Translate this page into:
Aphicidal activity of five plant extracts applied singly or in combination with entomopathogenic bacteria, Xenorhabdus budapestensis against rose aphid, Macrosiphum rosae (Hemiptera: Aphididae)
⁎Corresponding authors. a.noureldeen@tu.edu.sa (Ahmed Noureldeen), uttam5454@gmail.com (Uttam Kumar),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The rose aphid Macrosiphum rosae (L.) is now a globally spread insect species damages rose plants affecting quality and productivity. Botanical insecticides are excellent alternative to synthetic pesticides, as they have minimal environmental persistence and toxicity, and are more compatible with the biocontrol agents than synthetic pesticides. This study aimed to evaluate extracts of five plant species i.e. Citrullus colocynthis, Tagetes erecta, Rosmarinus officinalis, Thymus vulgaris, and Withania somnifera; and entomopathogenic bacteria (EPB), Xenorhabdus budapestensis against M. rosae, as individual and concomitant treatments to determine their compatibility under laboratory conditions. Results indicated that the five plants extracts and EPB applied individually had immense contact or residual toxicity against M. rosae. Methanol extract of T. erecta significantly proved to be more effective as aphicide than ethanol and acetone extracts of five tested plants. Similarly, the results also show a direct, significant relationship between the mortality rates and both EPB cell suspension concentration and exposure time when applied individually. Moreover, three days after treatment, the combination of EPB and each plant extract resulted in a significantly higher M. rosae mortality than the EPB or plant extract alone. We conclude that five plants extracts especially T. erecta had compatible capacity with EPB, thus it could be used in integrated aphid management programs.
Keywords
Botanicals
Entomopathogenic bacteria
Rose aphid
Macrosiphum rosae
Bioassay
Compatibility
Secondary metabolites
1 Introduction
Rose is known as the “Queen of Flowers” over the world (Datta, 1997). In the floriculture industry, the rose is the most significant crop. Roses are used as cut flowers, potted plants, and garden plants, with an annual value of $10 billion. Their petals are also used as a source of natural scents and flavorings, which contributes to their economic value. Furthermore, cut rose flower is regarded as one of the best cash crop ornamental flowers. The damask rose (Rosa damascene) is the most important species used to produce rose water, attar of rose, concrete, and essential oils; all of which are valuable and important base material for perfume and cosmetic industries (Zuker et al., 1998; Ayci et al., 2005). R. damascena trigintipetala Mill. is grown at Taif, Saudi Arabia, where a unique local variety known as the Taify rose is valued for its oil content and adaptability to the local climate. Aphids, thrips, whiteflies, and various lepidopteran larvae are some of the insects that damage roses. The buds, leaves, and flowers of rose plants are extremely vulnerable to these insect infestations. As a result, infestations with these insect pests limit rose yields (Karlik and Tjosvold, 2003). Rose aphid, Macrosiphum rosae L. (Hemiptera: Aphididae); potato aphid, M. euphorbiae (Thomas); cotton aphid, Aphis gossypii Glov.; and green peach aphid, Myzus persicae; are all major aphid pests that infest and harm rose plants (Karlik and Tjosvold, 2003). M. rosae is a key pest of rose, causing direct damage by sap sucking which results in deformed leaves and new bloom stems, stunted growth, gall formation and changing the composition of plant biochemicals (Jalalizand et al., 2012; Singh et al., 2014). Indirect loss is incurred, however, when honeydew is secreted, which promotes mold growth on blossoms and leaf surfaces, lowering plant photosynthetic activity and thus yield (Jalalizand et al., 2012). Therefore, aphid infestation lowers the market value of rose flowers and has a negative impact on plant flowering capacity, resulting in losses of 20–40 % (Jayma and Ronald, 1992). Aphids are also responsible for the transmission of various plant viruses, as well as providing entry points for fungal spores and bacteria inside rose plants through holes punched during feeding (Chau et al., 2004). Taif's rose has been known to have severe M. rosae infestations (Sayed and Montaser, 2012; Sayed and Alghamdi, 2017). Growers frequently use synthetic chemical insecticides to protect flowers from aphid infestation. Synthetic pesticides are usually expensive and leave long-lasting residues on exposed surfaces, even though they provide immediate and effective control for the time being; besides this sucking insect pest associated with rose plant has been long known to develop resistance (Metcalf, 1980). Furthermore, the scenario