Application of solarization for sanitization of sewage sludge compost
⁎Corresponding author. saimo@sakarya.edu.tr (Saim Ozdemir),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Performance of the solarization process on inactivation of pathogenic bacteria was explored to provide the sanitization standards set for the sewage sludge compost. For evaluation of the microbiological quality of sludge compost and the sanitation efficiency of solar application, Escherichia coli, thermotolerant coliforms, Clostridium, and Enterococci were selected as the indicator microorganisms. Unsolarized (control) treatment was conducted at room temperature (25 ± 2 °C) in laboratory conditions. Solarization treatment was performed under heating conditions, where the sufficient ultraviolet radiation was directly provided by sun. The results indicated that solarization remarkably increased the temperature of sludge compost to maximum 65 °C at 5 cm of compost depth. The inactivation rates of indicator microorganisms exposed to the solarization treatment were recorded significantly higher than the unsolarized treatment. Among the indicator microbial agents, E. coli was found as the most susceptible microorganism and lowered from 4 log CFU g−1 to undetectable levels after 6 days of solarization process. However, the reduction rate of the unsolarized group of this bacteria was determined to be less than the 1-log within 15 days of the entire experimental period. For the solarized compost, the viability of Enterococci ranged from 2.31 log CFU g−1 to 1.80 log CFU g−1 within 6 days and reduced to below the detection limit (2 × 101 cells as CFU) after 12 days of solar application. The reduction rates of thermotolerant coliforms and Enterococci were slow (kmax = 3.22 and 3.27 day−1, respectively), but they were reduced to below the detection limit within 12–15 days. The inactivation curves demonstrated that Clostridium showed more resistance to heat provided by solarization compared with other indicator microorganism species. The Clostridium reduction in solarized treatment was determined as 3-log, while the unsolarization treatment provided 0.7-log reduction. Findings of this study clearly corroborated that the temperature profile generated by the solarization process was adequate for elimination and/or inactivation of various microbial pathogens to achieve the desired standards within two weeks.
Keywords
Clostridium
E. coli
Enterococci
Thermotolerant coliform
Sewage sludge compost
Sanitization
Solarization
1 Introduction
Sewage sludge is a specific by-product of wastewater treatment facilities and their amount, worldwide, has considerably increased over the past years (Salihoglu et al., 2007; Boudjabi et al., 2019). Although agro-based utilization of sewage sludge is generally regarded as the most ideal option of management, a wide variety of pathogenic microorganisms in its composition imposes a serious threat to the environmental health (Ozdemir et al., 2013; Alvarenga et al., 2016). Sewage sludge provides an infinite habitat that promotes diverse microorganism including various co-existing phyto-pathogenic and human pathogenic bacteria (Ogleni and Ozdemir, 2010). For this reason, these pathogens must be destructed initially before the disposal to avoid cross-contamination of environment and crops.
Many researchers reported that sewage sludge composting is the most suitable process that decreases the pathogenic population (Pourcher et al., 2005; Alvarenga et al., 2016), but whole material may not be efficiently inactivated (Wolna-Maruwka, 2008), so that it is difficult to create appropriate homogeneous conditions ensuring a temperature higher than 50 °C for one week or more (Dede and Ozdemir, 2015). Additionally, inconsistent temperature profile within the compost pile may not be sufficient to eliminate all pathogens in the most cases (Pourcher et al., 2005). For the aim of achieving a high-level of disinfection and meeting the microbiological standards of the compost, it is crucial to maintain a sufficient period for a high-temperature isotherm in the sanitization process. Otherwise, additional options of hygienization, such as high temperatures (Romdhana et al., 2009), drying (Belloulid et al., 2017) or application of alkali conditions (Salihoglu et al., 2007), could be required to provide permissible levels.
Considering the destruction mechanism of enteric (intestinal) organisms, temperature rise caused by bio-metabolic activity (Wolna-Maruwka, 2008) or external sources particularly above the thermophilic range are regarded as the key parameters affecting the inactivation of pathogens in a sludge and compost matrix (Oropeza et al., 2001). Romdhana et al. (2009) reported that a temperature in the range of 44–48 °C is the upper boundary for the bacterial growth. They also emphasized that a temperature of 55 °C for 15 days or more is adequate for the heat inactivation of pathogenic bacteria during the sludge composting process. Likewise, Weil et al. (2013) reported that in a controlled-temperature water bath studies conducted at the temperature of 48.8, 54.4, and 60 °C provided complete inactivation of enteric pathogenic bacteria after 36, 8, and 0.5 h, respectively.
