Translate this page into:
Apoptotic genes expression in mice hepatocytes during malaria infection
*Address: Department of Biology, Teachers college, King Saud University, P.O. Box 271942, Riyadh 11352, Saudi Arabia. Tel.: +966 505233673 salkahtani@ksu.edu.sa (Saad Alkahtani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 17 June 2010
Abstract
Malaria represents a major global health problem by causing multi-cellular dysfunctions in the infected host. The aim of the present study was to investigate the apoptotic gene expression in liver of mice during malaria disease by mRNA expression of three genes involved in apoptosis; Bax, Bcl-2 and Caspase-3 and other parameters at different time points after infection with Plasmodium chabaudi in the liver cells of female C57BL/6 mice. Mice were injected intraperitoneally (ip) with 106 P. chabaudi-infected erythrocytes and then scarified at days (0, 1, 4 and 8, respectively). Quantitative real-time PCR and immunoblotting were used to quantify apoptotic genes and protein kinetic, respectively. The levels of Bax, Bcl-2 and Caspase-3 were significantly (P < 0.05) increased only at days 1 and 8 compared with day 0 in both the liver cells and protein. The levels of parasitemia was significantly (P < 0.01) increased at day 8 compared with day 0 in the blood. These results have shown that P. chabaudi induced apoptosis in the liver cells. Thus, this study suggests that the induced apoptotic gene expression was due to the out come of malaria.
Keywords
Apoptosis
Liver
Plasmodium chabaudi
Mice
1 Introduction
Despite decades of intense research, malaria remains a major global health problem with an estimated mortality about 2 millions every year (Greenwood et al., 2005; World Malaria Report, 2005; Bergmann-Leitner et al., 2009). The few available anti-malaria drugs are becoming increasingly ineffective due to increasing the resistance of parasites (Carlton et al., 2001). All efforts to develop an effective anti-malaria vaccine have failed to date (Hviid and Barfold, 2008). Basic research is therefore – more than ever – urgently required to understand both protective host mechanisms and pathological complications induced by malaria (Callaway, 2007). A rather convenient malaria model system is the Plasmodium chabaudi blood stage infection in mice, since P. chabaudi shares several characteristics with P. falciparum, the most dangerous human malaria species (Hernandez-Valladares et al., 2005).
The liver plays a central role in malaria: It is not only the site of preerythrocytic development of Plasmodium parasites, but also acts as an effector against malarial blood stages as recently shown (Wunderlich et al., 2005; Krücken et al., 2005a). In particular, the Kupffer cells, which represent about 90% of all host macrophages, are able to eliminate parasite-derived hemozoin and even Plasmodium-infected erythrocytes (Krücken et al., 2005b; Guha et al., 2006a). On the other hand, the spleen is believed to participate in cleaning parasites from the circulation and providing a strong hematopoietic response during acute infections (Dkhil, 2009).
Apoptosis, programmed cell death, is an active form of cell death which is linked intimately with both physiology as well as pathology in variety of cellular systems (Gourley et al., 2002; Alkahtani et al., 2009). The dysregulation of liver apoptosis during malaria is a critical event in liver pathology. Apoptosis does not only play an essential role in development and tissue homeostasis but is also involved in a wide range of pathological conditions. Reactive oxygen species (ROS) have been involved in the apoptosis induced by different stimuli as well as the pathologic cell death that occurs in many diseases (Oh et al., 2004).
Apoptosis may occur via a death receptor-dependent (extrinsic) or independent (intrinsic or mitochondrial) pathway. The death receptor pathway comprises Fas (CD95/Apo-1) and TRAIL (Apo-2) and this pathway is activated when ligands specific for either Fas or TRAIL bind to their respective receptors, resulting in activation of Caspase-8, which finally activates Caspace-3 (Lyke et al., 2004). On the other hands mitochondrial pathway of apoptosis is initiated by the down-regulation of anti-apoptotic proteins (such as, Bcl-2, Bcl-xl) and/or up-regulation of pro-apoptotic proteins (such as, Bax, Bad, Bid), resulting in the opening of mitochondrial permeability transition pores and release of apoptosis inducing proteins (cytochrome c, apoptosis inducing factor, etc.) from mitochondria (Akanmori et al., 1996; Barton, 1996; Gourley et al., 2002; Alkahtani et al., 2009).
