Translate this page into:
Appraisal of the anti-arthritic potential of Strelitzia reginae inWistar rats via modulating molecular inflammatory cascadesIL-6,IL-17a, IL-1β,TNF-α, IFN-γ, RANKL, OPG, and IL-4 and attenuating oxidative stress biomarkers
⁎Corresponding author. maliksaadullah@gcuf.edu.pk (Malik Saadullah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The study aimed to investigate the potential anti-arthritic effects of methanolic aerial extract of the plant Strelitzia reginae (MSRA), because of its potential anti-arthritic effects.The research utilised Wistar albino rats, and all groups except the control group had arthritis in their right hind paws caused by 0.1 ml of CFA (Complete Freund’s adjuvant) emulsion. For 21 days, all rats (except those in the normal and arthritic groups) were administered the extract at doses of 600, 300, and 150 mg/kg/day in conjunction with 10 mg/kg of piroxicam. By enhancing biochemical markers, reducing paw edoema, and reestablishing immunological organs and body weight, the extract shown substantial effects. Polymerase chain reaction (PCR) results demonstrated a significant decrease in receptor activator of nuclear factor kappa-beta ligand (RANKL), interlukin-17a (IL-17a), tumour necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and interferon-γ (IFN-γ). The study also found that when polyarthritic groups were treated with dosages of MSRA, the expression of osteoprotegrin (OPG) and IL-4 was upregulated. Around 600 mg/kg/day, the highest levels of OPG (1.24 ± 0.025 ng/ml) and IL-4 (80.31 ± 0.23-fold) were seen. The levels of catalase (CAT) and superoxide dismutase (SOD) increased (p < 0.05) in the groups treated with 600 mg/kg MSRA and piroxicam, while malondialdehyde (MDA) decreased.
Keywords
Rheumatoid arthritis
Strelitzia reginae
Oxidative stress biomarkers
1 Introduction
In its most basic form, rheumatoid arthritis is a kind of symmetric polyarthritis that affects both the big and tiny diarthroid joints in the body. The synovium becomes invaginated by CD4 + T lymphocytes, B cells, and macrophages in rheumatoid arthritis, causing distinct lymohoid aggregates to form with a germinal core (Firestein, 2003). Approximately 0.5 % to 1 % of the global population is impacted by rheumatoid arthritis. There are three distinct stages to the course of a disease. Fluid retention and synovial hypertrophy occur during the initial, asymptomatic phase of the process, which is characterised by the activation of inflammatory cells and cytokines.The activation of T cells in the second phase (the amplification phase) leads to the infiltration and destruction of subchondral bone and articular cartilage; this process is gradual, permanent, and symptomatic. In the end, the last stage leads to ankylosis of the joints and long-term abnormalities (Scionti et al., 2016). In rheumatoid arthritis, the metabolic enzymes lipo-oxygenase (LOX) and cyclo-oxygenases (COX) and the proinflammatory cytokines interleukin-1β, IL-6, and tumour necrosis factor-alpha (TNF-α) find a balance. The breakdown of proteoglycans and collagen is aided by pro-inflammatory cytokines, which hinder their formation. In addition, prostaglandins—most notably prostaglandin E2—are produced by cyclo-oxygenase COX 2, which in turn causes inflammation, pain, and impairment (El-Miedany and El Rasheed 2002). Therefore, rofecoxib and celecoxib are selective COX-2 inhibitors used to treat rheumatoid arthritis (RA) because they lessen inflammation and discomfort without causing the gastrointestinal side effects sometimes seen with nonsteroidal anti-inflammatory drugs (NSAIDS) (Farzaei et al., 2016; Saleem et al., 2020a,b). An herbaceous perennial with evergreen foliage, Strelitzia reginae is a monocotyledon plant. Subtropical temperate climates are ideal for its growth (Yassen and Badawy ِ Abou-Sreea 2016).
When it came to chemical bioprospecting of Strelitzia reginae, there was basically very little information available in the scientific literature. Therefore, this study set out to assess the anti-arthritic potential of Strelitzia reginae's methanolic aerial extract. In this investigation, the dosages of 600, 300, and 150 mg/kg/day were chosen by considering the rats' body weight. Furthermore, by employing the CFA-induced arthritic model, the molecular and cellular mechanisms that alleviate arthritic and inflammatory activity were ascertained. By reducing oxidative stress indicators, regulating inflammatory cytokines, and improving histological implications, the study demonstrated substantial anti-arthritic potential.
2 Materials and methods
2.1 Drugs and chemicals
Complete Freund’s adjuvant (InvivoGen®, France), trizol (Trizole reagent; Thermo-Scientific®, UK), Oligo-primers (Macrogen®, USA), nuclease-free water (Ambion-Thermo-Fisher®, USA), methanol (Merck®) and Maxima Syber Green/ROX Master Mix 2X (Thermo-Scientific®, UK) were purchased.
2.2 Collection of plant and preparation of extraction
The Strelitzia reginae plant was bought from a nursery in Patoki, Pakistan. Strelitzia reginae was recognised by renowned taxonomist Mansoor Hammed of the University of Agriculture Faisalabad and given the voucher number 1-173-2022. It was sent to the herbarium after that. To remove any dirt or insects, the gathered plant was washed with distilled water. The next step was to let the plant dry in the shade for ten days. There were 20 kilogrammes of dried plant material. The aerial and root portions of the plant were separated. The parts that were used for the extraction process were ground into a coarse powder separately, and then the triple maceration was applied. Using 50 cc of 70 % methanol using the same method as before, the residue was again extracted. A volumetric flask was used to collect the supernatant, which was then evaporated using a rotary evaporator (Stuart®, UK) at 40 °C under reduced pressure (Oroian et al., 2020). The methanolic aerial parts of extract were named MSRA (methanolic extract of aerial parts of Strelitzia reginae).
2.3 Experimental animals
The study was carried out by choosing previously acclimatized healthy Wistar rats with a weight between150 and 220 g. There were six different groups made with a random allocation of rats. The rats were confined in the animal house of the faculty of pharmaceutical sciences at GC University Faisalabad. The experiments were conducted by following the rule and regulations provided by the institutional Bio ethics committee vide letter number GCUF/ERC/72.