Solarization, a process that provides temperature rise in soil, and is routinely performed for controlling of soil borne plant pathogens (Wu et al., 2009). It is an easy and efficacious technology that has been implemented for hydrothermal disinfection of the soil which is covered with polyethylene sheets under the conditions of high environmental temperature and intensive solar radiation. The fundamental principle of the solarization process is to keep the radiant energy in the humid soil that is under the plastic cover, and to preserve this temperature for several weeks. Solarization can increase the soil temperature up to certain levels that are sufficient to destroy many disease-causing microorganisms. The high efficiency in removing indicator microorganisms by solar application has been already highlighted in the literature. For instance, in a recent study, Sossou et al. (2016) obtained a removal efficiency of 3.2-log reduction for E.coli and 1.6-log reductions for Enterococcus faecalis in a 5-h solarization period. Similarly, in a pilot-scale experimental study, Ozdemir et al. (2013) reported that a significant reduction could be achieved in thermotolerant fecal coliforms and Clostridium and complete inactivation could be attained in E. coli. The inactivation of pathogens in residual compost is a time-consuming and energy-wasting process, thus it is needed to apply more practical and economic method. In this regard, solar application is an easy to handle and cost-effective disinfection technique that can able to reduce enteric microorganism in fecal material (Ozdemir et al., 2013; Sossou et al., 2016), and its combination with compost treatment could allow a safe end-product for environmental protection.
So far, only a few of studies (Wu et al., 2009; Ozdemir et al., 2013; Sossou et al., 2016) dealing with inactivation of sludge originated enteric microorganism have earlier addressed the solar heat inactivation using dewatered sludge, toilet compost and soil applied sludge. The most recent research (Sossou et al., 2016) focused mainly on the rapid inactivation of enteric microorganism in compost using composting toilet. However, as a further step, the sanitizing effect of solar application on the inactivation of municipal sludge compost microorganism has not been widely investigated. For this reason, the present study aims at fulfilling the gap in this field by focusing upon solarization effect on inactivation of susceptible and resistant enteric bacteria in sewage sludge compost.
Based on the foregoing facts, the specific objectives of this study were as follows: (1) to explore the temperature rise potential of solarization in compost pile; (2) to examine the sanitization potential of accumulated heat to enteric microorganism; (3) to investigate the inactivation kinetics of susceptible and resistant bacterial indicators; and (4) to assess the effectiveness of the proposed treatment method on the inactivation rate of enteric microorganism from sewage sludge compost.
2 Materials and methods
2.1 Collection and preparation of sewage sludge
The sewage sludge used in the experimental study was collected from a Wastewater Treatment Plant (average wastewater flow rate = 80,000 m3 day−1) of the city of Sakarya, located in the northwest region of Turkey (latitude 40° 50′ N, longitude 30° 19′ E, and 31 m above the sea level). The treatment plant is operating with extended aeration. After thickening process, sludge is mechanically dewatered by decanter, allowing about 20% dry matter content. The dewatered sludge obtained from this facility was first composted in a 60 L-capacity laboratory-scale composter by mixing with 1:1 (v/v) grounded hazelnut husk (particle size of 0–4 mm) bulking agent. The composting experiment was carried out for a period of 28 days, representing the active composting phase. No specific aeration system was installed, but pile was aerated by mixing the content manually every day. Temperatures of the compost pile were above the 52 °C threshold during 3 days of the first 15 days of the composting experiment. The initial concentrations of selected indicators in compost mixture were 4.0 log CFU g−1 for E. coli, 5.1 log CFU g−1 for thermotolerant coliform, 2.31 log CFU g−1 for Enterococci, and 4.0 log CFU g−1 for Clostridium.
Following the active phase of composting, compost samples were homogeneously separated into parts. One part of compost sample was loaded into the PVC solarization container up to a height of 40 cm. Solarization treatment was directly exposed to ultraviolet radiation provided by sun at local environmental conditions, while unsolarized (control) treatment, which was conducted with the same amount of compost, was kept at room temperature (25 ± 2 °C) in the laboratory during the experimental study (Fig. 1).