Oxidative stress is known to induce apoptosis in many cellular systems, including the liver but apoptosis in liver during malaria infection has been overlooked. However, induced-malaria apoptosis in other cellular systems has been studied in mice (Roberts et al., 2001; Wunderlich et al., 2005), monkeys (Jones et al., 1999), and human (Picot et al., 1997). The present study was undertaken to investigate apoptotic molecules in mice liver to elucidate the apoptosis pathway mechanisms during malaria infection.
2 Material and methods
2.1 Animals and infection
Normal female C57BL/6 mice 10–14 weeks old were obtained from the central animal facilities of Heinrich Heine University, Düsseldorf, Germany, and housed in plastic cages. Mice were bred and maintained under specified pathogen free conditions. Mice were fed with standard diet (Wohrlin, Bad Salzuflen, Germany) and water ad libitum. The experiments were approved by the State authorities and followed German law on animal protection.
2.2 Blood stage malaria
We used a non-clonal line of P. chabaudi (Wunderlich et al., 1988) exhibiting a very similar, but not identical restriction length polymorphism pattern to P. chabaudi chabaudi (Krücken et al., 2005b). Erythrocytic stages of P. chabaudi were passaged weekly in NMRI mice. From these mice, blood was taken and 106 P. chabaudi-infected erythrocytes were injected i.p. in the mice. Parasitemia was evaluated in Giemsa-stained blood smears. The total number of erythrocytes was determined in a Neubauer chamber. A total of five mice were scarified at each day (0, 1, 4 and 8, respectively) by cervical dislocation. Livers were removed and cut into smaller pieces and kept at −80 °C.
2.3 RNA-isolation
Approximately 250 mg frozen liver was homogenized with an ultraturrax in 5 ml Trizol (Peqlab Biotechnology, Erlangen, Germany) for one minute. After mixing with 1 ml chloroform for 15 s, the suspension was incubated for 15 min at room temperature and centrifuged at 3000g for 45 min. After isopropanol precipitation of the supernatant, the pellet was washed twice with 80% ethanol and air-dried and dissolved in 200 μl RNase-free water. RNA concentrations were determined at 260 nm, and the purity of RNA was checked in 1% agarose gel.
2.4 Quantitative real-time PCR
All RNA samples were treated with DNase (Applied Biosystems, Darmstadt, Germany) for at least 1 h and then converted into cDNA following the manufacturer’s protocol using the Reverse Transcription Kit (Qiagen, Hilden, Germany). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the ABI Prism® 7500HT Sequence Detection System (Applied Biosystems, Darmstadt, Germany) with SYBR Green PCR Mastermix from Qiagen (Hilden, Germany). We investigated the genes encoding the mRNA for following proteins: 18S; Bax; Bcl-2; Caspase-3. All primers used for qRT-PCR were commercially obtained from Qiagen. PCR reactions were conducted as follows: 2 min at 50 °C to activate uracil-N-gylcosylase, 95 °C for 10 min to deactivate UNG, 40 cycles at 94 °C for 15 s, at 60 °C for 35 s and at 72 °C for 30 s. Reaction specificity was checked by performing dissociation curves after PCR. For quantification, mRNA was normalized to 18S rRNA. The threshold Ct value is the cycle number selected from the logarithmic phase of the PCR curve in which an increase in fluorescence can be detected above background. The ΔCt is determined by subtracting the Ct of 18S rRNA from the Ct of the target (ΔCt = Ct-target − Ct-18S rRNA). The relative mRNA levels of non-infected mice are described as a ratio of target mRNA copy to 18S rRNA copy = . The -fold induction of mRNA expression on days (0, 1, 4 and 8 p.i. was determined using the -method (−ΔΔCt = ΔCt day 0 p.i. − ΔCt day 8 p.i.).
2.5 Protein extraction
Approximately 10 mg of mice liver was homogenized in a cold homogenizer tube containing 2 ml of homogenization buffer. Homogenates were spun at 300 rpm for 10 min. The supernatants were removed and stored at −80 °C. The concentration of total protein in each sample was estimated spectrophotometrically (GeneQuant pro, Amersham, USA) at 595 nm. Equal volumes of 2× sample buffer and protein (30 μg/μl) were mixed in an Eppendorf tube and heated to 95 °C for 5 min before loading (Hossain et al., 2000; Mathas et al., 2003).
2.6 SDS–PAGE and immunoblotting
2.6.1 Antibodies
2.6.1.1 Primary antibodies anti-(Bax; BCL-2; Caspase-3 and GABDH)
Primary antibodies were obtained from Cell Signalling, USA and used to detect the Bax; Bcl-2 and Caspase-3 proteins and GABDH protein as a reference. Polyclonal antibodies were produced by immunizing rabbits and diluted with 5% skimmed milk in PBS containing 0.1% Tween 20 (1:1000).