demands sustainable solutions due to other issues such as health risks, negative side effects, insect comeback and disturbance, and environmental pollution caused by the continued use of synthetic chemical pesticides (Parmar, 1993; Freeha et al., 2017). One such option that has the potential to change modern day insect pest control is the employment of natural and biodegradable compounds, predators, parasitoids, and entomopathogenic microorganisms (Ghodke et al., 2013; Dixit et al., 2015). Secondary metabolites such as phenols, flavonoids, quinones, terpenoids, alkaloids, and tannins are produced by plants to protect themselves from herbivorous and microbial attacks. Due to their efficiency against various life stages of many insect pests, extracts or essential oils of medicinal or aromatic plants are frequently utilized for pest management (Ahmed et al., 2020). There is an increasing requirement for new active compounds and ingredients for pest control that have less negative environmental effects (Rodríguez-González et al., 2019). Botanical pesticides and plant extracts have recently demonstrated a significant role in pest control due to their low cost, lack of residual effects, environmentally friendly nature, wide availability, and high toxicity against numerous insect pests such as aphids. Furthermore, due to their molecule complexity, they are unlikely to cause pesticide resistance in diseases and pests (Bedini et al., 2020). Medicinal plant extracts have been successfully utilized against a range of hemipteran pests, particularly various aphid species (Vishwanath, 2002; Sood et al., 2005). Many plant-derived essential oils, such as neem, rosemary, lavandula, thyme, and ziziphora, have insecticidal properties that cover a broad range of soft-bodied arthropod pests (Murray, 2006; Alexenizer and Dorn, 2007). Bacteria have long been regarded as potential pest control alternatives to conventional chemical insecticides. Secondary metabolites (SMs) or small-molecule natural products are synthesized by these microorganisms. Agrochemical, food, and pharmaceutical sectors are all interested in many of these SMs since they have a diversity of biological functions. Entomopathogenic bacteria (ENB) have attracted a lot of attention as biological control agents over the last two decades. The Gram-negative bacteria Xenorhabdus spp., which are members of the Enterobacteriaceae family and are symbiotically coupled with entomopathogenic nematodes of the Steinernematidae family (Boemare et al., 1993; Forst et al., 1997), have demonstrated a composite relationship between these bacteria and their nematodes. The extreme toxicity against several insect species has been justified as a result of their association (Akhurst, 1983; Herbert and Goodrich-Blair, 2007; Herbert et al., 2009). As nematodes infest their insect hosts, they release symbiotic bacteria from their intestinal tracts into the heomocoel, where the bacteria grow and kill the insects within 48 h. The bacteria subsequently turn the insect into suitable food for nematode and release toxins (Akhurst and Bedding, 1986) that keep the dead insect from decaying while the nematodes feed and multiply on it (Sicard et al., 2004; Yang et al., 2012). The bacterium Xenorhabdus has been proven to grow successfully in the laboratory, and both cell suspensions and cell-free supernatants of this bacterium have been reported to have a detrimental effect on several insect pests, causing mortality. Xenorhabdus szentirmaii is a unique source of antimicrobial peptides that are effective against practically all known plant pathogens (Fuchs et al., 2014). Many biocontrol agents are affected by the majority of synthetic pesticides. These control agents interact in a synergistic, additive, or antagonistic approach. Synergistic interactions would improve EPB efficacy while lowering insecticide adverse effects. Plant-derived insecticides are more effective when used in combination with microbial or synthetic insecticides than when used alone (Isman, 2006). As a result, integrated pest management (IPM), which combines biocontrol agents and biopesticides, is gaining popularity and has been shown to be an environmentally friendly management strategy in which biocontrol agents can be mixed with plant-derived extracts (Kalita and Hazarika, 2018). Although there have been numerous studies on the toxicity effects of plant extracts and EPBs applied singly on insect pests, little work has been carried out to find the lethal combined effects of those two agents on aphid control. Therefore, this study was aimed to evaluate, for the first time, the compatibility of extracts from five medicinal plant species located in the Taif region of Saudi Arabia and EPB, Xenorhabdus budapestensis, against the rose aphid, M. rosae, under laboratory conditions.