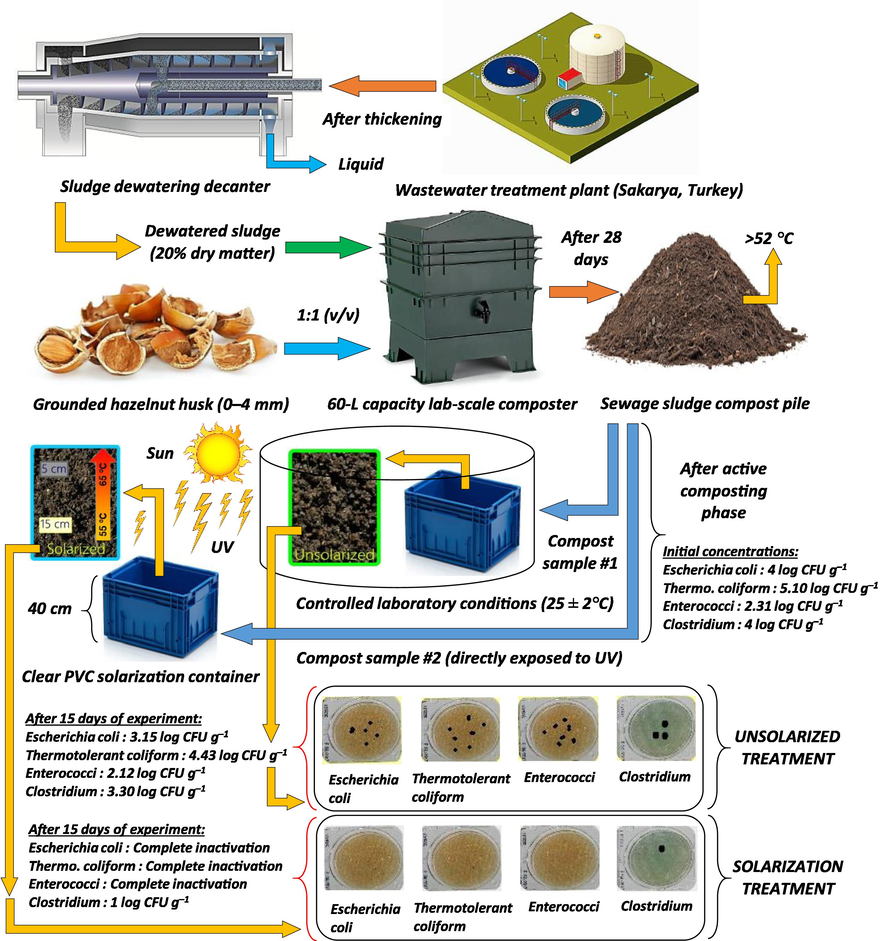
- Schematic representation of the solar application to sludge compost for microbial disinfection.
2.2 Sampling period and procedure
The experiment was carried out from August 1 to 15, 2015. Meteorological data of the experimental period were obtained from Adapazari meteorological station, Sakarya, Turkey (Fig. 2). Compost temperatures at two different depths (between 0–10 cm and 10–30 cm) were measured on a daily-basis (every day 14:00p.m.) by using a soil thermometer (TF002, Agromak, Turkey) within two-week sampling period. Compost samples were taken from 5 and 30 cm depths in each sampling day and homogenously mixed sub samples obtain from mixture were used for microbial analysis. The effects of solarization on microbial reduction were evaluated by obtaining samples (1 g for each treatment) at every 48 h within 15 days of the entire experimental period.
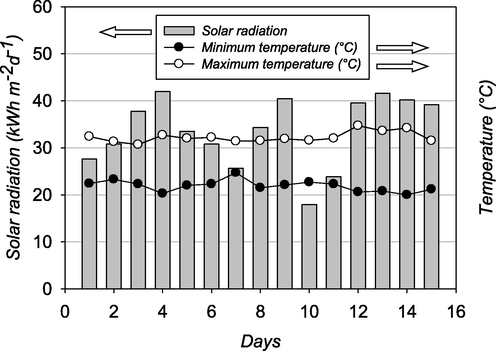
- Ambient solar radiation, the maximum and the minimum daily temperatures during the solarization period.
2.3 Indicator microorganisms and microbial procedure
For quantitative evaluation of the microbiological quality of sludge compost and the sanitation efficiency of the present solar application, Escherichia coli, thermotolerant coliforms, Clostridium, and Enterococci were chosen as the indicator microorganisms. In this study, Escherichia coli and thermotolerant coliforms were identified on m-FC medium following to the membrane filtration based on the procedure specified in ISO 9308-1 (ISO, 2014). Enterococci was enumerated by membrane filtration in accordance with the ISO standard 7899-2 (ISO, 2000). Moreover, Clostridium was counted based on ISO 14189 standard (ISO, 2013).