2.4 CFA-induced arthritis (Complete Freund’s adjuvant-induced)
We divided the rats into six groups of two each. Separate from Group II, which was dubbed AC (arthritic control), Group I was called NC (normal control). Likewise, Group (III) was given piroxicam (10 mg/kg/day) as the usual control group, while Groups (IV), (V), and (VI) were given MSRA doses of 150, 300, and 600 mg/kg/day, respectively. On the initial day, the right subplantar area of the hind paw was targeted to induce arthritis in all mice that were not administered NCG. The procedure to induce arthritis was injecting 0.1 ml of CFA emulsion intradermally. (Sigma-Aldrich, Washington, DC, USA). Over the course of seven days, the animals did not receive any therapeutic dosages. Oral gavage was then initiated and maintained until day 28 of therapy.
2.5 Phytochemical assessment by high performance liquid chromatography (HPLC)
To determine the various compounds present in MSRA, HPLC was performed following the method established according to a study carried out (Saleem et al., 2020a). Thus, a solution sample was prepared by mixing 50 mg of MSRA with 40 ml of 60 % methanol. The mixture was acidified with HCL and heated at 90 °C for a period of 2 h. The filtration of the sample was performed using a 0.2 μm syringe filter. The filtered sample solution was injected into reverse- phase HPLC by using columns (C-18) and detector (UV–Vis). Moreover, the mobile phase was made up of and 6 % v/v acetic acid and acetonitrile. The retention times of different phytochemicals were recorded and determined by comparing them with standard chromatograms.
2.6 Evaluation of polyarthritis
Throughout the course of the research, we noticed a few of the following physical characteristics: At 1, 7, 12, 16, 20, and 28 days, an electronic vernier calliper was used to assess paw diameters, body weight, and arthritis score in order to diagnose polyarthritic edoema (Zhang et al., 2017).
2.7 Hematological and biochemical investigation
After 29 days of starvation and a light ether anaesthesia, the animals were prepared for sacrifice. For haematological and biochemical tests, blood samples were taken, correspondingly. In order to measure C-reactive protein (CRP), a commercially available kit from Antec Diagnostic Products®, UK, was also utilised. The levels of creatinine, urea, alkaline phosphatase (ALP), alanine transaminase (ALT), rheumatoid factor (RF), and aspartate aminotransferase (AST) were also measured using an automated chemistry analyzer (Microlab 300®) and protocols developed by Analyticon Biotechnologies AG of Germany. Similarly, a complete blood count (CBC) was also determined using an automated hemocytometer (Sysmex®, Roche, Germany).
2.8 Immune organ weight
The sacrificed rats were cut open through the abdomen. The thymus and spleen were taken out, cleaned with distilled water, and weighed.
2.9 In vivo estimation of oxidative stress biomarkers
Oxidative stress biomarkers such as malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD) levels were calculated from CFA-induced arthritis-developed rats. For this, the rats were anaesthetized, killed, their livers were taken and homogenates with 10 % w/v were formulated. In addition, the liver homogenate was also used to determine protein content. At 550 nm, the total superoxide dismutase (SOD) was determined by following colorimetric technique. Likewise, the CAT activity was estimated by following the colorimertic method at 405 nm. Moreover, the MDA quantification was accomplished by applying thiobarbituric acid-reacting substance method (TBARS) at 532 nm (Jurczuk et al., 2004).
2.10 qRT-PCR
The following inflammatory biomarkers were evaluated in cases of arthritis: IL-17a, TNF-α, IL-4, IL-6, interferon-γ, OPG, and RANKL. Blood was drawn using a heart puncture and deposited in EDTA tubes for analysis. Geno All® of Korea supplied the RiboEx™LS kit that the TRizol technique utilised to extract RNA. In addition, the amount of RNA was measured using a Nano drop spectrophotometer. Additionally, the WizScript® cDNA synthesis kit (maker's protocol) was used to synthesise complementary DNA (Cui et al., 2022).
2.11 Statistical analysis
The results were presented as the average plus or minus the standard deviation after a statistical analysis. The data was analysed using two-way analyses of variances (ANOVA). This included percentage inhibition, paw diameter, body weight fluctuation in arthritis-induced rats, and all other relevant data was analyzed by one way ANOVA and Tukey’s test. A similar analysis was conducted using GraphPad Prism® 6.01 to assess the impact of plant extracts and the gold standard medicine, piroxicam, on the arthritic score of rats throughout many study days.
3 Results
3.1 High performance liquid chromatography (HPLC) Evaluation
Fig. 1 shows that the aerial sections of the methanolic extract included the most essential components identified by HPLC evaluation: p-coumaric acid, kempherol, and gallic acid.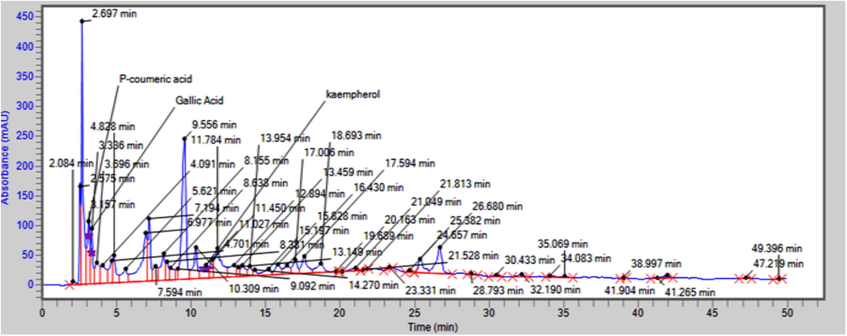
The HPLC spectrum shows phytochemicals detected in methanolic roots extract of Strelitzia reginae.
