Translate this page into:
Application of a new adsorption kinetic model for the removal of Zn(II) ions present in aqueous solutions with Malatya clay
*Corresponding author: E-mail address: nilgunonursal@siirt.edu.tr (N. Onursal)
-
Received: ,
Accepted: ,
Abstract
Efficient removal protocols are essential to mitigate the significant environmental issue posed by heavy metal pollution. A prevalent and efficient technique for this is adsorption. Zinc [Zn (II)] is a critical element that must be eliminated from water sources before its concentration attains hazardous levels. This research investigates the kinetics of Zn (II) adsorption on natural Malatya clay (MC) and presents a novel model for said process. The current study used naturally occurring MC as an adsorbent. The material was synthesized and characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), and Brunauer-Emmett-Teller (BET) studies. Tests for adsorption were performed at 298, 308, and 318 K to examine the influence of temperature, pH, and adsorbent dosage. Five models were included in the kinetic and isotherm analyses: Elovich, intraparticle diffusion, pseudo-second-order (PSO), pseudo-first-order (PFO), and a novel model. The least squares method was used to calculate adsorption capacity and regression (R2) values for the best, most accurate model. The adsorption capacity increased with temperature, culminating at pH 6. The PSO model, with an R2 > 0.99, surpasses all kinetic models except for the Elovich, Weber-Morris (WM), and PFO. The Langmuir isotherm study confirmed chemical adsorption, indicating the highest monolayer adsorption capacity of 43.29 mg/g at 318 K. The proposed kinetic model demonstrated high R2 values and flexibility, effectively characterizing Zn (II) adsorption on heterogeneous and multilayer surfaces. The findings suggest that MC possesses significant potential for the removal of Zn (II). The suggested kinetic model, which accommodates various surface and adsorption circumstances, offers a fresh and dependable framework for adsorption research. Considering these results, the innovative model and Malatya clay merit substantial attention as effective strategies for alleviating zinc contamination in aquatic environments.
Keywords
Heavy metal
Langmuir isotherms
Malatya clay
New adsorption kinetic model
Zinc
1. Introduction
Urbanization, with an ongoing increase in global population, has significantly accelerated the use of heavy metals and raised pollution levels. The rising concentration of heavy metal pollution in the environment is harmful to several organisms (Altunkaynak et al., 2023). To mitigate these consequences and protect public health and ecological systems, robust waste monitoring and management protocols are essential in urban areas. Most of the wastewater generated because of industrial activities contains toxic heavy metals (Kayranli, 2021). One of these heavy metals is zinc. Based on data from 2019, zinc production was almost 10.5 Mt, thanks to electrolytic, purification, processing, roasting, pyrometallurgical, and electrodeposition processes. Zinc is used in many areas due to its strong binding. It is one of the most widely used metals, especially in galvanic plating, medicine, mining, battery, painting, smelting, agricultural processes, pesticide, rubber, and pigment industries (Dal et al., 2021). Due to the ion pollution of heavy metals, the presence of such materials at high levels in the environment is potentially dangerous and the realization of this danger has led to serious concerns. Currently, careless metal release into the environment results in the degradation of soil, water contamination, and vehicle-induced air pollution (Sen and Gomez, 2011).
The WHO states that the permissible zinc content in drinking water is 5 mg/L (Venable, 1884). Heavy metals are inherently present in the earth’s crust and, thus, cannot be fully eliminated. They infiltrate our bodies through drinking water, food, and air, although in minimal quantities (Abdus-Salam and Adekola, 2018). Certain elements, like copper, selenium, and zinc, are essential for sustaining human metabolic processes (Kangalgi̇l and Yardımcı, 2017). Selenium is vital to several biological activities, including thyroid hormone metabolism, immune system modulation, and antioxidant defense mechanisms. It also participates in many enzymes as a cofactor. Diseases such as insulin resistance, cancer, cardiovascular diseases, diabetes, and neurological disorders and aging are thought to intensify in the absence of this element (Akdeniz et al., 2016).
Zinc is a vital element that significantly influences several biological processes in both animals and plants (Tchounwou et al., 2012). The excess supply of these metals beyond the required quantities induces various issues. Excessive zinc consumption may lead to neurological issues like sleepiness, depression, ataxia, and seizures. Therefore, it is crucial to remove these components from water as effectively as possible. Wastewater treatment and harmful material removal have become issues for almost the whole world. This is because both groundwater and surface water are polluted with human-induced impurities (Nasseri et al., 2021).
There are several techniques for removing heavy metal ions, including ion exchange, oxidation, chemical deposition, evaporation, solvent extraction, adsorption, and reverse osmosis (Cherono et al., 2021). Some of the conventional methods are quite costly in addition to their low efficiency at very low metal concentrations such as 1-100 mg/L. In addition, some of them cause sludge formation and create an additional cost burden for disposal (Nematollah et al., 2014). This is because these toxic sludges are wastes and pretreatment systems that require high energy consumption (Koroğlu, 2007). One of the most popular methods owing to its affordability and simplicity of use, is adsorption (Cherono et al., 2021). Due to its exceptional separation efficiency, adsorption is regarded as one of the most efficient methods for the elimination of heavy metals.