Serial dilutions of the sample were performed in the isotonic solution, and 1 g of each sample was consecutively diluted tenfold in 9 mL of this solution for the microbial analysis. Dilutions from 10−1 to 10−5 of 100 μL of 1:10 dilution were conducted in triplicates on different microbiological media. The inoculated plates were incubated at 37 °C for 24 h, after incubation, the emergent microbial colonies on each plate was quantified and each value was recorded (Ogleni and Ozdemir, 2010).
2.4 Microbial inactivation modeling
For the quantification of decline in survival curves, the following shoulder/tail inactivation models (i.e., linear approach, logistic (or Fermi) function, and residual population density-based inactivation model, respectively) expressed in Eqs. (1)–(3) are used (Geeraerd et al., 2000; McKellar and Lu, 2003; Sant’Ana, 2016):
In this study, GInaFiT inactivation model fitting tool was implemented to derive the best-fit microbial inactivation models for the selected indicator microorganisms (Geeraerd et al., 2005). The analyses were implemented within the framework of GInaFiT version 1.6 a freeware Add-in for Microsoft® Excel® 2016 (Microsoft Inc., Redmond, WA) package operating on a Casper Excalibur (Intel® Core™ i7-7700HQ CPU, 2.81 GHz, 16 GB of RAM, 64-bit) Windows 10 PC platform. In all calculations, spreadsheets of Microsoft® Excel® running under the same operating system was used as an open database connectivity data source. According to the preliminary computational analysis, the best-fit models were log-linear regression, log-linear regression plus tail, and log-linear regression plus shoulder plus tail, respectively, for E. coli, thermotolerant coliforms, and Enterococci and Clostridium.
2.5 Statistical procedure
In this study, the root mean sum of square error (RMSE) was used as the primary statistical performance indicator for judging the quality of the best-fit inactivation curves. The inactivation rate kmax (slope of the exponential portion of the survival curve) and T90 values (time to inactivate 90% of the population) were computed using the best-fit models obtained with the help of GInaFiT. Log10 transformed bacterial quantifications were subjected to two-way analysis of variance (ANOVA) analysis to interpret the influence of time and sludge depth on the pathogen inactivation. In the computational analysis, an alpha (α) level of 0.05 (or 95% confidence) was considered to appraise the statistical significance of the shoulder/tail-based models.
The data sets of solarized and unsolarized (control) treatments obtained for indicator microorganisms were statistically evaluated via proper parametric (unpaired (or two-sample) t test and F (variance ratio) test) or non-parametric tests (the Mann–Whitney (MW) U (or the Wilcoxon rank-sum) test or the Kruskal–Wallis (KW) test with the Dwass-Steel-Chritchlow-Fligner method). Prior to applying these tests, the Shapiro–Wilk W and the Levene’s tests were respectively implemented as preconditions to ensure whether the subsets (herein solarized and unsolarized treatments for indicator microorganisms) had a normal or non-normal distribution, and variances (or standard deviations) of the paired groups were homogeneous or unequal. In case of the data sets were not normally distributed, a non-parametric test (MW or KW) was conducted instead of a parametric test. Test results were appraised with two-tailed p values to indicate the statistical significance between the experimental groups (Ozdemir et al., 2020).
3 Results and discussion
3.1 Temperature increase by solarization process
The variation of temperatures recorded at the depths of 5 and 15 cm in the solarized compost during the experimental period is depicted in Fig. 3. As seen from the respective profiles, at the beginning of the solarization process, the temperature quickly rose above 50 °C on day 3 for both monitored compost depths. During the days with higher solar radiation, compost temperatures were recorded above the thermophilic temperature of 60 °C at 30 cm depth and even reached 65 °C at 5 cm of compost depth. As expected, those compost temperatures were significantly higher (p < 0.01) than the compost kept at laboratory conditions. Compost temperatures in unsolarized experiment were close to ambient temperature fluctuated around 20–25 °C at each sampling day. For 2-week summer term, solarization of compost in the PVC container was very effective for increasing the compost temperature (Fig. 3) and showed a lethal effect for E. coli, thermotolerant coliforms, Enterococci, and Clostridium species (Romdhana et al., 2009; Sossou et al., 2016).
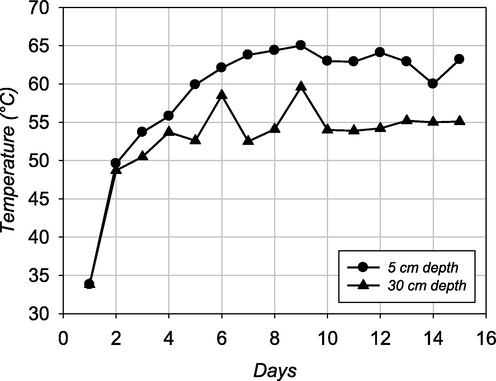
- Recorded compost temperatures at 5 and 30 cm depths of solarized treatment during experimental period.