3.2 Paw swelling assessment
After administering CFA subplantarly on the eighth day of the research, the most significant edoema was noted. The arthritic control (AC) group showed an increase in paw diameter from day eight to day twenty-one. Paw diameter was shown to be reduced by treatment with a methanolic extract of MSRA; the most significant impact was observed at 600 mg/kg when compared to AC, as indicated. At 600 mg/kg, the MSRA extract had the greatest impact compared to the arthritic control group, according to the x-ray examination of the hind paws (Fig. 2).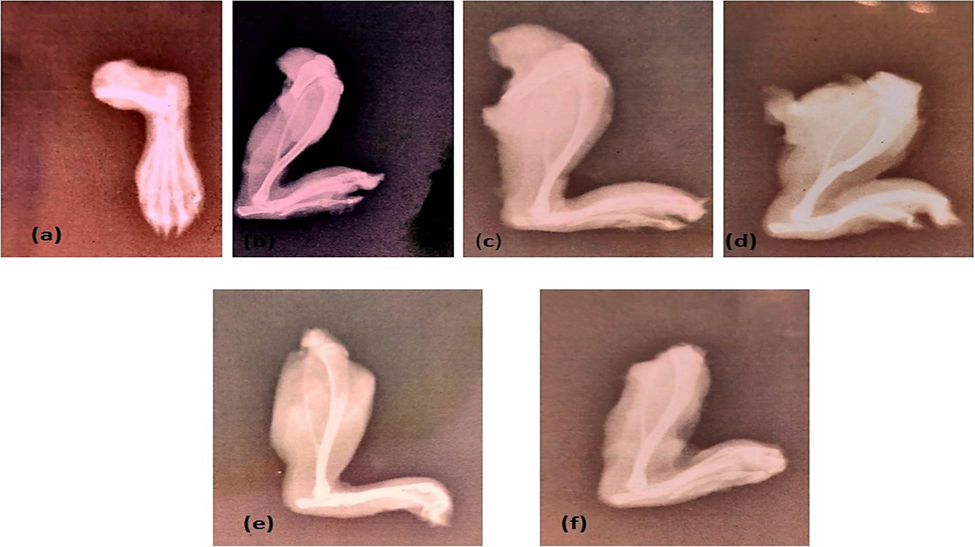
X-ray evaluation showed significant improvement in CFA-induced arthritic hind paws by methanolic aerial extract of Strelitizia reginae especially at 600 mg/kg dose. Here, (a) shows (NC), (b) represents (AC), (c) exhibited piroxicam-treated, (d) 150 mg/kg (e) 300 mg/kg and (f) 600 mg/kg represents effects of different doses.
3.3 Effect on body weight, paw swelling and arthritic score
Throughout the course of the research, participants' weight, paw edoema, and arthritis score were measured at various intervals. Body weight, paw edoema, and arthritic score were all positively affected by the 16–28 day treatment of piroxicam and MSRA dosages. Fig. 3b shows that the piroxicam-treated group had changes in body weight, paw edoema, and arthritic score at the 300 mg and 150 mg dosing levels, respectively.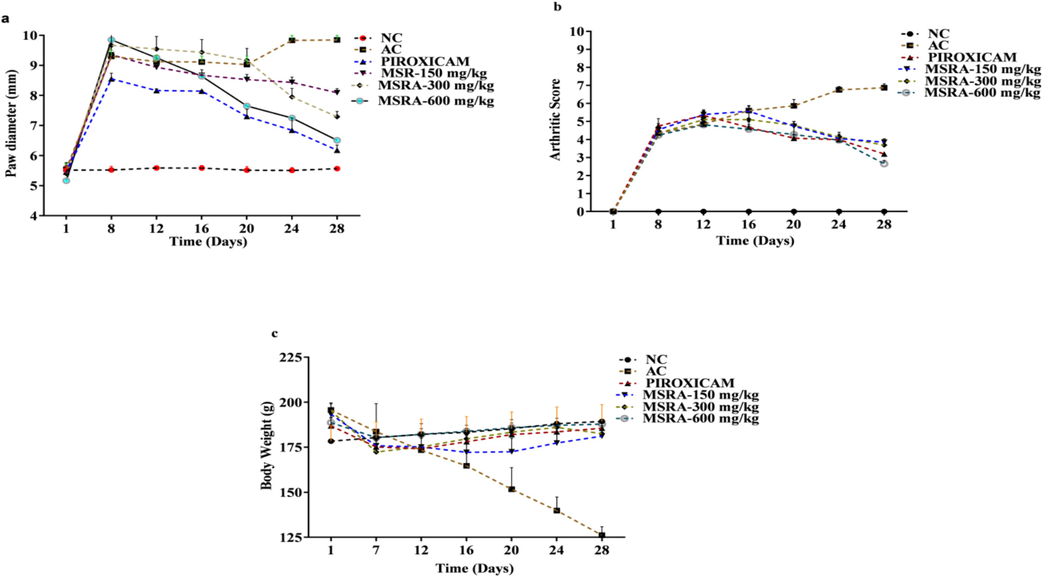
a-c The effect on body weight in CFA-induced arthritic rats by treatment with methanolic extract of aerial parts of Strelitzia reginae (MSRA). Here, a shows effect on paw diameter, b exhibited effect on arthritic score and c represents effect on body weight.
3.4 Effect on hematological parameters, kidney and liver function parameters
Rats given FCA had elevated levels of rheumatoid factor (RF), alkaline phosphatase (ALP), C-reactive protein (CRP), and alanine transferase (ALT). In rats treated with conventional medication and plant extracts, a significant decrease in ALP, ALT, CRP, and RF was seen (p < 0.0001). The most effective dose of MSRA in alleviating arthritis in rats was 600 mg/kg, which restored HB, platelets, RBCs, and WBCs significantly (p < 0.05) when compared to AC without HB, platelets, RBCs, and WBCs. MSRA extract treatment resulted in an increase of HB and RBCs in arthritic rats. Fig. 4 shows that white blood cell and platelet counts dropped compared to the normal control group (NC). Control groups without arthritis exhibited significantly higher levels of the systemic biomarkers C-reactive protein and RF. Additionally, as seen in Fig. 5, there were no statistically significant changes in creatinine and urea levels between the groups treated with piroxicam and plant extract.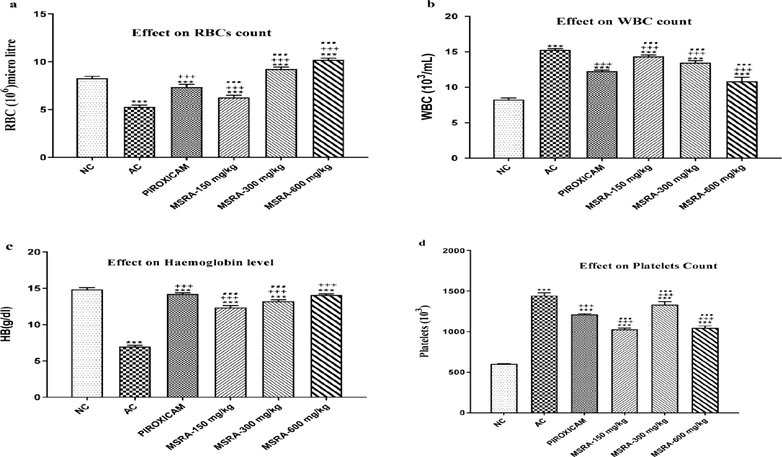
Effect of aerial methanolic extract of Strelitziareginae (MSRA) on hematology of FC-induced arthritic rats. One-way ANOVA followed by Tukey’s test was performed and values were expressed as mean ± S.D (n = 6). Where ***, +++, … exhibited significant difference (p < 0.05) in respect to (NC) normal control, (AC) arthritic control and piroxicam-treated group respectively. Here, FCA = Freund’s complete adjuvant, MSRA = Methanolic extract of aerial parts of Strelitzia reginae.