2 Materials and methods
2.1 Insects
The adults of rose aphid, M. rosae were originally collected from common rose farms of Taif region, Saudi Arabia. These aphids were reared on potted Taify rose plants grown in a growth chamber (25 ± 2 °C, 65 ± 3 % R.H. and a photoperiod of 16:8h L:D) for two months (several generations) before conducting the experiments. All experiments were performed under the above mentioned conditions in laboratory.
2.2 Plant extracts
Five medicinal plant species from four different families were collected in January 2021 from their natural habitat in the Al-Shafa region, Taif Province, as illustrated in Table 1. These tested plants were morphologically identified by the Herbarium Unit at the Biology Department, Faculty of Science, Taif University, Saudi Arabia. The collected fresh parts (1 kg from each plant species) were washed thoroughly under running tap water, cut into small pieces, fully shade dried for 10 days, and used for extraction. Dried plant materials were homogenized to fine powder by a mortar and pestle. Following Ahmed et al. (Ahmed et al., 2020) with some modifications, extractions were performed thrice at room temperature for three days by using the cold extraction/solvent extraction method. In brief, five grams of fine powder from each plant was extracted with 100 ml 95 % of different organic solvents i.e. ethanol, methanol and acetone. Afterward, each extract was centrifuged at 5000 rpm for 15 min and filtered 3 times with Whatman filter paper No. 1. Then, the volume was reduced by concentrating the filtered material through the rotary evaporator at 30 °C. Next, the filtrate was allowed to dry for 12 h in a fume hood at 28 °C. The dry extracts were dissolved in 1 % aqueous solution of dimethylsulfoxide (DMSO) and adjusted to a final concentration of 1000 μg/mL and then, the extracts were stored in glass bottles at 4 °C for further bioassays.
No.
Scientific Name
Common Name
Family Name
Extracted Part
1
Citrullus colocynthis (Cc)
Colocynth
Cucurbitaceae
Leaves
2
Tagetes erecta (Te)
African marigold
Asteraceae
Flowers
3
Rosmarinus officinalis (Ro)
Rosemary
Lamiaceae
Leaves
4
Thymus vulgaris (Tv)
Thyme
Lamiaceae
Leaves
5
Withania somnifera (Ws)
Ashwagandha
Solanaceae
Leaves
2.3 Gas chromatography–mass spectrometry (GC–MS) analysis of T. erecta flowers
The methanol extract of T. erecta flowers was analyzed with an Agilent GC–MS instrument using an Agilent HP-5MS column (30 m length) with helium as the carrier gas at a flow rate of 7 ml min−1. Agilent HP-5 ms is a (5 %-phenyl)-methylpolysiloxane phase with low bleed characteristics that is ideal for GC/MS. The column is bonded, cross-linked, and the solvent rinsable, with excellent inertness for active compounds with improved signal-to-noise ratio for better sensitivity and mass spectral integrity. The oven was set at the following temperatures: initial, 70 °C; ramp with 5 °C min−1 to 310 °C; hold for 1 min at 310 °C; ramp to 70 °C with 5 °C min−1. For the determination of the compounds, the analytical method was used: mass spectra (authentic chemicals, Wiley spectral library collection and NSIT library).
