 a
3.4 ± 0.74 bc
100 % Paraffin wax cups
2.4 ± 0.5 b
2.6 ± 0.24 bc
Paraffin wax + bee wax cups
1.8 ± 0.37 b
1.0 ± 0.31 c
Brown plastic cups (control)
1.0 ± 0.4 b
0.8 ± 0.2 c
The wax material which a minimum larval acceptance rate was the T2 and T6. However, all materials used were coupled with more larval acceptance rate after 72 h of grafting in comparison to the control group during both years 2020–21 and 2021–22.
4 Discussion
Honey bees are key agricultural pollinators that actively pollinate agricultural crops and plants, allowing for enormous food production around the world (Cho et al., 2022). One of the most commonly cited reasons for honey bee loss around the world is a decrease in queen quality. Different queen rearing methods are crucial in determining the effectiveness of honey bee output. This research has been carried out to evaluate and compare various cup materials for their aptness on the larval acceptance during the honey bee (A. mellifera) queen raising process.
Our finding demonstrated that the highest means value of accepted larvae was related with uncapping bee wax material and the larval acceptance rate with this substrate was much greater than that of larvae treated with all other treatments. When compared to control plastic cups, larval uptake was significantly higher in other cup materials. Current findings revealed that the use of uncapping bee wax enhances the larval acceptance rate for queen rearing objectives. The fact that it is the purest wax generated by bees during the capping of mature honey frames throughout nectar seasons may explain why liquid substances uncapping bee wax are advantageous during queen rearing (Cobey, 2005). In control plastic cells, the larval acceptance rate was low, ranging from 13.33% and 12.00%, only that similar to the control treatment in this study. In addition, Mattiello et al. (2022) reported that the size of the queen cell cup might influence the acceptance rate of grafted larvae and the size of the queen, which may affect the colony quality. The size of the cell cup appears to have a good impact on the weights of the queens' body parts.
Our findings also indicated that the larval acceptance rate was statistically significant in uncapping bee wax cups material as a comparison to other queen cell cup materials during the spring season in both years. However, another study revealed that larval acceptance rate was differed significantly between the duration of two different seasons because of weather conditions (Shafey et al., 2022). Further, the acceptance rate of larvae and queen cells was negatively affected by the maximum and minimum temperatures (Khan et al., 2021). Unexpectedly, our study did not show the highest and lowest larval acceptance rate in a different month of the spring season. Moreover, other studies reported that a maximum larval acceptance rate was observed in June than in the other months (Cengiz et al., 2009; Ahmad and Dar, 2013).
Because of the greater sample size (n = 30), this analysis is likely to be more significant, and future research should include a larger sample size. The explanations why uncapped bee wax performed best as grafting materials are unknown and will need to be examined further in field and laboratory research tests. Furthermore, in the rather warm and dry environment of cell building colonies (30–35 °C), uncapping may have been more loved and kept for a longer time by bees than the other ingredients used.
As a result, it may be stated that the bees preferred their own manufactured by-products over other materials utilized in queen rearing. These findings could aid local beekeepers and growers in obtaining robust queens of superior quality through the best beekeeping techniques.
5 Conclusion
Among all the cup materials investigated for queen generation in this study, artificial queen cell cups constructed of uncapping bee wax had the highest larval acceptance and queen emergence. As a result, it has been determined that employing uncapping bee wax as an artificial queen cell cup material in A. mellifera mass queen raising under the environmental circumstances of Islamabad, Pakistan, during the spring season of March and April can generate the most significant number of queens. The use of uncapping bee wax can boost the acceptability of grafted larvae during queen raising, according to the findings of this study. Further studies are needed to evaluate queen cups from a variety of sources, in particular, could be utilized to boost the number of queen honey bees raised.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors appreciate the support of the Research Center for Advanced Materials Science (RCAMS) at King Khalid University Abha, Saudi Arabia through a project number RCAMS/KKU/001-21. This research work was also supported by Agricultural Linkages Program – project entitled “Superior Quality honeybee queen production through Non- traditional Techniques” under Grant No. ALP NR- 047 of Honeybee Research Institute (HBRI), National Agricultural Research Centre (NARC), Pakistan Agricultural Research Council (PARC), federal Ministry of National Food Security and Research Islamabad, Pakistan.