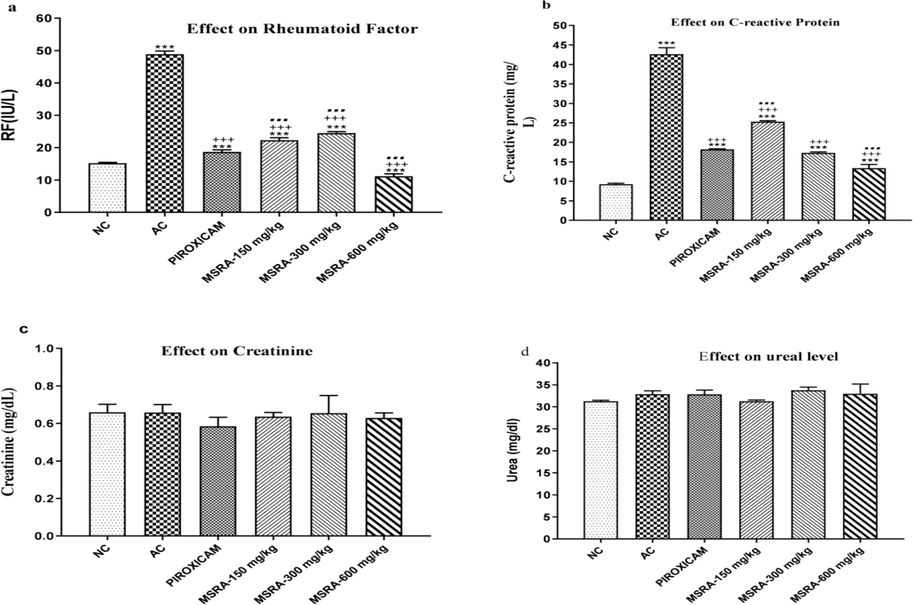
In rats with hind paw-induced arthritis, the effect of an aerial methanolic extract of Strelitzia reginae (MSRA) on C-reactive protein (CRP), rheumatoid factor (RF), and parameters measuring renal function was investigated. A one-way analysis of variance (ANOVA) and Tukey's test were used for statistical analysis. The mean ± S.D. values were calculated from a sample size of 6. Except for the normal control, arthritic control, and Piroxicam, there was a significant difference (p < 0.05) between ***, +++, and… Specifically, “NC” stands for “normal control,” “AC” for “arthritic control,” “FCA” for Freund's complete adjuvant, and “MSRA” for a methanolic extract of Strelitzia reginae aerial parts.
The arthritic control group also had significantly higher levels of ALP and ALT. Fig. 6 shows that compared to the piroxicam-treated groups, the ALP and ALT levels reduced at 600 mg dosages, Fig. 7.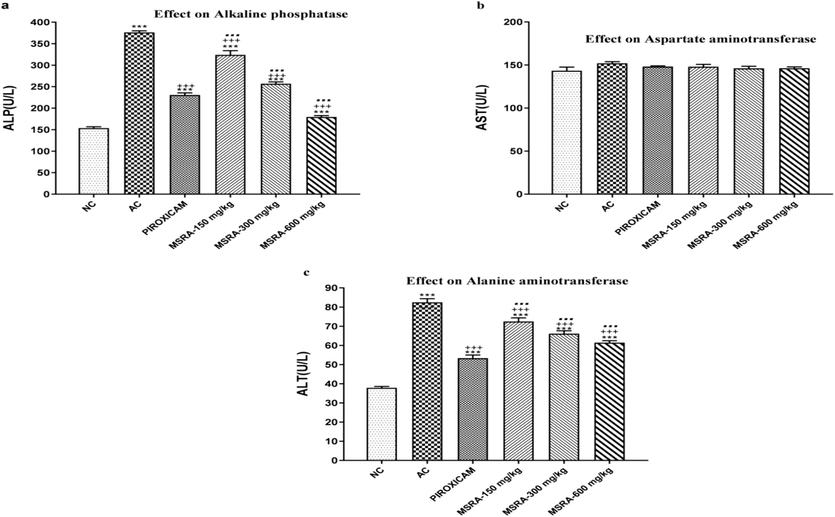
In a study of rats with hind paw-induced arthritis, the researchers looked at how the aerial methanolic extract of Strelitzia reginae (MSRA) affected liver function tests.The results were evaluated using one-way ANOVA followed by Tukey's test, and the average plus or minus the standard deviation (n = 6) were used as the values. In comparison to the normal control, the arthritic control, and piroxicam, ***, +++, and… showed a significant difference (p < 0.05) accordingly. Specifically, “NC” stands for “normal control,” “AC” for “arthritic control,” “FCA” for Freund's complete adjuvant, and “MSRA” for a methanolic extract of Strelitzia reginae aerial parts.