2.4 EPb
In this study, one strain of symbiotic bacterium included in the bioassay was provided from the Fodor Laboratory at Pannonia University, in Keszthely, Hungary. The symbiotic bacterium, Xenorhabdus budapestensis DSM 16,342 (EMA), which was isolated from the EPN Steinernema bicornutum was used for determining its compatibility with plant extracts against M. rosae. The bacterium was regularly cultivated on LBTA (Luria Bertani agar) indicator plates [10 g of peptone, 5 g of yeast extract, 5 g of sodium chloride, 15 g of agar, 25 mg of bromothymol blue, 40 mg of 2,3,5-triphenyltetrazolium chloride and 1 L of distilled water (pH 6.8)] in the dark at 25 °C. In preparing the bacterial cell suspension, as an inoculum for a 100-mL culture, a single dark blue/red colony was placed separately into test tubes containing 5 ml of LB liquid media. Exactly 100-mL aliquots of culture were shaken overnight at room temperature in 500-mL Erlenmeyer flasks before being transferred to flasks containing 400 ml of the same media and shaken at 200 rpm for five days. To obtain the bacterial pellet, the multiplied bacterial culture was centrifuged (13,000 rpm for 30 min) at 4 °C. A 0.22 m Millipore filter was used to separate the supernatant and pellet which was resuspended in sterile distilled water. Adjustment of the bacterial cell suspension at OD600 to 1.0 was performed using a spectrophotometer (SpectroStar Omega, BMC Labtech, Aylesbury, UK). A 10-fold serial dilution spread plate was used, and the bacterial suspension concentration was 108 (CFU/mL). Six dilutions of bacterial cell suspension were adjusted to 108, 107, 106, 105, 104, and 103 CFU/mL.
2.5 Bioassays
2.5.1 Toxicity of plant extracts
Each of the extract prepared by using different solvents (1000 μg/mL) was diluted by distilled water to obtain five concentrations of 800, 600, 400, 200 and 100 μg/mL. Contact and residual toxicity methods were adopted to evaluate the activity of each extract against M. rosae. For contact assay, 20 adult wingless aphids were dipped in each respective extract/concentration for 5 s and released on freshly cut leaves placed in plastic petri dishes (100 × 15 mm) with moistened cotton tissues to maintain humidity. While in residual toxicity test, fresh rose leaf discs (2 cm diameter) were cut off, dipped for 20 s in each respective extract/concentration and dried in air for half an hour. After the dipping application, 3 leaf discs were placed in a petri dish (100 × 15 mm) lined with moistened cotton tissues. Next, 20 adult wingless aphids were released on these leaf discs. For both toxicity assay tests, 1 % DMSO prepared in distilled water was used as the control. Then, the dishes were kept in a plant growth chamber at 25 ± 2 °C, 65 ± 3 % R.H. and a photoperiod of 16:8 L:D. Accordingly, each replicate (plate) contained 20 individual aphids, with a total of 5 replicates per concentration. Aphid mortality was assessed at an exposure of 24, 48 and 72 h by gentle probing with a fine brush and observing the lack of insect movement and the change in the body to a post-mortem color (Sadeghi et al., 2009). The mortality rates in the treatments were corrected with that in the control according to Abbott’s formula (Abbott, 1925).
2.5.2 Pathoginicity of EPB
The toxicity of EPB cell suspension was evaluated on M. rosae via contact and residual toxicity methods. The experiment was conducted as previously described in the plant extract bioassay with application of prepared six concentrations of bacterial cell suspension instead of plant extract and distilled water was used as control.