Conventional heavy metal removal techniques often prove ineffective when addressing very low metal concentrations (1-100 mg/L). Consequently, they are unsuitable for trace-level detoxification. Numerous proven methods exist for the removal of heavy metals; nevertheless, they may be costly and energy-demanding, especially in the management of hazardous sludge and its disposal. Research on efficient and cost-effective methods for zinc removal has been limited despite its significance as an essential micronutrient and potential contaminant and its broad industrial applications. The recent need for biocompatible materials for applications in several fields, including healthcare, medicine, water treatment, and purification, has generated increased interest in the domain of nanotechnology (Solmaz et al. 2024). The efficiency and sustainability of wastewater treatment systems, particularly at low zinc concentrations, may be enhanced using novel, cost-effective adsorbents with superior zinc selectivity. This may be accomplished in addition to minimizing sludge generation and overall treatment expense. A sustainable approach for heavy metal pollution management involves using Malatya clay to clean contaminated water and eliminate Zn (II) ions. Natural materials like Malatya clay provide an eco-friendly alternative to traditional water treatment procedures, which are becoming unsustainable owing to the escalation of heavy metal contamination in aquatic environments resulting from urbanization.
Malatya clay is an efficient adsorbent for the removal of zinc ions from water, according to recent initiatives to reduce environmental heavy metal pollution. Environmental cleaning solutions will be enhanced by more optimization via research.
2. Materials and methods
2.1 Clay
The clay used in chemistry pertains to aluminosilicates and finds applications across several sectors, including manufacturing, building, ceramics, and cleaning. However, they are quite complex materials whose structures have been recently elucidated (Balali-Mood et al., 2021). The clay used in this study was obtained from the Malatya province of Eastern Anatolia region of Turkey. The clay in stone form was ground and pulverized in a grinder, then passed through a 120-micron sieve and dried by heating in an oven at 80°C for 12 hours. It was made ready to be used in the experiments as natural clay.
2.2 Heavy metals
Organisms today are far more susceptible to encountering heavy metals than they were a century ago, due to industrialization. Exposure to heavy metals such as lead, arsenic, mercury, chromium, cadmium, zinc, and copper may result in causing toxicity to humans. This study aimed to devise a method for the extraction of Zn(II) ions from aqueous solutions. While the toxicity of heavy metals has been acknowledged for an extended period, recent experimental findings indicate that Ni, Zn, and Cu—three of the most prevalent metals are, in fact, vital for human health (Madejová, 2003). Zinc, a transition metal, is also a micronutrient that is very important. It is a trace element that ensures the proper functioning of both the body and immune systems (Dal et al., 2021).
2.3 Adsorbate solutions
The chemicals that were used were purchased from Sigma and Merck. The required amount of Zn(NO3)2 was dissolved in distilled water to create metal ion solutions with different concentrations. The pH of the solutions was also adjusted using 0.1 M HCl and 0.1 M NaOH solutions.
2.4 Experimental studies
The solution (25 mL) containing 1-100 mgL-1/Zn (II) was added to a 50 mL flask that contained 25 mg of clay. The solution was shaken at temperatures 298, 308, and 318 K for 2 hours. The concentration of residual metal ions after adsorption was measured using the Atomic Absorption Spectrophotometer (AAS). Equation 1 was used to calculate the amount of Zn (II) ions adsorbed per unit mass. This calculation determined the optimal initial concentration of Zn (II) ions for attaining maximum adsorption.
where m is the amount of adsorbent (g), V is the volume (mL), Ci is the starting concentration, and Ce is the equilibrium concentration.
2.5 Devices and models used in adsorption study
The amount of Zn(II) was measured by AAS (Unicam 929, USA), inductively coupled plasma optical emission spectrometry (ICP-OES), Perkin-Elmer Optima 5300), and inductively coupled plasma mass spectrometry (ICP-MS). For surface analysis, SEM of the EVO 40 LEQ type was used. The Shimadzu TGA-50 was used to conduct thermogravimetric analysis (TGA) and differential thermal analysis (DTA) at temperatures between 20°C and 750°C. The BET (Brunauer-Emmett-Teller) surface area was calculated using Quantasorb surface analyzer technology. The infrared spectra were recorded using an FTIR spectrophotometer of the Mattson-1000 type.
3. Results
3.1 Effect of contact time on adsorption
The impact of agitation duration was assessed within the time frame of 30 to 360 minutes. Fig. 1 illustrates the impact of shaking duration. The quantity of adsorbed Zn (II) (qe) increased with prolonged contact time.
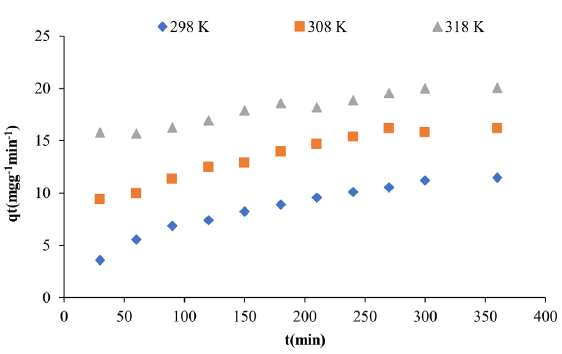
- Effect of contact time at different temperatures on Zn(II) adsorption.