2.6.1.2 Secondary antibodies (anti-rabbit IgG) HRP-linked antibodies
Secondary antibodies were obtained from Cell Signalling, USA. Antibodies were labeled with peroxidase and assayed using enhanced chemiluminescence (ECL) western blotting detection reagents obtained from Amersham, RPN2106PC, USA.
The mix of proteins and 2× sample buffer were separated on 30% polyacrylamide gel using a PowerPac Basic system (S.N 37S/7159, Italy) at 50 V for 1 h and then at 100 V near the end of the electrophoresis. Proteins were then blotted on nitrocellulose membrane. The nitrocellulose membrane was washed several times with phosphate buffered saline (PBS) and then incubated in 5% skimmed milk in PBS containing 0.1% Tween 20 to blocking of non specific sites. Membranes were incubated with primary antibodies overnight at 4 °C, and then with secondary antibodies for 3 h. Membranes were then washed three times (5 min each) in PBS-T, all steps under mild agitation. The detection of the reacted antigen/antibody products was performed using enhanced chemiluminescence Western Blotting Detection Reagents according to the manufacturer’s instructions (Amersham) (Oh et al., 2004).
2.7 Statistical analysis
Two-tailed Student’s t-test and Fisher’s exact test were used for statistical analysis.
3 Results
3.1 Characteristics of P. chabaudi infection
Infected mice with 106 P. chabaudi-infected erythrocytes became evident on day 4 and increased to reach its peak (48%) on day 8 and then plummeted rapidly to about 0.4% on day 12 (Fig. 1).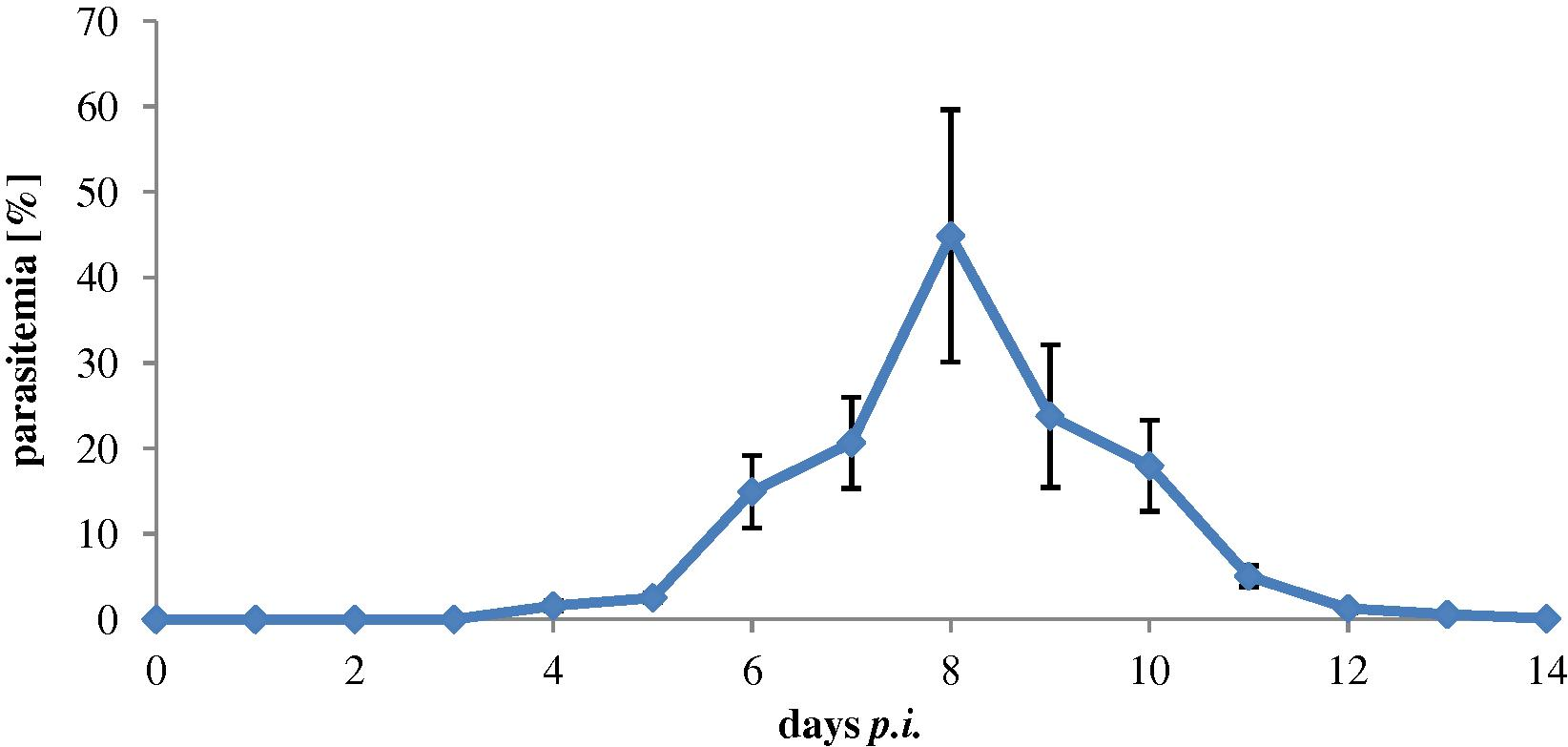
Parasitemia (48%) of female C57BL/6 mice (n = 20) infected with 106 P. chabaudi-infected erythrocytes.