2.5.3 Combination activity between plant extracts and EPB
From the resulted data of bacterial bioassay, the concentration of 1 × 105 CFU/mL was used to be combined with a low concentration of each methanol extract (100 μg/mL) as contact application method against M. rosae. The mixture of each plant extract and bacterium was prepared from 2.5 ml of plant extract (200 μg/mL with 2 % DMSO) and 2.5 ml of bacterium suspension (2 × 105 CFU/mL distilled water) to obtain 5 ml of mixture with a final concentration of 100 μg/mL (Plant extract) and 1 × 105 CFU/mL (Bacterium). Therefore, 11 treatments were carried out: 1 × 105 CFU/mL (bacterium alone), 5 treatments with 100 μg/mL (for each plant extract), and 5 treatments of mixtures of bacterium with the 5 tested plant extract. Two control groups were used in this experiment: control 1—aphids were dipped in distilled water to correct mortality of bacterium alone; control 2—aphids were dipped in DMSO (1 %) to correct mortality of each plant extract alone and of mixtures. The contact was applied as previously described in the plant extract bioassay. Each treatment or control was repeated 5 times, where each replicate contained 20 aphid individuals. Then, all Petri dishes were investigated after 72 h for mortality. All treatments were carried out under the controlled conditions of 25 ± 2 °C, 65 ± 3 % RH, and 16L: 8D photoperiod.
2.6 Statistical analysis
The median lethal concentration (LC50), 95 % confidence limits of lower and upper values, slope, intercept, and chi-square (χ2) were estimated using probit analysis of mortality versus concentration by SPSS software program, version 23 (Spss, 2015). Statistical analysis for all experiments was performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA). Meanwhile, two-way ANOVA with Duncan’s test was used to compare among corrected mortalities caused by individual treatments of plant extracts and EPB (p < 0.05). Moreover, one-way ANOVA was conducted to assess the compatibility of each plant extract and EPB on aphid toxicity.
3 Results
3.1 Toxicity of plant extracts
The contact and residual assays demonstrated that all five plant extracts had variable degrees of toxicity against rose aphid in three solvents (Tables 2, 3). The contact efficacy of the five plant extracts on M. rosae mortality was revealed in Table 2. The percent corrected mortality rate of adult aphids was found to be directly related to the plant extract species, concentration, solvent, and exposure time. Among the five tested plant extracts, results indicated that highest mortality (66.3 %) was significantly recorded by T. erecta extract; followed by T. vulgaris (57.8 %), R. officinalis (57.3 %) and C. colocynthis (53.7 %) which were not significantly differ; while W. somnifera afforded lower mortality (48.5 %). The results also revealed superiority of methanol extracts over acetone and ethanol ones implying none of the acetone or ethanol extracts at five concentrations killed more aphid individuals than methanol plant extracts. Moreover, for all plant extracts, the 800 μg/mL concentration was most effective in achieving maximum toxicity compared to the other four concentrations. Furthermore, the data also indicated that mortality is correlated to prolonged time exposure (Table 2). The mortality rates after 24 and 48 h were 2 % for the control, whereas, it recorded 4 % after 72 h exposure. The maximum corrected mortality rate (98.9 %) was recorded by methanol extract of T. erecta at 800 μg/mL and 72 h post treatment, followed by acetone and ethanol extracts with 96.9 % mortality percentage each at the same concentration and exposure time. Moreover, methanol extract of T. erecta at low concentrations (200 and 100 μg/mL) afforded extreme mortality after 72 h exposure which was 91.8 % and 89.6 %, respectively. Likely, methanol extract of R. officinalis at 72 h exposure and 800 μg/mL induced 94.4 % mortality, whereas, acetone and ethanol extracts induced 92.2 % mortality each at the same duration and concentration, while at 100 μg/mL in the same solvent it gave considerable mortality (55.6 %). The lowest mortality percentage was recorded for extracts of W. somnifera at all five concentrations. The methanol and acetone extracts of W. somnifera performed slightly better than its ethanol counterpart with mortality rates 53.9 %, 50.5 % and 41.1 %, respectively. At 100 μg/mL concentration, ethanol W. somnifera extract produced minimum mean percentage mortality of 17.8 % after 24 and 48 h exposure.