3.2 E. coli inactivation
The driving force of time-temperature kinetics has been known as the main process for bacterial inactivation in heat applications for sanitization experiments (Singh et al., 2011; Sossou et al., 2016). In a laboratory-scale time–temperature experiments, Lang and Smith (2008) examined the bacterial decay of E. coli strains at 70 and 55 °C and found the complete inactivation after 10 s and 20–60 min respectively. However, in a full-scale processing conditions, complete inactivation time was reported to be extended beyond the hours to day in thermophilic temperature ranges (Singh et al., 2011). In the present study, E. coli population decreased rapidly under the solarization treatment, and the reduction rate showed a first-order (linear) removal kinetic with substantially higher reduction effectiveness at solarized compost samples compared to the unsolarized treatments (Fig. 4a). E. coli population was reduced from 4 log CFU g−1 to below the detection limit values of 2 × 101 cells (as CFU) after 6 days of solarization. The number of E. coli of compost samples that did not exposed to solarization reduced, but the reduction rate was determined to be less than the 1-log. The results indicated that the temperature increase in solarized compost samples was caused a lethal temperature range for E. coli. Consistent with previous researches, E. coli was more susceptible to heat inactivation (Watcharasukarn et al., 2009) assured by the solarization. For the increasing temperatures, the inactivation kinetics (kmax = 1.04 day−1 and T90 = 2.91 days) obtained in the present study were agreed with those observed for the E. coli in the similar solarization conditions applied for the municipal sewage sludge (kmax = 0.65–0.85 day−1 and T90 = 2.47–4.60 days) depending on sludge depth (Ozdemir et al., 2013). The reductions in the number of microorganism showed a positive correlation with time. On the other hand, compost temperatures were induced by higher ambient temperature and daily solar radiation level. Therefore, inactivation rates (kmax) increased with compost temperature increase, and T90 values reduced (Table 1). The numerical results corroborated the positive impact of the solarization on inactivation rate of E. coli.
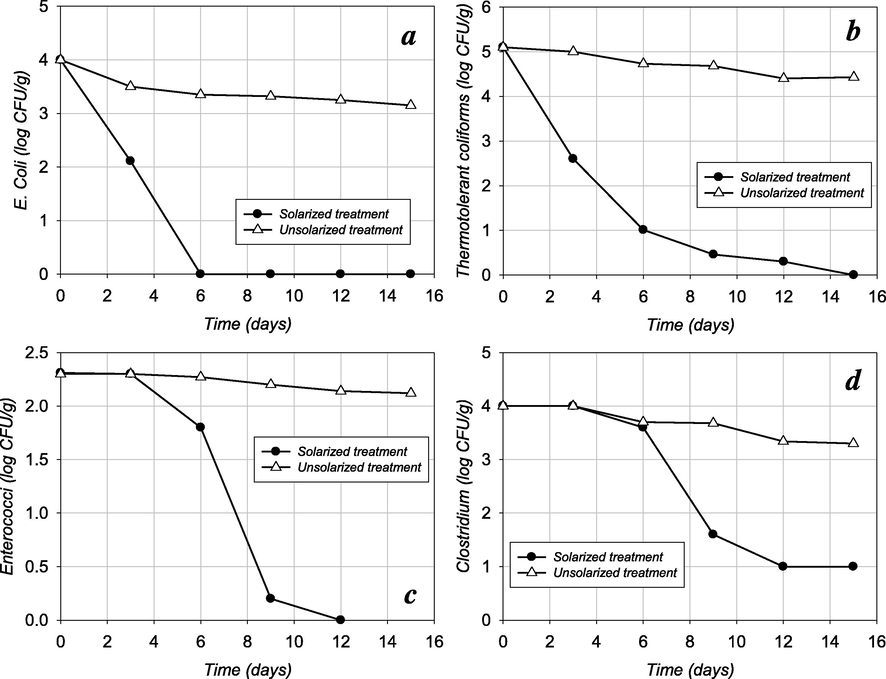
- Inactivation of indicator microorganisms in the sludge compost under solarized and unsolarized treatments: (a) E. coli; (b) Thermotolerant coliforms; (c) Enterococci; and (d) Clostridium.