3.2 Apoptotic genes expression in liver
Quantitative real-time PCR and Western blot analysis were used to detect changes in mRNA levels of different apoptotic genes in the liver. The level of mRNA expression for Bax as a pro-apoptotic gene and Caspase-3 as an executioner gene were significantly (P < 0.05) increased at days (1 and 8) after infection compared with day 0 (Fig. 2). The level of mRNA expression for Bcl-2 as anti-apoptotic gene was significantly (P < 0.05) and dramatically increased at all three time points after infection compared with day 0 (Fig. 2). Representative Western blots of Bax; Bcl-2 and Caspase-3 proteins in liver are shown in (Fig. 3). The results show a decreased Bax protein on days 1 and 4 compared with day 0 but the increment was clear on day 8 compared with days 1 and 4 indicating increased level of Bax protein in the liver during malaria infection. The levels of Bcl-2 and Caspase-3 were increased at all days after infection compared with day 0. The GABDH protein was used as a control.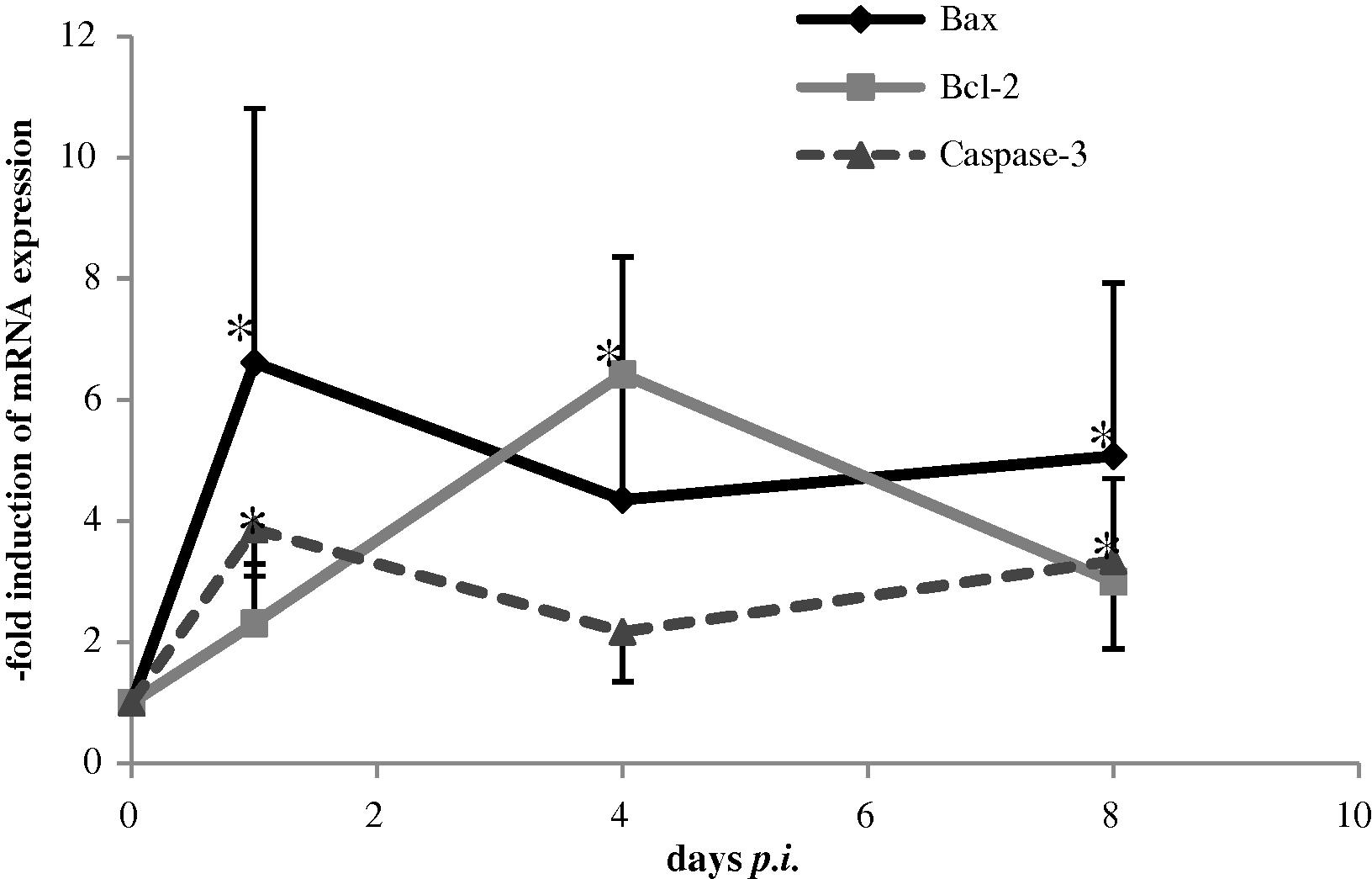
RT-PCR of Bax, Bcl-2 and Caspase-3 genes expression in liver of Plasmodium chabaudi infected mice. The expression of genes were measured at different times. The data present are the mean ± SE (n = 5). *Significant value at (P < 0.05).
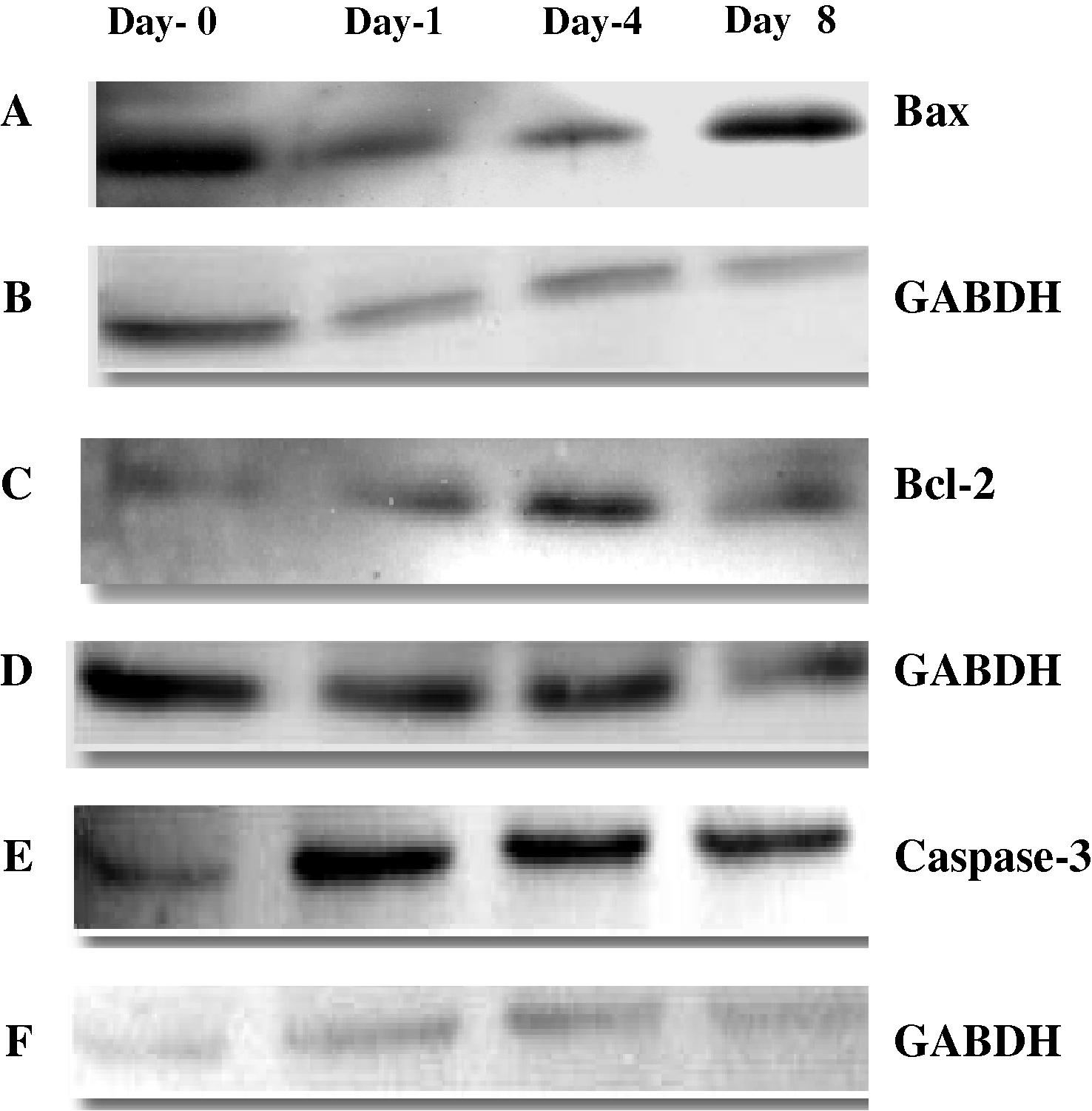
Western blot analysis of (A); Bax (C); Bcl-2; (E); Caspase-3 and (B, D and F); GABDH obtained from cytosol of control (day-0) and infected mice at days (1, 4 and 8). Equal amount of protein (30 μg/lane) was electrophoresed by SDS–PAGE. Protein was transferred onto nitrocellulose membrane and probed with primary antibodies as described in the text. The immunoplot is a representative of three independent experiments with similar results.
4 Discussion
This study demonstrates that the infected mice with P. chabaudi induced apoptotic genes expression by assessing mRNA expression of three genes involved in apoptosis Bax, Bcl-2 and Caspase-3 at different time points. Apoptosis is a complex cellular process whereby cells commit “suicide” in response to a wide variety of stimuli (Bergmann-Leitner et al., 2009). Although apoptosis was originally thought to be entirely distinct mechanisms of cell death, several works have shown that the processes are regulated by many of the same biochemical intermediates, including death factors and reactive oxygen species (Sanchez-Alonso et al., 2004). The