Plant extract
Solvent
Exposure time (h)
a Corrected Mortality (%)
Solvent Means
Plant Extract Means
Concentration (μg/mL)
800
600
400
200
100
C. colocynthis
Acetone
24
b51 ± 1.9
47 ± 2
44 ± 4
35.6 ± 1.4
27.8 ± 2.5
56.1b
53.7c
48
66.7 ± 2.5
62.2 ± 3.2
60 ± 4.1
48 ± 3.7
40 ± 0
72
85.6 ± 1.4
81.1 ± 1.4
77.8 ± 2.5
68.9 ± 1.4
45.6 ± 5.7
Ethanol
24
48 ± 2.5
45 ± 2.2
42 ± 3.7
35.6 ± 5.4
20 ± 3.2
44.4c
48
57.8 ± 3.8
51.1 ± 4.4
46 ± 5.1
44.4 ± 3.5
33.3 ± 4.9
72
61.1 ± 4.9
52.2 ± 4.2
48.9 ± 4.4
47.8 ± 5.9
33.3 ± 4.9
Methanol
24
62.2 ± 2.1
60 ± 3.2
54.4 ± 3.7
46 ± 2.4
34.4 ± 4.4
60.5 a
48
66 ± 2.9
64 ± 2.4
56.7 ± 2.1
50 ± 3.2
45.6 ± 5.7
72
92.2 ± 3.3
88.9 ± 3
83.3 ± 7.5
57.8 ± 4.2
46.7 ± 2.8
Concentration Means
65.6 a
61.3 ab
57b
48.2c
36.3 d
T. erecta
Acetone
24
57.8 ± 3.8
45.9 ± 5
42.9 ± 6.9
38.8 ± 4.6
28.6 ± 3.2
66.2 ab
66.3 a
48
76.5 ± 2.6
73.5 ± 2.9
69.4 ± 4.6
69.4 ± 3.2
59.2 ± 4.6
72
96.9 ± 2.1
94.4 ± 1.8
93.8 ± 2.6
83.3 ± 5.3
62.5 ± 7.1
Ethanol
24
60 ± 4.1
57.8 ± 3.8
44.9 ± 2.9
31 ± 8.4
20.4 ± 3.8
63.7b
48
76.5 ± 3.1
72.4 ± 3.8
69.4 ± 4.6
59.2 ± 4.6
48.9 ± 7.2
72
96.9 ± 2.1
92.7 ± 3.1
83.3 ± 7.1
72.9 ± 4.2
69.6 ± 7.2
Methanol
24
76.5 ± 2.6
57.8 ± 3.8
48.9 ± 3.6
38.8 ± 4.6
28.6 ± 3.2
68.9 a
48
91.8 ± 5
88.8 ± 4.7
83.7 ± 6.9
63.2 ± 6.9
48.9 ± 7.2
72
98.9 ± 1
95.8 ± 1.9
92.7 ± 3.1
91.8 ± 5
89.6 ± 3.3
Concentration Means
81.3 a
75.5b
69.9 bc
60.9c
50.7 d
R. officinalis
Acetone
24
69 ± 4.8
54 ± 6.2
50 ± 7.1
46 ± 5.1
38 ± 3.7
58.6b
57.3b
48
75.6 ± 1.4
66.7 ± 1.8
58.9 ± 3.3
51.1 ± 4.8
40 ± 2.7
72
92.2 ± 6.5
70 ± 5.2
63.3 ± 3.3
55.6 ± 2.5
48.9 ± 2.7
Ethanol
24
56 ± 2.9
46 ± 2.9
40 ± 3.2
40 ± 3.2
29 ± 4.8
53.1c
48
73.3 ± 2.1
64.4 ± 3.8
54.4 ± 2.7
44.4 ± 7.9
33.3 ± 3.5
72
92.2 ± 3.8
65.6 ± 5.1
57.8 ± 6.5
53.3 ± 4.2
46.7 ± 4.2
Methanol