| Indicator bacteria | Maximum specific decay or inactivation rate (kmax:day−1) | Time to inactivate 90% of the population (T90:day) | Root mean square error (RMSE) |
|---|---|---|---|
| Escherichia coli | 1.04 | 2.91 | 0.156 |
| Thermotolerant coliforms | 3.22 | 3.35 | 0.953 |
| Enterococci | 3.27 | 10.1 | 0.530 |
| Clostridium | 1.61 | 7.90 | 0.299 |
3.3 Thermotolerant coliform inactivation
As seen from Fig. 4b, the number of thermotolerant coliform decreased rapidly and was quantified below 1-log after 6 days of solar application, while the population in unsolarized (control) treatment remained almost stable during the experimental period (Fig. 4b). The average number of thermotolerant coliforms was quantified as 5.10 log CFU g−1 at the beginning of the solar application and reduced substantially to 1.01 log CFU g−1 within the first week of the process. After the bacterial concentration decreased to 1.01 log CFU g−1, the rate of destruction within the further stage was recorded to be slower. This can be ascribed to the fact that heat resistant fraction of the bacteria need be exposed to higher dose of inactivation factor with longer exposure time. This is compatible with the findings of Lang and Smith (2006) who reported the complete inactivation of thermotolerant strains within 40 s and 60 min at 70 and 55 °C, respectively. Representing the real-world applications, the complete inactivation was achieved on day 15 at the end of the experimental period of present study, which is in line with the Singh et al. (2011) who reported more than 5 days for complete removal from compost mixture at 55 and 60 °C. For the thermotolerant coliforms considered in this study, a positive correlation (p ≤ 0.01) between bacterial reduction kinetics and temperature rise achieved by the solarization confirmed the desired sanitizing condition for the sludge compost (Ogleni and Ozdemir, 2010).
3.4 Enterococci inactivation
Unlike E. coli and thermotolerant coliform, the number of Enterococci bacteria in the sludge compost remained almost stable (except with a very small fluctuation from 2.31 log CFU g−1 to 2.30 log CFU g−1 for solarized treatment) within 3 days of the entire experimental period (Fig. 4c). The initial mild temperatures and appropriate compost substrates were considered suitable conditions for the viable cell count for those bacteria species. The viable cell counts of Enterococci revealed that the viability of these bacteria with time ranged from 2.31 log CFU g−1 to 1.80 log CFU g−1 for solarized compost (within 6 days) and decreased to concentrations below the detection limit of 2 × 101 cells (as CFU) after 12 days of solar application. The destruction of the indicator organisms could be explained by the increase in temperature and other possible environmental conditions (Ozdemir et al., 2013). The reduction of the Enterococci count below the detectable number was noted after 12 days of solar application. Supporting results were also observed by Klein et al. (2010) who reported rapid inactivation of Enterococci at 60 °C (kmax = 1.2 day−1 and T90 = 2.1 days) compared to the incubation at 37 °C (kmax = 0.05 day−1 and T90 = 46 days).
3.5 Clostridium inactivation
Clostridium was selected as another indicator microorganism to determine the effect of the implemented solarization process to removal kinetics of resistant bacterial species. The removal rate during the solarization is shown in Fig. 4d. The decrease in viable cell under the solar application was found positively correlated with the temperature rise (p ≤ 0.01). While the solarization treatment provided 3-log reduction, the Clostridium reduction in unsolarized treatment was determined as 0.7-log. However, according to the inactivation curves, T90, and kmax values (Table 1), the Clostridium was found to be more resistant to heat inactivation provided by solarization. It is noted that the sigmoidal inactivation curve, which indicates the resistance of this microorganism to negative environmental conditions, has also been reported by others (Viau and Peccia, 2009; Fisher and Phillips, 2009). Moreover, Usui et al. (2017) reported that temperature rise beyond 55, 60, and 70 °C and exposure times of 5, 10, and 30 days had a positive correlation with the complete elimination of Clostridium bacteria in the sludge.
Although the significant reductions were achieved in viable cell counts, solar application was not able to lower the Clostridium population below undetectable levels of 2 × 101 cells (as CFU) (Table 1, Fig. 4d). The possible reason can be ascribed to the adaptive characteristics of the remained fraction of these species to thermophilic conditions, where temperature rises were not faster. Miller et al. (2009) reported that gradual temperature increases stimulate the adaptive mutation, as indicated by the existence of tailing in the inactivation curve. Similarly, presence of heat-adapted strains of Salmonella spp. in a manure compost maintained at 55 °C were observed during the 30 days of experimental period (Shepherd et al., 2010). In the present study, the positive correlation between the temperature rise and 3-log reduction in the number of Clostridium indicated that the solarization process could be applied as a suitable sanitization alternative for the sludge compost.