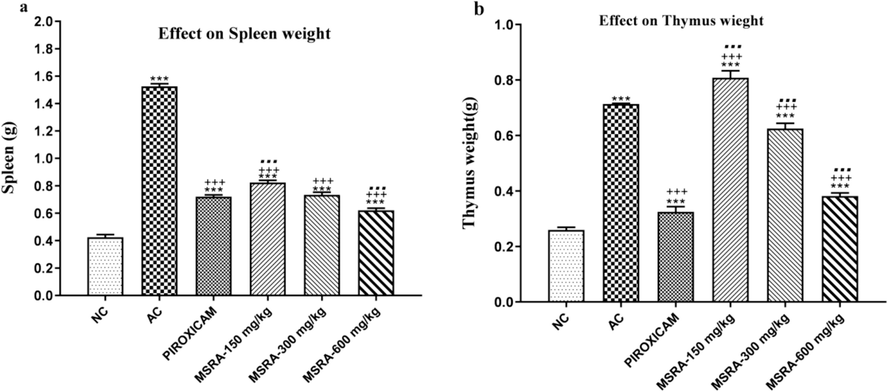
Immune system mass in arthritic rats as a function of Strelitzia reginae methanolic extract. We used one-way ANOVA and Tukey's test to evaluate the data, and we calculated the values as the mean ± S.D. (n = 6).There was a significant difference (p < 0.05) between the normal control, the arthritic control, and the group treated with piroxicam when the letters ***, +++, and… were used.Strelitzia reginae aerial parts methanolic extract (MSRA).
3.5 Effect on immune organ weight
The weights of thymus and spleen were significantly higher in arthritic control group(p < 0.05)as compare to normal control. It was noted the significant weight restoration of the thymus and spleen by treating the arthritic rats with plant extracts and maximum action was exhibited at the dose of 600 mg/kg.
3.6 Effect on oxidative stress markers
The AC group showed a significant decrease in SOD and CAT activity (p < 0.0001) when compared to the normal control group. However, as seen in Fig. 8, the SOD and CAT activities were considerably enhanced by the combination of 600 mg of MSRA and piroxicam.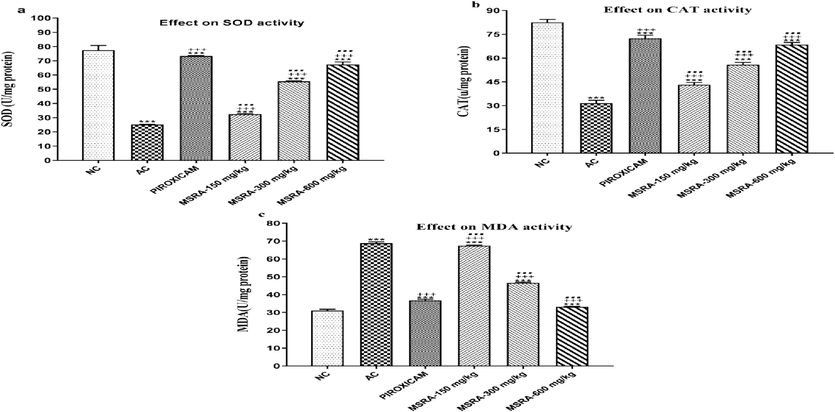
A-c Reaction between oxidative stress indicators in arthritic rats and a methanolic extract of Strelitzia reginae aerial parts. Biomarkers for oxidative stress were markedly enhanced by the MSRA extract. In contrast to the groups treated with NC, AC, and piroxicam, respectively, ***, +++, and… showed a significant difference (p < 0.05). The data is examined using one-way ANOVA, then Tukey's test, and the results are shown as the mean ± S.D. (n = 6). Specifically, “FCA” stands for Freund's complete adjuvant, “MSRA” for methanolic extract of aerial portions of Strelitzia reginae, “NC” for normal control, and “AC” for arthritic control.
When comparing the AC group to the usual control group, a significant increase in MDA levels (p < 0.0001) was also noted. Fig. 8 shows that compared to the arthritic control group, MSRA at 600 mg plus piroxicam significantly reduced MDA levels.
3.7 Effect on gene expression
During the course of the investigation, genetic markers for inflammation were expressed in Wistat rats.Arthritic control group levels significantly increased compared to normal control group levels of IL-6, TNF-α, IL-17a, IFN-γ, IL-1β, and RANKL expression (p < 0.0001). Nonetheless, at doses of 600 and 300 mg/kg, MSRA extract reduced this increase in arthritic rats. Fig. 9a shows that rats treated with 600 mg/kg of extract outperformed those treated with piroxicam.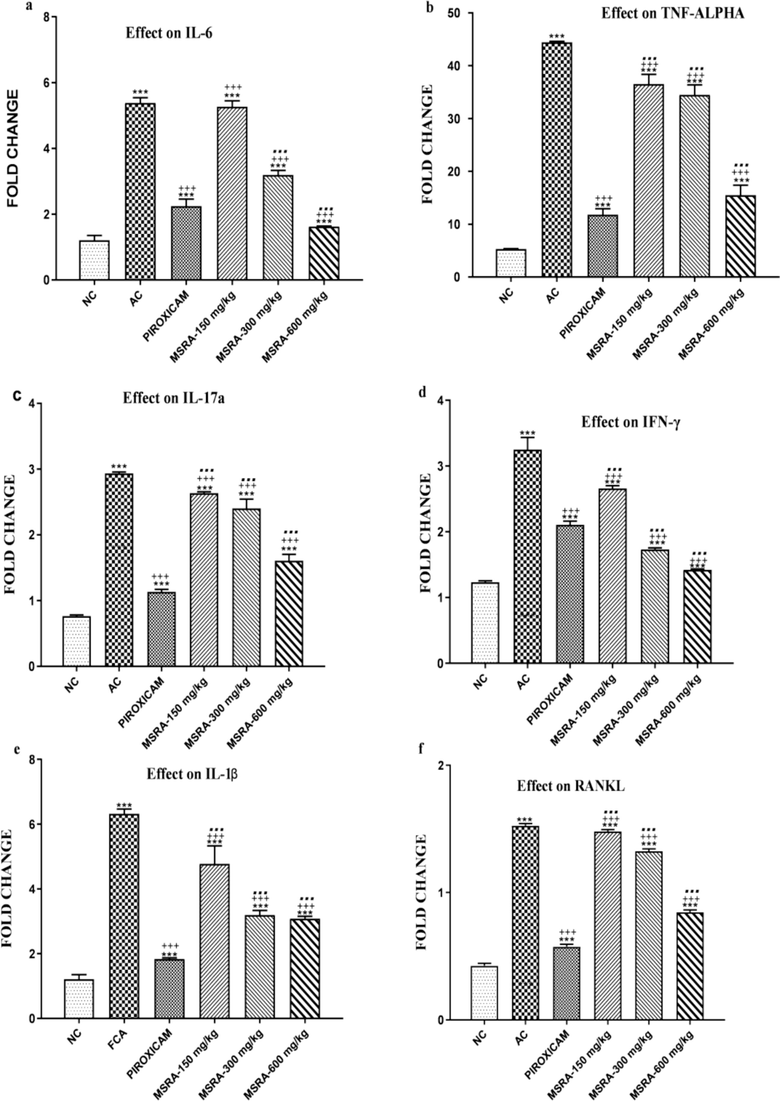
a-f Modulation of inflammatory cytokine expression in CFA-induced polyarthritic rats by a methanolic extract of Strelitzia reginae aerial parts. At 600 mg/kg, MSRA had the greatest impact in reducing the expression of IL-6, TNF-α, IL-17a, IFN- γ, IL-1β, and RANKL mRNA. In comparison to the normal control, the arthritic control, and the piroxicam-treated groups, ***, +++, and… showed significant differences, respectively. The data are analysed using one-way ANOVA followed by Tukey's test, and the results are shown as the mean ± S.D. (n = 6). Specifically, “NC” stands for “normal control,” “AC” for “arthritic control,” and “MSRA” for “methanolic extract of aerial parts of Strelitzia reginae.”.