3.2. Effect of pH on adsorption
To investigate the pH effect, solutions were prepared at 25 mg Malatya clay (30°C) at different initial pH ranges (1, 2, 3, 4, 4.5, 5, 5.5, 6, 6.5, 7, 8, 10) and after adding 0.1 mol L-1 KCl and using NaOH and HCl solutions, a change in the ionic strength of the solutions was observed. The clay and aqueous solutions used as adsorbents were kept in the reaction medium for 2 hours and then centrifuged and the samples were read in the device. The best pH was 6.
Fig. 2 illustrates the influence of pH on the adsorption of Zn (II) onto Malatya clay. Zn (II) ions may exist as Zn2+, Zn(OH)+, Zn(OH)2, or ZnO22- depending on the pH in aqueous solutions. At low pHs, when the hydronium (H3O+) ion concentration is high, silane (ΞSi-O-) in clay can be found as Zn (II) ions.
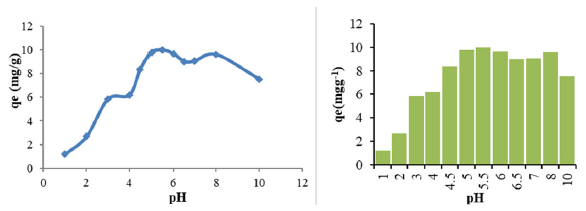
- The effect of pH on the removal of Zn2+ with Malatya Clay.
An interaction occurs between the groups and hydronium and silanol groups are formed (Eq. 2).
At medium pHs, silane groups react with Zn(II) ions to form silane complexes (Eq. 3).
In this study, the highest adsorption capacity was obtained between pH ≈ 6.9. At higher pHs, Zn(II) is transformed into zincate (ZnO2-) form and does not react with silsn (ΞSi-O-) groups. Thus adsorption decreases.
3.3 Adsorbent characterization
3.3.1 X-ray diffraction analysis (XRD)
The XRD diffractogram of Malatya clay used as an adsorbent in the experiments in the range of 0-70 2θ has been given in Fig. 3(a). Similarly, the XRD diffractogram of Malatya clay treated with ethylene glycol in the 0-30 2θ range has been given in Fig. 3(b). The XRD diffractogram of clay heated at 350°C in the range of 0-30 2θ has been given in Fig. 3(c). Lastly, the XRD diffractogram of clay heated at 550°C in the range of 0-30 2θ has been given in Fig. 3(d). Detailed clay analyses revealed that the sample contains montmorillonite and illite - vermiculite.
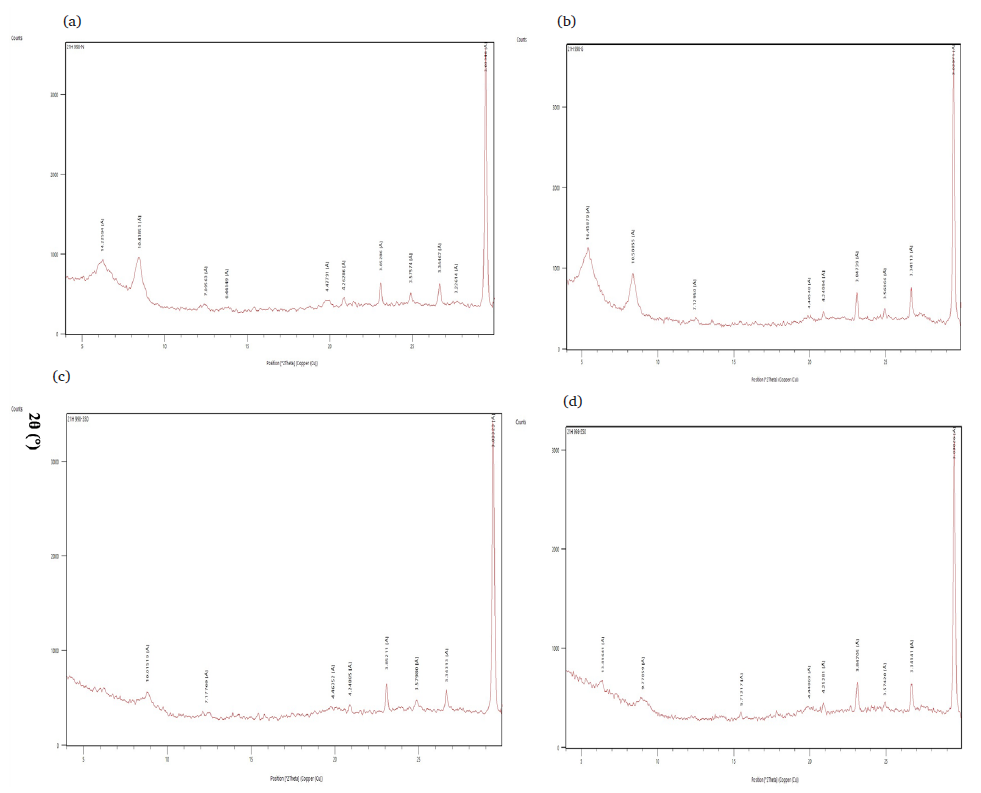
- (a) XRD diffractogram of Malatya clay in the 0-30 2? range. (b) XRD diffractogram of Malatya clay treated with ethylene glycol in the 0-30 2? range. (c) XRD diffractogram of Malatya clay heated at 350 oC in the 0- 30 2? range. (d) XRD diffractogram of Malatya clay heated at 550 oC in the 0-30 2? range.