References
- Standardization of managed honey bee (Apis mellifera) hives for pollination of Sunflower (Helianthus annuus) crop. J. King Saud Univ.-Sci.. 2021;33(8)
- [Google Scholar]
- New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci.. 2020;21(2):382.
- [Google Scholar]
- Mass rearing of queen bees, apis mellifera l. (hym: apidae) for bee colony development raised under the temperate conditions of kashmir. Bioscan. 2013;8(3):945-948.
- [Google Scholar]
- Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res.. 2013;52(1):1-30.
- [Google Scholar]
- Some characteristics of queenbees (Apis mellifera L.) rearing in queenright and queenless colonies. J. Animal Veterinary Adv.. 2009;8(6)
- [Google Scholar]
- Cho, S., Lee, S.H., Kim, S. (2022). Determination of the Optimal Maturation Temperature for Adult Honey Bee Toxicity Testing.
- A versatile queen rearing and banking system-Part 1 the“ Cloake Board Method” of queen rearing. Am. Bee. J.. 2005;145(4):308-311.
- [Google Scholar]
- Experimental evaluation of the reproductive quality of Africanized queen bees (Apis mellifera) on the basis of body weight at emergence. Genet. Mol. Res.. 2013;12(4):5382-5391.
- [Google Scholar]
- The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.) Apidologie. 2011;42(1):1-13.
- [Google Scholar]
- Comparative evaluation of Doolittle, Cupkit and Karl Jenter techniques for rearing Apis mellifera Linnaeus queen bees during breeding season. J. Appl. Nat. Sci.. 2017;9(3):1658-1661.
- [Google Scholar]
- Effects of rearing period and grafting method on the queen bee rearing. J. Appl. Animal Res.. 2005;27(1):45-48.
- [Google Scholar]
- The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften. 2003;90(10):477-480.
- [Google Scholar]
- Nutritional profile and potential health benefits of super foods: a review. Sustainability. 2021;13(16):9240.
- [Google Scholar]
- Morphometric study of newly emerged unmated queens of honey bee Apis mellifera L. in Isia Governorate, Egypt. Arthropods. 2013;2(2):80.
- [Google Scholar]
- Nutritional efficacy of different diets supplemented with microalga Arthrospira platensis (spirulina) in honey bees (Apis mellifera) J. King Saud Univ.-Sci.. 2022;34(2):101819.
- [Google Scholar]
- Honey bee (Apis mellifera jemenitica) colony performance and queen fecundity in response to different nutritional practices. Saudi J. Biol. Sci. 2022
- [Google Scholar]
- Queen cells acceptance rate and royal jelly production in worker honey bees of two Apis mellifera races. PLoS ONE. 2021;16(4)
- [Google Scholar]
- Effects of rearing season on the quality of queen honeybees (Apis mellifera L) raised under the conditions of aegean region. Mellifera. 2004;4(7)
- [Google Scholar]
- Effect of queen cell size on morphometric characteristics of queen honey bees (Apis mellifera ligustica) Ital. J. Anim. Sci.. 2022;21(1):532-538.
- [Google Scholar]
- Honey bee queen health is unaffected by contact exposure to pesticides commonly found in beeswax. Sci. Rep.. 2021;11(1):1-12.
- [Google Scholar]
- Effect of queen mandibular pheromone on initiation and maintenance of queen cells in the honey bee (Apis mellifera L.) Can. Entomol.. 1996;128(2):263-272.
- [Google Scholar]
- Naz, S., Malik, M.F., Hussain, M., Iqbal, R., Afsheen, S. (2022). 2. To check the socio-economic importance of honey bee for developing countries in current financial crisis. Pure Appl. Biol. (PAB) 11(3), 851-860.
- Shafey, A.S., Shebl, M.A., Mahmoud, M.F., Kamel, S.M. (2022). Evaluation of Colony Parameters for Queen Rearing under Arid Ecosystem Conditions.
- Thomas, S. (1985). Honeybee Ecology: A Study of Adaptation in Social Life.
- The Biology of the Honey Bee. Harvard University Press; 1991.