The arthritic control group showed a considerably lower level of mRNA expression of osteoprotegrin (OPG) and IL-4 compared to the normal control group (p < 0.0001).Nevertheless, as shown in Fig. 10a, the arthritic control group showed no increase in OPG levels when treated with piroxicam at a dosage of 600 mg/kg.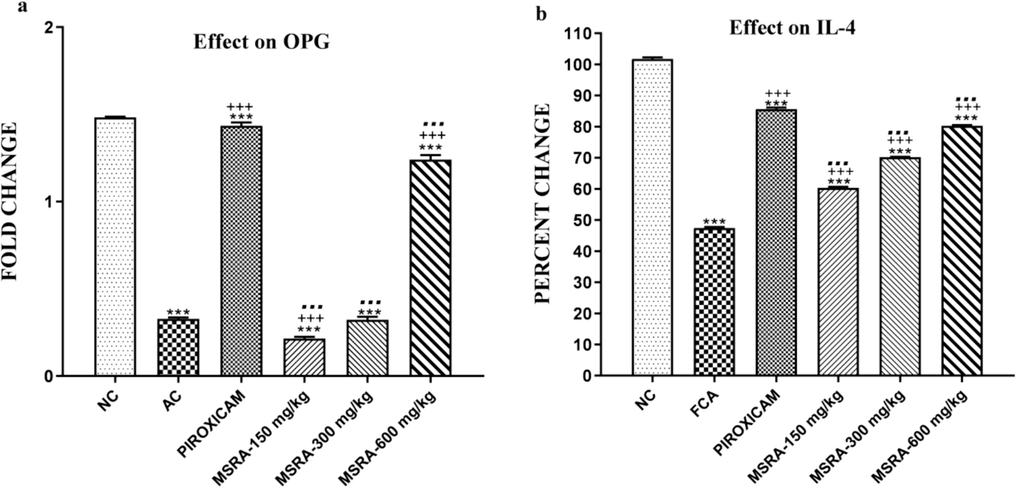
Anti-inflammatory cytokine expression in CFA-induced polyarthritic rats and the effect of methanolic aerial extract of Strelitzia reginae (MSRA). At 600 mg/kg, MSRA therapy leads to the greatest upregulation of OPG and IL-4 expression levels. In relation to the NC, AC, and piroxicam-treated groups, ***, +++, and… showed a significant difference (p < 0.05) accordingly. The data are shown as the mean ± S.D. (n = 6), and they were analysed using one-way ANOVA followed by Tukey's test.We have “FCA” (Friend's complete adjuvant), “MSRA” (Methanolic extract of aerial portions of Strelitzia reginae), “NC” (normal control), and “AC” (arthritic control).
4 Discussion
The study's overarching goal was to identify the probable cause of polyarthritis in Wistar rats and assess the anti-arthritic capabilities of the Strelitzia reginae plant. The investigation was conducted using a CFA-induced arthritis model. The use of HPLC allowed for the qualitative evaluation of the phytochemical ingredients of MSRA. The measurements of the paws and the differences in body weight were also documented. Additionally, the effects of MSRA were revealed by recording the biochemical and haematological markers. Hydraulic acid, gallic acid, and kempherol were among the phytochemicals identified by high-performance liquid chromatography (HPLC) as having medicinal, antioxidant, and anti-inflammatory properties. The anti-inflammatory properties of gallic acid have also been deduced from many research (Kroes et al., 1992). Among the flavonoids present in fruits and vegetables is the well-known antioxidant kaempferol. The source is Silva dos Santos et al. (2021).The phenolic hydroxycinnamic acid (P-coumaric acid) is present in many foods and drinks, including fruits, vegetables, and beverages. According to Abramovič (2015), many studies have found that HCs have antioxidant properties and shield crucial components from oxidation. One of the most used methods for assessing the possibility of anti-arthritic effects is the CFA-induced arthritic model. Animal studies have shown that it can imitate rheumatoid arthritis, and its similarities to the processes of human arthritis have led to its widespread acceptance. The stated source is Liu et al. (2022).There are two potential pathways that might culminate in the onset of arthritis following CFA treatment. The acute stage, which lasts for the first 10 days, is characterised by the development of primary lesions. During the subsequent 11–28 days, known as the chronic stage, synovitis and lesions on the opposite side of the joint will appear (Saleem et al., 2020b). The results from the polyarthritic rats show that thrombocytopenia and total leukocyte count (TLC) are higher in arthritic patients. The correlation between thrombocytosis and rheumatoid arthritis is substantial. Hutchinson et al. (1976) found that active intravascular coagulation causes an increase in platelet formation in this condition. The results of this investigation demonstrate that piroxicam and plant extracts can restore TLC and thrombocytes. Thirty to sixty percent of arthritis patients reportedly experience anaemia symptoms (Perumal et al., 2017). In rheumatoid arthritis, anaemia manifests as a low red blood cell count. Shabbir et al. (2018) found that polyarthritis is associated with elevated IL-6 expression, another factor that contributes to anaemia. The results showed that the groups treated with the plant extract were able to restore red blood cell count and anaemia.