3.3.2 SEM (scanning electron microscope) analysis
Fig. 4 shows an SEM-EDAX image of Malatya clay. Comparing the EDX results in Fig. 4 with the XRF results yields similar findings.
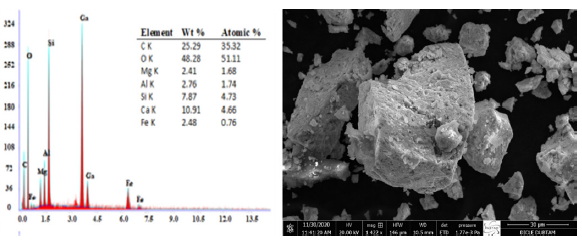
- SEM-EDAX image of Malatya clay.
3.3.3 BET (Braun-Emmet and Teller) analysis
The sample underwent vacuum treatment at 105°C for 20 hours to ascertain pore size and multipoint surface area. The BET technique was used using nitrogen gas adsorption at a temperature of 77.3 K and low pressure, resulting in a measured surface area of 60.266 m2/g.
3.3.4 XRF (X-ray diffractometer) analysis
Table 1 presents the findings of the XRF analysis performed on the Malatya clay used in the experiment. The subsequent sequence of elemental oxide percentages is shown as indicated by the XRF analysis of the clay, i.e., SiO2 > CaO > Al2O3 > Fe2O3 > MgO > K2O > TiO2 > MnO = Na2O = P2O6.
| Sample Mark |
LOI % |
Al2O3 % |
CaO % |
Fe2O3 % |
K2O % |
MgO % |
MnO % |
Na2O % |
P2O5 |
SiO2 % |
TiO2 % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Malatya Clay | 26.60 | 6.02 | 27.8 | 4.6 | 0.8 | 4.2 | 0.1 | 0.1 | 0.1 | 29.2 | 0.3 |
XRF: X-ray fluorescence.
Analysis of the data in Fig. 3 and Table 1 reveals a correlation between SEM-EDX and XRF findings, as well as between microscopic and macroscopic data, indicating mutual support.
3.4 Effect of adsorbent doses
Different amounts of adsorbents were determined, and 10 ppm Zn(II) solution was added. After the reaction was finished, the samples were read in the device and then the appropriate amount of adsorbent was determined. Other parameters were studied with the determined adsorbent amount. The amount of adsorbent influences the adsorption process; a higher dosage causes a stronger connection between the contact surface and the adsorbate, which in turn absorbs a bigger quantity of the pollutant (Sheela and Nayaka, 2012). The impact of adsorbent dose on Zn(II) adsorption has been shown in Fig. 5.
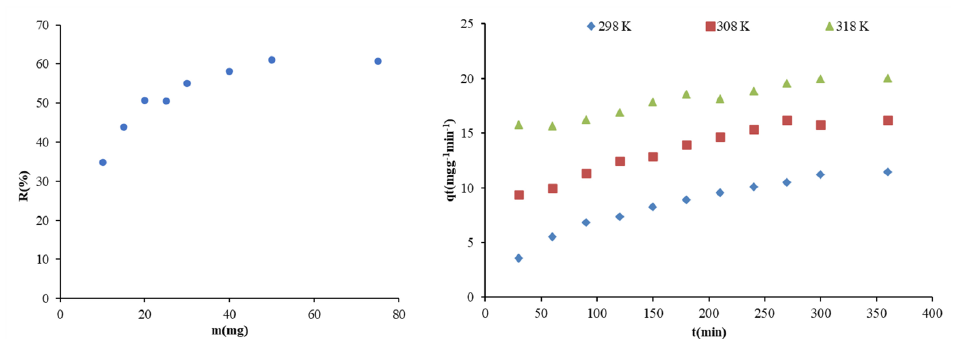
- The effect of adsorbent dose and contact time on Zn(II) adsorption.
To calculate the amount of Zn(II) adsorbed per unit, adsorption efficiency, and the weight of adsorbent (q) was applied according to Equations 4 and 5, respectively.
Where i and Ce denote the initial and post-treatment Zn (II) concentrations in the sample (mg/L), V represents the volume of solution (L), and m signifies the quantity of adsorbent (g), respectively.
3.5 Effect of contact time
The increase in adsorption over time was assessed in the study using equation 6.
Here C = concentration, k = rate constant, n = degree of the rate equation.
The influence of contact duration, varying from 0 to 360 minutes, was analyzed to assess the effect of Zn (II) ions on Malatya clay. The peak value was observed at 360 minutes (Fig. 5). The control phase and rate mechanism of Zn(II) ion adsorption on Malatya clay were investigated using numerous kinetic models. The models included the intra-particle diffusion model, pseudo-second-order model, pseudo-first-order model, and an extra model (Aydın Temel, 2018).
3.5.1 Pseudo-first order model (PFO)
Equation 7 is a prevalent representation of the pseudo-first order model.