data presented here connect with previous results that malaria infection significantly induces liver apoptosis mediated by oxidative stress mechanisms (Guha et al., 2007). The pathogen of malaria, Plasmodium, enters erythrocytes and thus escapes recognition by the immune system and then induces oxidative stress to the host erythrocytes and triggers erythrotosis which trigger activation of caspases (Alkahtani, 2009; Föller et al., 2009).
Generally, ROS generation and cytotoxins can increase in many pathological situations and cause cell death, often in a dose-dependent manner high dosages of the toxicant usually result in necrosis leading to loss of ion homeostasis, and secondly to the inability to maintain mitochondrial respiration and ATP levels essential for cellular survival (Alkahtani, 2009). Cell apoptosis is regulated via two major pathways: the intrinsic or mitochondrial pathway and extrinsic or death receptor pathway. Both pathways converge at the level of active effector caspases which cleave various cellular target proteins and then leading to apoptosis. In the liver, the apoptosis could result from a combination of both pathways: the intrinsic apoptosis pathway by generation of oxidative stress and the extrinsic apoptosis pathway by activation of Kupffer cells which can secrete TNF (Alkahtani, 2009). The mitochondrial apoptotic pathway plays a critical role in liver cell death during malaria infection (Guha et al., 2007). The intrinsic apoptotic pathway is initiated when Bcl-2 homology 3 (BH3) domain proteins (the called-so BH3-only proteins) sense cellular stress or developmental cues and lead to the release of cytochrome c and other pro-apoptotic cofactors from the inter-membrane space of mitochondria which is consider to be an essential cofactor required for the activation of caspases and is regulated by the Bcl-2 family of proteins upstream of caspase activation (Baliga and Kumar, 2007). Once in the cytosol, cytochrome c interacts with the apoptotic protease activating factor-1 (Apaf-1) and the procaspase-9 forming the apoptosome complex (Yuan and Yankner, 2000). The result is the cleavage and activation of procaspase-9 and other procaspases that are responsible for the executive stages of apoptotic cell death. The Bcl-2 family of proteins localize at membrane compartments during apoptosis and can either promote or inhibit apoptosis. Briefly, Bcl-2 is an apoptosis suppressing factor that