One marker of disease activity in RA patients is the blood level of C-reactive protein (Deyo et al., 1980). According to Scott (2000), RF positive is just as significant as other markers for the development of synovitis, the arthritic condition that causes joint destruction and functional impairment. In the arthritis control group, CRP and RF levels were found to be higher. The levels of ALP and ALT are increased by bone erosion and inflammatory chemicals in the body, but they are decreased in the groups treated with plant extract and piroxicam. The levels were significantly decreased after being treated with piroxicam and plant extracts (Comar et al., 2013).
Arthritic rats have heavier immune organs, as shown in earlier studies (Zhang and An, 2007). Similarly, the present study found that administering plant extract to arthritic rats resulted in a return to normalcy of immune organ weights and systemic indicators.
Along with antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT), malondialdehyde (MDA) is now acknowledged to be a significant oxidative stress indicator. Lipid per oxidation produces malondialdehyde (MDA), a clinically characterised end product of oxidative stress. By transforming radicals of superoxide anion into oxygen and hydrogen peroxide, superoxide dismutase (SOD) is an essential enzyme. As a result, it plays a crucial role in regulating ROS levels within cells. In a similar vein, CAT helps cells avoid oxidative stress by breaking down hydrogen peroxide into oxygen and water. The stress indicators in the present investigation showed an increase in rats that were induced arthritis, but they were effectively recovered with exercise treatment with a combination of plant extract and piroxicam (Camkurt et al., 2017).
A typical symptom of rheumatoid arthritis is cachexia. The overproduction of TNF-α and interleukin-1β is likely the primary cause of this. Packed together, tumour necrosis factor-α and interleukin-1β produce cachexia. Changes in the ratio of protein synthesis to breakdown in muscles are brought about by these causes. According to Walsmith and Roubenoff (2002), when there is an imbalance like this, muscle atrophy occurs. A research found that inflammation driven biochemical processes are increased when activated macrophages spread pro-inflammatory cytokines. The research indicates that cytokines such as TNF-α, IL-1, and IL-6 are vital in the development of pain. The present investigation found that administering a plant extract to arthritic rats reduced levels of pro-inflammatory cytokines (Zhang and An, 2007).
Evidence suggests that RA patients have elevated COX-2 expression in their synovial tissues. This increased expression is caused by pro-inflammatory cytokines including IL-1 and TNF-α. Using a plant extract to treat arthritic rats significantly restores pro-inflammatory factors (Crofford, 1999).A cytokine with pleiotropic effects is tumour necrosis factor alpha (TNF-α). It is a homotrimer protein of 157 amino acids that is produced by T-lymphocytes, activated macrophages, and natural killer cells. Evidence suggests it controls the development of some inflammatory autoimmune illnesses.Initiating biological programmes like inflammation and cell death is its job, and it does it via producing molecular signals. The protein processes a death domain (DD) after binding to TNFR1 and TNFR2 (Jang et al., 2021). This study's arthritic groups exhibited an increase in TNF-α levels, which is indicative of the production of inflammatory cytokines. Rats with arthritis showed a significant decrease in this factor after receiving dosages of plant extract. Based on what we know about the biology of type II inflammatory response, interleukin-4 is a cytokine that protects against certain illnesses. Basophils, eosinophils, NKT cells, and CD4 T cells stimulate ILC2, leading to IL-4 synthesis in the body, according to the cellular study based on IL-4 sources. IL-4 is well-known for its role in stabilising lymphocyte activities, which it regulates. A recent research shown that a dosage of 600 mg/kg of the anti-inflammatory cytokine IL-4 significantly increased its levels in rats with arthritis (Raphael et al., 2015). The control of bone metabolism is greatly influenced by osteoprotegerin (OPG). In addition to being an inhibitor of bone resorption, it is a member of the TNF-receptor family. It prevents RANKL from binding to its receptor RANK by binding to its ligand RANKL. The immune system is modulated by the OPG/RANKL system. The hormones control the system by changing the amount of calcium in the cellular environment. The production of RANKL mRNA is triggered by several cytokines, including as IL-1, TNF-α, and IL-17, which all contribute to the activation of T cells. When RANKL is secreted, it triggers inflammatory responses, bone resorption, and osteoclastogenesis. In contrast, IL-4, OPG, and IL-10 all work to reduce inflammation, which in turn helps to stop bone resorption and osteoclast development (Boyce and Xing, 2007). According to the results of this study, OPG levels are increased following MSRA therapy, and RANKL expression is suppressed as a result of OPG. At the initial stage of inflammation, IL-6 is generated in a local lesion. As it travels through the circulation, it moves from the area of inflammation to the liver. Acute phase proteins such haemoglobin, fibrinogen, α1-antichymotrypsin, serum amyloid A (SAA), and CRP are induced by it. Nevertheless, IL-6 plays a role in decreasing albumin, fibronectin, and transferrin serum levels. Amyloidosis and other chronic inflammatory illnesses can develop when SAA levels remain high for an extended period of time. Hypozincemia, caused by the overexpression of the zinc importer ZIP14, is also IL-6′s fault. In addition to stimulating the release of platelets from the bone marrow, it aids in the maturation of megakaryocte cells. Inflammation intensity is indicated by changes in red blood cell and platelet counts as well as acute phase protein levels.The present investigation found that arthritic groups had elevated IL-6 expression. The goal of the piroxicam and plant extract therapy is to reduce tumour necrosis factor alpha levels (Tanaka et al., 2014). A key role of immunoregulatory molecules known as anti-inflammatory cytokines is to modulate the response to pro-inflammatory cytokines. Cytokines IL-4, IL-10, IL-1 receptor antagonist, IL-11, and IL-13 have a significant role in reducing inflammation.Because it inhibits the production of inflammatory cytokines including IL-1, TNF-α, and IL-6, IL-10 is thought of as a very effective anti-inflammatory cytokine.Beyond that, IL-10 regulates the overexpression of endogenous cytokines and the downregulation of pro-inflammatory cytokine receptors on many levels. Further, IL-10 is implicated in the alleviation and potential reversal of neuropathic pain. Patients suffering from chronic pain may have low concentrations of anti-inflammatory cytokines such as IL-4 and IL-10. The results of this study clearly show that administering plant extract to arthritis rats raises their IL-4 levels (Zhang and An, 2007).