3.6 Sustainable advantages of solarization for disinfection of organic waste
The municipal wastewater treatment operations generate substantial amount of sludge that should be properly disposed (Alvarenga et al., 2016; Davis et al., 2016). This nutrient-rich sludge could be beneficially recycled for agricultural purposes after composting to meet nutrient demand and enrich the soil organic matter (Boudjabi et al., 2019). Adapting more sanitized sludge compost to crop cultivation is believed to promote recycling facilities, encourage waste management, and land application (Ozdemir et al., 2013; Belloulid et al., 2017). In this regard, considering the combined advantages of waste disposal and the extended beneficial recycling alternatives, integration of composting with environmentally friendly solar disinfection practices might be very feasible for the closed waste management strategies (Davis et al., 2016).
Solarization is an efficient sanitization option to produce microbiologically safe compost, however, the method needs to be further tested for different pathogenic microorganisms and their resistant species such as spore-forming bacteria. Moreover, regardless of the efficacy of the sanitization process, it is also considerable to be aware of the fundamental microbial principles when studying on waste products, such as sewage sludge, to minimize the recontamination that is caused by pathogenic microorganisms.
In this study, a new disinfection alternative for sewage sludge compost using solar energy was introduced, and its performance was analyzed by focusing on different indicator microorganisms in the summer period of temperate region. In the future studies, system performance could be analyzed in terms of different environmental conditions such as compost humidity, CO2 concentration, or solar radiation rate. More detailed configuration could be developed by the designer for the aim of increasing the temperature rise and accumulation of heat in the solarization system to define technical options that enable to achieve higher performance.
4 Conclusions
In this study, the effectiveness of the solarization process on inactivation of pathogenic microorganisms, such as Escherichia coli, thermotolerant coliforms, Clostridium, and Enterococci, was investigated for meeting the hygienization standards for the sewage sludge compost. Based on the experimental findings, the main conclusions were drawn as follows:
-
(1)
Microbiological reductions indicated that the solar application was able to provide effective removal of indicator microorganisms, and the temperature increase was the primary factor providing microbial inactivation during the solarization process.
-
(2)
Complete inactivation of E. coli, thermotolerant coliform, and Enterococci was achieved with the solarization following to the composting, while the Clostridium was not reduced to the undetectable level during experimental period due to the adaptive characteristics of the residual viable cells to thermophilic regime.
-
(3)
Based on the experimental results and the published literature, it is concluded that the integration of solarization process with composting could improve disinfection efficiency and microbiological quality of sludge compost to meet the regulations set for Class A Biosolids for the agricultural reuse (Sanin et al., 2011).
-
(4)
The application of solarization system is a simple, just comprises to keep the solar energy beneath the plastic cover to warm up the material and maintain this temperature for several days to weeks depending on the temperature rise above the sanitization range.
-
(5)
High ambient temperature and intense solar radiation during the summer period could increase and improve the hydrothermal disinfection performance of the system. Therefore, this process can be commonly applied in regions with high solar radiation and high temperatures during the summer season. It doesn’t need external energy consumption and therefore considered as low-tech and environmentally friendly system.
References
- Beneficial use of dewatered and composted sewage sludge as soil amendments: Behaviour of metals in soils and their uptake by plant. Waste Biomass Valor.. 2016;7:1189-1201.
- [Google Scholar]
- Solar greenhouse drying of wastewater sludges under arid climate. Waste Biomass Valor.. 2017;8:193-202.
- [Google Scholar]
- Sewage sludge fertilization alleviates drought stress and improves physiological adaptation and yield performances in Durum Wheat (Triticum durum): a double-edged sword. J King Saud Univ.-Sci.. 2019;31:336-344.
- [Google Scholar]
- Closing the loop: integrative systems management of waste in food, energy, and water systems. J. Environ. Stud. Sci.. 2016;6:11-24.
- [Google Scholar]
- Comparison of composted biosolid substrate for containerized turfgrass production. Environ. Technol.. 2015;36:1651-1656.
- [Google Scholar]
- The ecology, epidemiology and virulence of Enterococcus. J. App. Microbiol.. 2009;155:1749-1757.
- [Google Scholar]
- Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol.. 2000;59:185-209.
- [Google Scholar]
- GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol.. 2005;102:95-105.