heterodimerizes with Bax and neutralizes the effects of the latter. When Bcl-2 is present in excess, cells are protected against apoptosis. In contrast, when Bax is in excess and the homodimers of Bax dominate, cells are susceptible to programmed death. Therefore, it is the ratio of Bax to Bcl-2 which determines the fate of a cell (Sanchez-Alonso et al., 2004). Infections with intracellular pathogens may provide an appropriate stress-related signal which would normally trigger the intrinsic pathway of apoptosis and, thereby, disturb or even prevent the further development of the affected host cells as a response to intracellular pathogens (Graumann et al., 2009). The results also, demonstrated that the protein levels of Bax as pro-apoptotic molecules decreased after infection compared with control, indicating that an apoptotic cell can eventually undergo secondary necrosis and rupture its contents into the surrounding medium because of the lack of scavenging cells, and thus the phagocytic step after apoptosis may not occur (Sanchez-Alonso et al., 2004). In addition, Pro-(Bax, Bid, Bad) and anti-apoptotic Bcl-2 members (Bcl-2, Bcl-XL) regulate the mitochondrial pathway. Since the anti-apoptotic Bcl-2/Bcl-XL proteins are localized to the outer mitochondrial membrane, they work to prevent Cyt c release from mitochondria. Recently, it has reported been that the p18/Bax fragment cleaved from full-length Bax (21-kDa) is as efficient as full-length Bax in promoting Cyt c release (Oh et al., 2004).
Due to their critical functions during apoptosis, Bcl-2 proteins are deregulated by several protozoa, which manipulate host cell apoptosis. Infections: In addition, experimental silencing of anti-apoptotic Bcl-2 and Mcl-1 decreases the survival of C. parvum-infected or T. parva-infected cells, respectively. It confirms that Bcl-2 family members indeed fullfil anti-apoptotic functions in cells infected by these intracellular protozoa. It is important to note, however, that this does not necessarily warrant the conclusion that the parasite-induced up-regulation of anti-apoptotic Bcl-2 proteins is instrumental in protecting the infected host cells from apoptosis. Up-regulation of Bcl-2 proteins following infection can be achieved indirectly via parasite-induced secretion of host growth factors that support the survival of infected cells or both infected and also non-infected bystander cells. Alternatively, distinct