Here, K1 is the first-order rate constant, qe is the equilibrium adsorption capacity in milligrams per gram of adsorbent, and qt is the quantity of adsorbed material at any given time in milligrams per gram of adsorbent. The value of K1 is obtained by plotting log (qe - qt) against t to determine the graph’s slope. Fig. 6(a) illustrates the plot of the first-order rate equation for Zn (II) ions on Malatya clay.
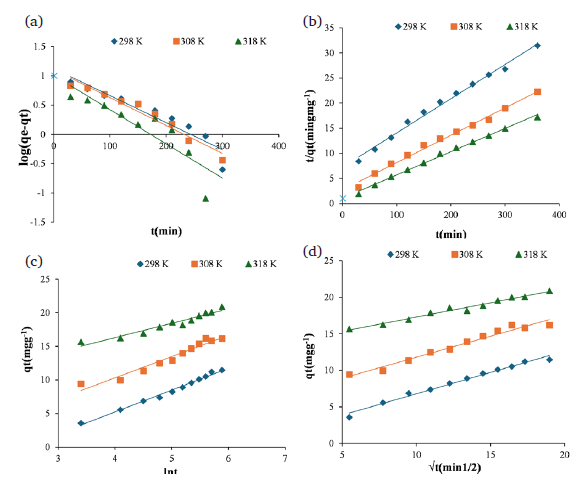
- Kinetic rate models (a) Pseudo- first-order rate plot (b) Pseudo-second-order model (c) Elovich Model and (d) Intraparkula-diffusion model.
3.5.2 Pseudo-second order model (PSO)
The equation of the kinetic rate equation (Eq. 8) of the pseudo second order model is given below.
The constant of the pseudo-second-order rate equation is represented by k2. Determining k2 and qe is simple by graphing t/qe against t. The plot of Zn (II) ion on Malatya clay, based on the pseudo-second-order rate equation, has been shown in Fig. 6(b).
3.5.3 Elovich model:
The Elovich kinetic model characterizes chemical adsorption (Baytar et al., 2018), the Elovich equation (Eq. 9) can be written as follows:
Here, qt denotes the adsorption capacity at time t (mg/g), α represents the Elovich model constant indicating the rate of chemical adsorption (mg/g min), t signifies the contact duration (min), and β refers to the activation energy for chemical adsorption as well as a constant for surface coverage (g/mg). The parameters α and β may be determined from the slopes and displacements of the lines shown in Fig. 6(c).
3.5.4 Intraparticle-diffusion model
The transfer of adsorbate to adsorbent particles is governed by the processes of film diffusion, interparticle diffusion, and adsorption. This approach may enhance the understanding of adsorption dynamics. The intra-particle diffusion model is defined as follows (Eq. 10) (Baytar et al., 2018).
The values of Kid and C are ascertained from the slope and intercept of the plot correlating t1/2 and qt, where qt represents the adsorption capacity at time t (mg/g); K denotes the rate constant of the intraparticle diffusion model (mg/g h); t signifies the contact time (h); and C is a constant (mg/g) that indicates the thickness of the boundary layer (Fig. 6d).
The results obtained from the kinetic data applied to the Pseudo-second order model, Pseudo-second order model, Intrapartikula-diffusion model and Elovich model have been given in Table 2.
|
Temperature (K) |
Pseudo first order | Pseudo second order | |||||
|---|---|---|---|---|---|---|---|
| k1 (min-1) | R2 | k2 (g/mg min) | qmax (mg/g) | R2 | |||
| 298 | 0.0085 | 0.8052 | 0.0261 | 14.66 | 0.9920 | ||
| 308 | 0.0083 | 0.8376 | 0.0800 | 18.35 | 0.9910 | ||
| 318 | 0.0076 | 0.4814 | 0.0220 | 21.60 | 0.9958 | ||
| Elovich | Weber-morris | ||||||
| A (g/g min-1) | B (g/g) | R2 |
K id (mg.g-1.min.1/2) |
C (mg/g) | R2 | ||
| 298 | 0.32 | 0.3075 | 0.9940 | 0.5838 | 0.9452 | 0.9853 | |
| 308 | 1.51 | 0.3161 | 0.9486 | 0.5756 | 6.0417 | 0.9652 | |
| 318 | 80.88 | 0.4684 | 0.9390 | 0.3923 | 13.3700 | 0.9785 | |
3.6 Concentration effect
Adsorption isotherms using equilibrium values of Zn (II) adsorption using Malatya clay have been given in Fig. 5. In addition, adsorption isotherm data were applied to Langmiur, Freundlich, Temkin, and Dubinin-Radushkevich isotherms.
Langmuir equation is as given below (Eq. 11).
Here qe is the adsorption capacity at equilibrium (mg g-1); Ce is the remaining solution concentration in equilibrium solution (mg L-1); qmax is the amount of adsorbed substance per unit clay (mg g-1); and KL is the Langmuir constant (Lmg-1).
The separation constant and parameter, RL, introduced by Weber and Chakkravarti, is used to ascertain the distinctive quality of the Langmuir isotherm.
Equation 12 is calculated as given (Veli and Alyuz, 2007).