24
63.3 ± 2.2
54 ± 3.3
47 ± 3
44 ± 2.4
40 ± 6.3
60.1 a
48
66 ± 2.9
57.8 ± 2.2
53.3 ± 4.2
51.1 ± 2.7
40 ± 2.7
72
94.4 ± 1.8
86.7 ± 2.8
83.3 ± 1.8
64.4 ± 6.7
55.6 ± 3.5
Concentration Means
75.8 a
62.8b
56.4 bc
49.9c
41.3 d
T. vulgaris
Acetone
24
67 ± 1.2
56 ± 1
47 ± 2
40 ± 2.7
10 ± 3.2
57.3b
57.8b
48
76.7 ± 3.2
73.3 ± 2.1
71.1 ± 2.7
57.8 ± 4.2
11.1 ± 3.5
72
86.7 ± 1.4
81.1 ± 2.2
75.6 ± 3.8
62.2 ± 5.7
44.4 ± 4.9
Ethanol
24
71.1 ± 2.1
60 ± 1.6
50 ± 3.2
44.4 ± 7.9
22.2 ± 4.6
56.3 bc
48
73 ± 1.2
64.4 ± 4.2
57.8 ± 6.5
46 ± 4
30 ± 3.2
72
73.3 ± 3.2
72.2 ± 2.5
66.7 ± 3.5
57.8 ± 5.4
55.6 ± 3.5
Methanol
24
65 ± 2.7
54 ± 3.7
51 ± 3.3
43 ± 3.7
33.3 ± 3.5
59.7 a
48
72.2 ± 3.5
57.8 ± 4.8
55.6 ± 3.5
44.4 ± 7.9
40 ± 3.2
72
86.7 ± 2.8
81.1 ± 3.3
77.8 ± 6.1
68.9 ± 4.2
64.4 ± 5.4
Concentration Means
74.6 a
66.7 ab
61.4b
51.6c
34.6 d
W. somnifera
Acetone
24
57 ± 3.7
55 ± 4.5
50 ± 7.1
28 ± 3.7
26 ± 5.1
50.5b
48.5 d
48
72.2 ± 0
55.6 ± 0
50 ± 4.3
50 ± 2.5
33.3 ± 3.5
72
74.4 ± 1.4
62.2 ± 2.1
56.7 ± 1.1
54.4 ± 2.1
33.3 ± 3.5
Ethanol
24
46 ± 4
43 ± 5.4
37 ± 5.4
24 ± 8.1
17.8 ± 5.7
41.1c
48
60 ± 2.7
46.7 ± 1.4
38.9 ± 2.5
36.7 ± 2.2
17.8 ± 5.7
72
70 ± 1.4
60 ± 2.1
52.2 ± 2.8
42.2 ± 2.8
24 ± 8.1
Methanol
24
64 ± 4.8
55.6 ± 3.5
48.9 ± 5.4
46.7 ± 4.2
34 ± 4
53.9 a
48
70 ± 2.8
58 ± 4.1
51.1 ± 5.7
48 ± 5.8
42.2 ± 4.5
72
76.7 ± 1.1
63.3 ± 1.4
52.2 ± 3.3
52 ± 5.8
45.6 ± 3.7
Concentration Means
65.6 a
55.5b
48.6c
42.4 cd
30.4 d
Plant extract
Solvent
Exposure time (h)
a Corrected Mortality (%)
Solvent Means
Plant Extract Means
Concentration (μg/mL)
800
600
400
200
100
C. colocynthis
Acetone
24
b28.6 ± 1.6
24.5 ± 1.9
22.4 ± 2.5
20.4 ± 3.8
12.2 ± 2.5
28.9 bc
34.9 d
48
36.7 ± 2.6
33.7 ± 2.3
32.7 ± 2.5
22.4 ± 2.5
13.1 ± 5.6
72
51 ± 3.1
43.8 ± 5.3
39.6 ± 6.1