- [Google Scholar]
- 7899-2 Water Quality-Detection and Enumeration of Intestinal Enterococci. Part 2: Membrane Filtration Method. Geneva, Switzerland: International Organization for Standardization (ISO); 2000.
- 14189 Water Quality-Enumeration of Clostridium perfingens. Membrane Filtration Method. Geneva, Switzerland: International Organization for Standardization (ISO); 2013.
- 9308/1 Water Quality-Enumeration of Escherichia coli and Coliform Bacteria. Part 1: Membrane Filtration Method. Geneva, Switzerland: International Organization for Standardization (ISO); 2014.
- Inactivation of indicators and pathogens in cattle feedlot manures and compost as determined by molecular and culture assays. FEMS Microbiol. Ecol.. 2011;77:200-210.
- [Google Scholar]
- Time and temperature inactivation kinetics of enteric bacteria relevant to sewage sludge treatment processes for agricultural use. Water Res.. 2008;42:2229-2241.
- [Google Scholar]
- McKellar, R.C., Lu, X., (Eds), 2003. Modeling microbial responses in food. CRC Series in Contemporary Food Science, Boca Raton, FL, USA.
- Sigmoidal thermal inactivation kinetics of Listeria innocua in broth: influence of strain and growth phase. Food Control. 2009;20:1151-1157.
- [Google Scholar]
- Pathogen reduction effects of solar drying and soil application in sewage sludge. Turk. J. Agric. For.. 2010;34:509-515.
- [Google Scholar]
- Removal of fecal indicator organism and parasites (fecal coliforms and helmint eggs) from municipal biologic sludge by anaerobic mesophilic and thermophilic digestion. Water Sci. Technol.. 2001;44:97-101.
- [Google Scholar]
- Effect of solarization on the removal of indicator microorganisms from municipal sewage sludge. Environ. Technol.. 2013;34:1497-1502.
- [Google Scholar]
- Effects of poultry abattoir sludge amendment on feedstock composition, energy content, and combustion emissions of giant reed (Arundo donax L.) J. King Saud. Univ. Sci.. 2020;32:149-155.
- [Google Scholar]
- Decrease of enteric microorganisms from rural sewage sludge during their composting in straw mixture. J. Appl. Microbiol.. 2005;99:528-539.
- [Google Scholar]
- Monitoring of pathogenic microorganisms contamination during heat drying process of sewage sludge. Process. Saf. Environ.. 2009;87:377-386.
- [Google Scholar]
- Sant’Ana A.S., ed. Quantitative Microbiology in Food Processing: Modeling the Microbial Ecology. Chichester, West Sussex, UK: John Wiley & Sons; 2016.
- Sludge Engineering: The Treatment and Disposal of Wastewater Sludges. Lancaster, PA, USA: DEStech Publications Inc; 2011.
- Determining thermal inactivation of Escherichia coli O157: H7 in fresh compost by simulating early phases of the composting process. Appl. Environ. Microbiol.. 2011;77:4126-4135.
- [Google Scholar]
- Inactivation kinetics of indicator microorganisms during solar heat treatment for sanitizing compost from composting toilet. J. Water Environ. Technol.. 2016;14:37-46.
- [Google Scholar]
- Effect of heat-shock treatment on the survival of Escherichia coli O157: H7 and Salmonella enterica Typhimurium in dairy manure co-composted with vegetable wastes under field conditions. Bioresour. Technol.. 2010;101:5407-5413.
- [Google Scholar]
- Survival and prevalence of Clostridium difficile in manure compost derived from pigs. Anaerobe. 2017;43:15-20.
- [Google Scholar]
- Screening Escheria coli, Enterococcus faecalis, and Clostridium perfingens as indicator organism in evaluating pathogen reducing capacity in biogas plants. Environ. Microbiol.. 2009;58:221-2230.
- [Google Scholar]
- Inactivation of human pathogens during phase II composting of manure-based mushroom growth substrate. J. Food Protect.. 2013;76:1393-1400.
- [Google Scholar]
- Evaluation of the enterococci indicator in biosolids using culture-based and quantitative PCR assays. Water Res.. 2009;43:4878-4887.
- [Google Scholar]
- Estimation of microbiological sewage sludge subject to composting process in controlled conditions. Pol. J. Environ. Stud.. 2008;18:279-288.
- [Google Scholar]
- Inactivation of Escherichia coli in soil by solarization. Soil Sci. Plant Nutr.. 2009;55:258-263.
- [Google Scholar]