The investigation was limited and confronted with several obstacles. A major obstacle was the possibility of concentration and quality variations in the plant extract, which might impact the reliability of the results. Possible sources of variation include drying and extraction processes used to prepare the plants for study. Moreover, the study's reliance on the Wistar rat model for arthritis development and therapy raises concerns that the model may not adequately capture the complexities of human arthritis. There was also a lack of statistical power due to the limited sample size. Another potential limitation is that the trial did not go long enough to detect any treatment-related side effects. In addition, the study did not go further into the molecular mechanisms at play, instead concentrating on biochemical and haematological measures, oxidative stress indicators, and inflammatory cytokines. The results' applicability to humans is another concern brought up by the use of animal models. Although, it is critical for assessing the treatment's safety to conduct comprehensive investigations into the possible toxicity and side effects of the methanolic extract of Strelitzia reginae.
To make the results more applicable to a wider population, future research should employ a bigger and more representative sample. To evaluate the plant extract's safety and long-term effects, investigations need to be conducted over an extended period of time. To further understand the extract's therapeutic benefits, it may be necessary to investigate the molecular mechanisms at work using cutting-edge methods like genomics and proteomics. Thoroughly assessing the possible toxicity and side effects is also crucial. Validating the treatment's effectiveness and safety for human applications would also need investigating different animal models and, ultimately, carrying out clinical studies.
5 Conclusion
The current study showed significant anti-arthritic effects from the plant extract of Strelitzia reginae. It was noticed that the methanolic extract of plant displayed downregulation of pro-inflammatory cytokines such as TNF-α, IL-4, and IL-17a,IFN- γ, IL-1, and RANKL. In addition to this, the upregulation of anti-inflammatory cytokines like OPG and IL-4 was also determined. Also, the oxidative stress biomarkers were subdued in polyarthritic rats The oxidative stress biomarkers were also improved in CFA- induced arthritic rats. There was a significant restoration of immune organ weight observed at a dose of 600 mg/kg. There is not much scientific data available for Strelitzia reginae in the literature, so the need is to unlock the new therapeutic potential of the plant.
CRediT authorship contribution statement
Asad Ali: Investigation, Data curation. Malik Saadullah: Writing – original draft, Data curation, Conceptualization. M. Fakhar-e-Alam: Methodology, Investigation. Rida Siddique: Formal analysis, Data curation. M. Atif: Writing – review & editing, Formal analysis.
Acknowledgement
Researchers Supporting Project number (RSP2024R397), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abramovič, H., 2015. Antioxidant properties of hydroxycinnamic acid derivatives: A focus on biochemistry, physicochemical parameters, reactive species, and biomolecular interactions. Coffee in health and disease prevention. Elsevier: 843-852.
- Boyce, B.F., Xing, L., 2007. The Rankl/Rank/Opg pathway. Cur. Osteoporosis Rep. 5 (3) 98-104.
- Evaluation of malondialdehyde, superoxide dismutase and catalase activity in fetal cord blood of depressed mothers. Clin. Psychopharmacol. Neurosci.. 2017;15(1):35.
- [Google Scholar]
- Oxidative state of the liver of rats with adjuvant-induced arthritis. Free. Radic. Biol. Med.. 2013;58:144-153.
- [Google Scholar]
- The role of COX-2 in rheumatoid arthritis synovial tissues. Arthritis. Res. Ther.. 1999;1(Suppl 1):S30.
- [Google Scholar]
- Burdock (Arctium lappa L.) leaf flavonoids rich in morin and quercetin 3-O-rhamnoside ameliorate lipopolysaccharide-induced inflammation and oxidative stress in RAW264. 7 cells. Food. Sci. Nutrit.. 2022;10(8):2718-2726.
- [Google Scholar]
- Interference by rheumatoid factor with the detection of C-reactive protein by the latex agglutination method. J. Rheumatol.. 1980;7(3):279-287.
- [Google Scholar]
- A mechanistic review on medicinal plants used for rheumatoid arthritis in traditional Persian medicine. J. Pharm. Pharmacol.. 2016;68(10):1233-1248.
- [Google Scholar]
- The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci.. 2021;22(5):2719.
- [Google Scholar]
- Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food. Chem. Toxicol.. 2004;42(3):429-438.
- [Google Scholar]
- Kroes, B. v., A. Van den Berg, H. Q. Van Ufford, et al., 1992. Anti-inflammatory activity of gallic acid. Planta medica. 58 (06) 499-504.
- Dehydrozingerone alleviates hyperalgesia, oxidative stress and inflammatory factors in complete Freund’s Adjuvant-induced arthritic rats. Drug. Des. Devel. Ther. 2022:3015-3022.
- [Google Scholar]
- Oroian, M., Dranca, F., Ursachi, F., 2020. Comparative evaluation of maceration, microwave and ultrasonic-assisted extraction of phenolic compounds from propolis. J.F.S.T57 70-78.
- In vivo antiarthritic activity of the ethanol extracts of stem bark and seeds of Calophyllum inophyllum in Freund’s complete adjuvant induced arthritis. Pharm. Biol.. 2017;55(1):1330-1336.
- [Google Scholar]
- T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine.. 2015;74(1):5-17.
- [Google Scholar]
- Moringa rivae leaf extracts attenuate Complete Freund’s adjuvant-induced arthritis in Wistar rats via modulation of inflammatory and oxidative stress biomarkers. Inflammopharmacology. 2020;28:139-151.
- [Google Scholar]
- Polystichum braunii extracts inhibit Complete Freund’s adjuvant-induced arthritis via upregulation of I-κB, IL-4, and IL-10, downregulation of COX-2, PGE2, IL-1β, IL-6, NF-κB, and TNF-α, and subsiding oxidative stress. Inflammopharmacology.. 2020;28:1633-1648.
- [Google Scholar]
- Prognostic factors in early rheumatoid arthritis. Rheumatology (Oxford, England).. 2000;39:24-29.
- [Google Scholar]
- Ziziphora clinopodioides ameliorated rheumatoid arthritis and inflammatory paw edema in different models of acute and chronic inflammation. Biomed. Pharmaco.. 2018;97:1710-1721.
- [Google Scholar]
- The pharmacological action of kaempferol in central nervous system diseases: a review. Front. Pharmacol.. 2021;11:565700.
- [Google Scholar]
- IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect. Biol.. 2014;6(10):a016295
- [Google Scholar]
- Investigation of the effect of phlomisoside F on complete Freund's adjuvant-induced arthritis. Exp. Ther. Med.. 2017;13(2):710-716.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103315.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