Langmuir separation coefficient RL indicates the appropriateness of the chosen activated carbon. RL>1 is inappropriate. RL=1 indicates linearity, 0<RL<1 is appropriate, and RL=0 signifies irreversibility.
Feundlich equation has been given in equation 13.
Here; KF (mg 1-1/n) 1 1/n g-1) and n are Freundlich constants. Temkin isotherm has been given in equations 14 and 15.
Here b is the Temkin heat of adsorption constant (j.mol-1); KT, is the equilibrium bond constant (l.g-1).
Dubinin-Radushkevich (D-R) isotherm has been given in Equation 16.
In D-R equations (17 and 18); lnq(D-R), max D-R maximum adsorption capacity (mg g-1), B: D-R isotherm constant (kJ -2 mol -2); Ɛ: Polany potential; T: temperature (K); R: ideal gas constant (kJ mol -1 K -1); E is the D-R adsorption energy (kJmol-1). E indicates whether adsorption is chemical or physical (Sdiri et al., 2016).
Isotherm constants and correlation coefficients of isotherms were calculated for Zn(II) adsorption using the equations of isotherm models given in Fig. 7 and Table 3. When R2 values in Table 3 were analyzed, it was seen that the highest values (R2= 0.9977; 0.9967; 0.9975) were in the Langmuir adsorption model. In addition, the maximum adsorption capacity obtained from the adsorption of Zn(II) ions on Malatya clay was compared with other literatures and given in Table 3.
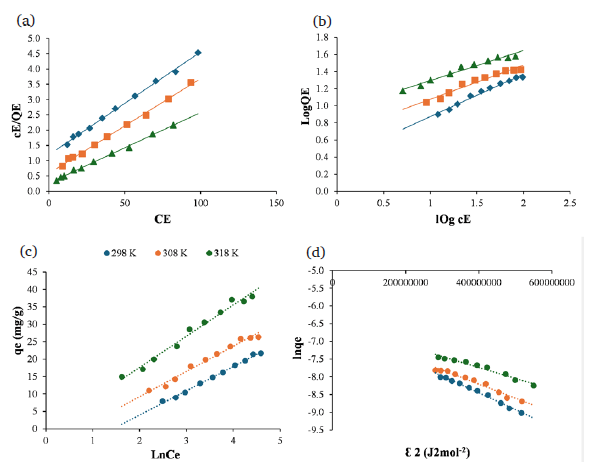
- (a) Langmuir, (b) Freundlich, (c) Temkin and (d) Dubinin- Raduskevich isotherm plots of Zn(II) adsorption of Malatya clay.
| Temperature | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| (K) |
KL (Lmg-1) |
qmax (mg/g) |
R 2 | KF | n | R2 | ||
| 298 | 0.0401 | 29.76 | 0.9975 | 2.38 | 2.01 | 0.9843 | ||
| 308 | 0.0182 | 32.26 | 0.9967 | 4.74 | 2.50 | 0.9584 | ||
| 318 | 0.0062 | 43.29 | 0.9977 | 8.79 | 2.84 | 0.9689 | ||
| Temkin | Dubinin-Raduskevich | |||||||
|
b (jmol-1) |
KT (L.g-1) |
R 2 |
q D-R, max (mg.g-1) |
E (KJmol-1) |
R2 | |||
| 298 | 284.8 | 1.029 | 0.9887 | 92.84 | 10.00 | 0.9660 | ||
| 308 | 353.5 | 0.483 | 0.9821 | 87.87 | 11.18 | 0.9821 | ||
| 318 | 380.0 | 0.239 | 0.9960 | 98.01 | 10.54 | 0.9887 | ||
| Comparison with Literature | ||||||||
| qmax (mgg-1) | Adsorbent | Literature | ||||||
| 2.495 | Bentonite (Gafsa, south of Tunisia) | Sdiri et al. (2016) | ||||||
| 4.395 | Bentonite (Bofe) | Araujo et al. (2013) | ||||||
| 5.09 | Brazilian Bentonite | De Araujo et al. (2013) | ||||||
| 8.21 | Turkish clinoptilolite | Oter and Akcay (2007) | ||||||
| 12.50 | Gray Clay | Vasconcelos et al. (2013) | ||||||
| 21.09 | Natural Bentonite | Bellir et al. (2013) | ||||||
| 68.49 | Bentonite | Sen and Gomez (2011) | ||||||
| 80.64 | Bentonite (Cankırı) | Veli and Alyüz (2007) | ||||||
| 154.60 | Montmorillonite | De Pablo et al. (2011) | ||||||
| 250.00 | Kaolin | Arias and Sen (2009) | ||||||
| 43.29 | Malatya clay | This Study | ||||||
4. Discussion
The clay used in this study is a good adsorbent compared to other clay types. It is clear from evaluating the kinetic study findings in Table 2 that the PSO model is the one that best fits the experimental results. As the temperature increased, the qe values also rose. This clearly proves that the heat field constitutes the adsorption kinetic process. The Langmuir isotherm model most accurately aligns with the experimental data, as shown by the isotherm research results presented in Table 3. The rise in qe over prolonged contact periods for the elimination of Zn (II) ions using Malatya clay suggests that diffusion mostly controls the adsorption mechanism. This result corresponds with current research, indicating that adsorption kinetics are mostly governed by surface diffusion in the first phases, ultimately attaining equilibrium when the adsorption sites get saturated (Haerifar and Azizian, 2013; Wang et al., 1992).