protozoa may also directly interfere with those signalling cascades that regulate expression of anti-apoptotic Bcl-2 family proteins. Previous studies on Bax mRNA expression levels did show a higher expression in in vitro bovine embryos in comparison with co-cultured or in vivo cultured embryos. Furthermore, gene expression analysis using RT-PCR indicates the significant up-regulation of Bax expression in liver of malaria infected mice suggesting the involvement of mitochondrial pathway of apoptosis (Guha et al., 2006b). However, besides the fact that different apoptotic markers cannot always be detected at the same time, very little is known on the exact timing of the successive steps in the apoptotic pathways and for this reason it is suitable to confirm this at the protein level. This is in agreement with the opinion of more and more researchers arguing that the detection of mRNA differences can only indicate a biological significance if protein expression and activity can confirm the results; this is certainly true for caspases because they are secreted as inactive procaspases, which are only active after further modification (Huert et al., 2007; Vandaele et al., 2008).
As observed in this study overexpression of apoptotic genes leads to programmed cell death. Up-regulation of Bax and/or down-regulation of Bcl-2 mRNA or protein levels has been observed in several experimental models including transient global ischemia, indicating that the expression of Bax and Bcl-2 genes may be regulated by p53 (Mishra et al., 2006).
In conclusion, under our experimental conditions, malaria infection with P. chabaudi induces apoptosis in the liver cells via Bax, Bcl-2 and Caspase-3 tests. In contrast, we have to bear in mind that apoptosis detection tests may reveals different results because some of specific targets are affected by different factors like caspases enzymes while other targets are not affected.
References
- Recombinant mouse IL-6 boosts specific serum anti-plasmodial IgG subtype titres and suppresses parasitaemia in Plasmodium chabaudi chabaudi infection. Parasite Immunol.. 1996;18:193-199.
- [Google Scholar]
- Antioxidation and hypomethylation effect on genotoxicity and programmed cell death induced in mice somatic cells by arsenic trioxide. J. Biol. Sci.. 2009;9(7):721-729.
- [Google Scholar]
- Detection of apoptosis induced by gentamicin in rat hepatocytes. Int. J. Zool. Res.. 2009;5(4):161-170.