In this study, a new empirical model for adsorption kinetics was established based on experimental data. Accordingly, the relationship between qt and t is an exponential function and is non-linear. The relationship has been expressed in (Eq. 19).
When es is passed to the right side of the equation, a non-linear equation is obtained.
Here; qt: Instantaneous amount of adsorbed substance (mg g-1), O: A constant related to the adsorption time (dk-1), t: Adsorption time (min), and S: A constant related to the instantaneous amount of adsorbed substance (mg g-1) expresses the expression.
When the natural logarithm of both sides in Eq. OS is taken, the linear form of the model is found. The resulting expression is a first-order equation in one unknown. This can be written as in Eq. 20.
Here, qt is the dependent variable, ln(qt/t) is the independent variable, the O term represents the slope, and the S term represents the drift. Here, O is a constant related to adsorption time and S is a constant related to qt. As O increases, equilibrium is reached in a shorter time. The shift in the graph gives the S constant. High S allows us to reach high qt values even at low concentrations. The Langmuir adsorption model had the highest R2 values (0.9977; 0.9967; 0.9975) (Table 3). This result demonstrates correspondence with the Langmuir adsorption isotherm. The monolayer adsorption capabilities (qmax) were found to be 32.26, 29.76, and 43.29 mg/g, according to the Langmuir isotherm. This indicates that adsorption is of a chemical origin since the adsorption energy varies from 8 to 16 kJ/mol and increases with rising temperature.
The results concerning the ideal adsorption of Zn (II) ions at about Ph:6 underscore the intricate relationship between the chemistry of zinc ions and the properties of Malatya clay. The pH level reflects the amphoteric characteristics of Zn ions, whose behavior is affected by the acidity or basicity of the surrounding solution (Cholico-González et al., 2020; Mustapha et al., 2019). In conclusion, sustaining a solution pH around 6 is essential for optimizing Zn (II) ion removal using Malatya clay, since both acidic and alkaline conditions might impede efficient adsorption due to competitive interactions or changes in ion speciation. Comprehending these dynamics is crucial for enhancing water treatment systems focused on heavy metal elimination.
5. Conclusions
This study indicates that Malatya clay may efficiently and cost-effectively eliminate Zn(II) ions. The dominance of the Langmuir isotherm indicates a robust adsorption process under optimal conditions (pH ≈ 6, contact length = 360 minutes). These findings support the use of Malatya clay in wastewater treatment and align with existing research on clay-based adsorbents. Investigation into its reusability and efficacy in systems, including several metals may be justified.
CRediT authorship contribution statement
N. Onursal: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Adsorption studies of zinc(II) on magnetite, baobab (Adansonia digitata) and magnetite–baobab composite. Appl. Water Sci.. 2018;8 https://doi.org/10.1007/s13201-018-0867-7
- [Google Scholar]
- İnsan Sağlığı ve Beslenme Fizyolojisi Açısından Çinkonun Önemi. Akademik Gıda. 2016;14
- [PubMed] [Google Scholar]
- Sulu Çözeltilerden Pb2+ İyonlarının uzaklaştırılmasında atık portakal kabuklarının kullanılması: Kinetik ve termodinamik Çalışmalar. Düzce Üniversitesi Bilim ve Teknoloji Dergisi. 2023;11:1105-1120. https://doi.org/10.29130/dubited.1089013
- [PubMed] [Google Scholar]
- Zinc adsorption in bentonite clay: Influence of pH and initial concentration. Acta Sci. Technol.. 2013;35 https://doi.org/10.4025/actascitechnol.v35i2.13364
- [CrossRef] [Google Scholar]
- Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay mineral: A kinetic and equilibrium study. Colloids Surf. A: Physicochem. Eng. Asp.. 2009;348:100-108. https://doi.org/10.1016/j.colsurfa.2009.06.036
- [Google Scholar]
- Endüstriyel sızıntı suyundan pb(II) giderimi İçin genleştirilmiş perlit kullanımı: Kinetik Çalışmalar. Turkish JAF Sci. Tech.. 2018;6:360. https://doi.org/10.24925/turjaf.v6i3.360-364.1748
- [Google Scholar]
- Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol.. 2021;12:643972. https://doi.org/10.3389/fphar.2021.643972
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- İğde Çekirdeğinden elde edilen aktif karbon kullanılarak sulu Çözeltilerden pb(II) adsorpsiyonun İncelenmesi: İzoterm ve kinetic. Bitlis Eren Üniversitesi Fen Bilimleri Dergisi. 2018;7:256-267. https://doi.org/10.17798/bitlisfen.422446
- [PubMed] [Google Scholar]