- [Google Scholar]
- Molecular adjuvants for malaria DNA vaccines based on the modulation of host-cell apoptosis. Vaccine. 2009;27(41):5700-5708.
- [Google Scholar]
- Mice and malaria mutants: unravelling the genetics of drug resistance using rodent malaria models. Trends Parasitol.. 2001;17:236-242.
- [Google Scholar]
- Apoptosis changes induced in mice splenic tissue due to malaria infection. J. Microbiol. Immunol. Infect.. 2009;42:13-18.
- [Google Scholar]
- Suicide for survival-death of infected erythrocytes as a host mechanism to survive malaria. Cell Physiol. Biochem.. 2009;24(3–4):133-140.
- [Google Scholar]
- Profound bias in interferon-gamma and interleukin-6 allele frequencies in western Kenya, where severe malarial anemia is common in children. J. Infect. Dis.. 2002;186:1007-1012.
- [Google Scholar]
- Mammalian apoptotic signalling pathways: multiple targets of protozoan parasites to activate or deactivate host cell death. J. Micinf.. 2009;11(13):1079-1087.
- [Google Scholar]
- Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. FASEB J.. 2006;20:E439-E449.
- [Google Scholar]
- Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. J. FASEB. 2006;20(8):1224-1226.
- [Google Scholar]
- Melatonin inhibits free radical-mediated mitochondria-dependent hepatocyte apoptosis and liver damage induced during malaria infection. J. Pineal. Res.. 2007;43(4):372-381.
- [Google Scholar]
- Arsenite induces apoptosis of murine T lymphocytes through membrane raft-linked signaling for activation of c-Jun amino-terminal kinase. J. Immunol.. 2000;165:4290-4297.
- [Google Scholar]
- Modification of gene products involved in resistance to apoptosis in metastatic colon cancer cells: roles of Fas, Apaf-1, NFκB, IAPs, Smac/DIABLO, and AIF. J. Surg. Res.. 2007;142:184-194.
- [Google Scholar]
- Malaria vaccines: immunity, models and monoclonal antibodies. Trends Parasitol.. 2008;24:392-395.
- [Google Scholar]
- Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in Aotus monkeys. Vaccine. 1999;17:23-24.
- [Google Scholar]
- Testosterone suppresses protective responses of the liver to blood-stage malaria. Infect. Immun.. 2005;73:436-443.
- [Google Scholar]
- Massive destruction of malaria-parasitized red blood cells despite spleen closure. Infect. Immun.. 2005;73:6390-6398.
- [Google Scholar]
- Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL- 8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun.. 2004;72:5630-5637.
- [Google Scholar]
- Inhibition of NF-kB essentially contributes to arsenic-induced apoptosis. Am. Soc. Hematol.. 2003;102(3):1028-1034.
- [Google Scholar]
- Hypoxia-induced Bax and Bcl-2 protein expression, caspase-9 activation, DNA fragmentation, and lipid peroxidation in mitochondria of the cerebral cortex of newborn piglets: the role of nitric oxide. J. Neurosci.. 2006;141(3):13339-13349.
- [Google Scholar]
- Cadmium induces apoptotic cell death in WI 38 cells via caspase-dependent Bid cleavage and calpain-mediated mitochondrial Bax cleavage by Bcl-2-independent pathway. J. BCP. 2004;68(9):1845-1855.
- [Google Scholar]
- Apoptosis related to chloroquine sensitivity of the human malaria parasite Plasmodium falciparum. Trans. Royal Soc. Trop. Med. Hygiene. 1997;91(5):590-591.
- [Google Scholar]
- Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev.. 2001;14:476-488.
- [Google Scholar]
- Polychlorinated biphenyl mixtures (Aroclors) induce apoptosis via Bcl-2, Bax and caspase-3 protiens in neuronal cell cultures. J. Toxlet. 2004;153(3):311-326.
- [Google Scholar]
- MRNA expression of Bcl-2, Bax, caspase-3 and -7 cannot be used as a marker for apoptosis in bovine blastocysts. J. Anireprosci.. 2008;106(1–2):168-173.
- [Google Scholar]
- World Malaria Report 2005 of the World Health Organization (WHO). <http://rbm.who.int/wmr2005/html/1-1.htm>.
- Testosterone-responsiveness of spleen and liver in female lymphotoxin β receptor-deficient mice resistant to blood stage malaria. Microbes Infect.. 2005;7:399-409.
- [Google Scholar]
- Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect. Immun.. 1988;56(9):2400-2406.
- [Google Scholar]