- Zinc removal from aqueous solutions by adsorption onto bentonite. Desal. Water Treat.. 2013;51:5035-5048. https://doi.org/10.1080/19443994.2013.808786
- [CrossRef] [Google Scholar]
- Adsorption of lead, copper and zinc in a multi-metal aqueous solution by waste rubber tires for the design of single batch adsorber. Heliyon. 2021;7:e08254. https://doi.org/10.1016/j.heliyon.2021.e08254
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adsorption behavior of pb(II), cd(II), and zn(II) onto agave bagasse, characterization, and mechanism. ACS Omega. 2020;5:3302-3314. https://doi.org/10.1021/acsomega.9b03385
- [CrossRef] [PubMed] [Google Scholar]
- Diyarbakır karacadağ kırmızı tepe skoryası ile cu(II) adsorpsiyon kinetiğinin incelenmesi. DÜMF Mühendislik Dergisi 2021 https://doi.org/10.24012/dumf.881650
- [PubMed] [Google Scholar]
- A kinetic and equilibrium study of zinc removal by Brazilian bentonite clay. Mat. Res.. 2013;16:128-136. https://doi.org/10.1590/s1516-14392012005000148
- [Google Scholar]
- Adsorption of heavy metals in acid to alkaline environments by montmorillonite and ca-montmorillonite. Chem. Eng. J.. 2011;171:1276-1286. https://doi.org/10.1016/j.cej.2011.05.055
- [Google Scholar]
- Mixed surface reaction and diffusion-controlled kinetic model for adsorption at the solid/Solution interface. J. Phys. Chem. C. 2013;117:8310-8317. https://doi.org/10.1021/jp401571m
- [CrossRef] [Google Scholar]
- Selenyumun insan sağlığı üzerine etkileri ve diyabetes mellitusla ilişkisi. Bozok Tıp Derg. 2017;7
- [PubMed] [Google Scholar]
- Mechanism of interaction and removal of zinc with lignocellulosic adsorbents, closing the cycle with a soil conditioner. J. King Saud Univ. Sci.. 2021;33:101607. https://doi.org/10.1016/j.jksus.2021.101607
- [CrossRef] [Google Scholar]
- Ağaçlı-Bolluca (İstanbul) yöresi seramik killerinin malzeme özelliklerinin araştırılması (Masters). Istanbul: Istanbul Technical University; 2007.
- FTIR techniques in clay mineral studies. Vib. Spectrosc.. 2003;31:1-10. https://doi.org/10.1016/s0924-2031(02)00065-6
- [Google Scholar]
- Adsorption isotherm, kinetic and thermodynamic studies for the removal of pb(II), cd(II), zn(II) and cu(II) ions from aqueous solutions using albizia lebbeck pods. Appl. Water Sci.. 2019;9 https://doi.org/10.1007/s13201-019-1021-x
- [CrossRef] [Google Scholar]
- Adsorption of zinc and copper (II) ions from aqueous solution using modified nano bentonite: Equilibrium, kinetics, and thermodynamic studies. Sep. Sci. Technol.. 2021;56:2708-2720. https://doi.org/10.1080/01496395.2020.1846055
- [CrossRef] [Google Scholar]
- Adsorption of Zn (II) from aqueous solution by using chitin extraction from crustaceous shell. J. Adv. Environ. Health Res.. 2014;2:110-119. https://doi.org/10.22102/jaehr.2014.40151
- [Google Scholar]
- Use of natural clinoptilolite to improve water quality: Sorption and selectivity studies of lead(II), copper(II), zinc(II), and nickel(II) Water Environ. Res.. 2007;79:329-335. https://doi.org/10.2175/106143006x111880
- [CrossRef] [PubMed] [Google Scholar]
- A natural clayey adsorbent for selective removal of lead from aqueous solutions. Appl. Clay Sci.. 2016;126:89-97. https://doi.org/10.1016/j.clay.2016.03.003
- [CrossRef] [Google Scholar]
- Adsorption of zinc (Zn2+) from aqueous solution on natural bentonite. Desalination. 2011;267:286-294. https://doi.org/10.1016/j.desal.2010.09.041
- [Google Scholar]
- Kinetics and thermodynamics of cadmium and lead ions adsorption on niO nanoparticles. Chem. Eng. J.. 2012;191:123-131. https://doi.org/10.1016/j.cej.2012.02.080
- [CrossRef] [Google Scholar]
- Removal of paracetamol from aqueous solution with zinc oxide nanoparticles obtained by green synthesis from purple basil (Ocimum basilicum l.) waste. Biomass Conv. Bioref.. 2024;14:10771-10789. https://doi.org/10.1007/s13399-024-05355-1
- [CrossRef] [Google Scholar]
- Heavy metal toxicity and the environment. Exp. Suppl.. 2012;101:133-164. https://doi.org/10.1007/978-3-7643-8340-4_6
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adsorption of zinc from aqueous solutions using modified Brazilian gray clay. AJAC. 2013;04:510-519. https://doi.org/10.4236/ajac.2013.49065
- [Google Scholar]
- Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater.. 2007;149:226-233. https://doi.org/10.1016/j.jhazmat.2007.04.109
- [CrossRef] [PubMed] [Google Scholar]
- Zinc in Drinking Water. J. Am. Chem. Soc.. 1884;6:214-216. https://doi.org/10.1021/ja02131a016
- [CrossRef] [Google Scholar]
- Reaction rate enhancement by surface diffusion of adsorbates. Biophys. Chem.. 1992;43:117-137. https://doi.org/10.1016/0301-4622(92)80027-3
- [CrossRef] [PubMed] [Google Scholar]