27.1 ± 3.3
25 ± 6.1
Ethanol
24
26.5 ± 2.6
23.5 ± 2.3
20.4 ± 3.8
12.2 ± 2.5
8.9 ± 4.1
32.6b
48
46.9 ± 3
42.7 ± 4.4
40.6 ± 4.5
28.6 ± 3.2
18.4 ± 3.2
72
56.1 ± 7.8
52 ± 9.9
48.9 ± 3.7
31.3 ± 2.6
31.3 ± 2.6
Methanol
24
28.6 ± 1.6
24.5 ± 1.9
20.4 ± 3.8
20.4 ± 3.8
12.2 ± 2.5
43.2 a
48
42.9 ± 7.1
37.8 ± 7.9
33.7 ± 9.1
28.6 ± 3.2
22.4 ± 4.1
72
90.6 ± 3.5
84.4 ± 4.7
79.2 ± 7.4
75 ± 9.7
47.9 ± 7.4
Concentration Means
45.3 a
40.8 ab
37.5b
29.6 bc
21.3c
T. erecta
Acetone
24
61.1 ± 2.5
56 ± 1.9
52 ± 3.7
50 ± 4.5
44.4 ± 3.7
61.1b
65.3 a
48
72 ± 2.5
56.7 ± 3.7
55.6 ± 3.5
53.3 ± 4.2
48 ± 3.7
72
95.6 ± 2.1
87.8 ± 4.1
80 ± 4.2
55.6 ± 3.5
48 ± 3.7
Ethanol
24
67.8 ± 3.7
58 ± 1.2
58 ± 3.7
55 ± 2.2
48 ± 3.7
67.1 a
48
68 ± 2.5
65.6 ± 2.7
68.9 ± 4.2
68.9 ± 4.2
56.7 ± 4.4
72
93.3 ± 2.1
92.2 ± 4.2
80 ± 3.8
63.3 ± 2.2
62.2 ± 5.7
Methanol
24
71 ± 1.9
62 ± 3
54 ± 2.4
50 ± 3.2
38 ± 3.7
67.8 a
48
75.6 ± 2.8
73.3 ± 2.1
64.4 ± 2.2
53.3 ± 4.2
44.4 ± 3.5
72
97.8 ± 1.4
93.3 ± 2.1
90.6 ± 3.5
80 ± 4.2
70 ± 2.2
Concentration Means
78 a
71.7b
67.1c
58.8 cd
51.1 d
R. officinalis
Acetone
24
24.5 ± 2.9
20.4 ± 2.6
18.4 ± 3.2
18.4 ± 3.2
8.9 ± 4.1
39.2b
42.6c
48
57.1 ± 2.6
55.1 ± 2.5
53.1 ± 4.1
38.8 ± 4.6
22.4 ± 5.2
72
64.6 ± 3
62.5 ± 2.6
58.3 ± 3.3
47.9 ± 7.4
37.5 ± 7.4
Ethanol
24
50 ± 5.7
45.9 ± 5
42.9 ± 13.1
18.4 ± 3.2
12.7 ± 3.7
44 a
48
52 ± 8.2
46.9 ± 3.2
42.9 ± 6.9
28.6 ± 4.6
20.4 ± 3.8
72
75 ± 1.9
70.8 ± 2.7
68.8 ± 3.3
47.9 ± 7.4
37.5 ± 3.3
Methanol
24
30.6 ± 4.7
26.5 ± 6.2
24.5 ± 6.9
22.4 ± 2.5
22.4 ± 4.1
44.7 a
48
58.2 ± 3.4
52 ± 3.8
42.9 ± 8.3
28.6 ± 3.2
28.6 ± 3.2
72
79.2 ± 1.6
76 ± 2.1
72.9 ± 4.2
68.8 ± 4.7
37.5 ± 7.4
Concentration Means
54.6 a
50.7 ab
47.2b
35.5c
25.3 d
T. vulgaris
Acetone
24
31.6 ± 2
25.5 ± 2
20.4 ± 3.8
20.4 ± 3.8
12.2 ± 2.5
32.7b
52.5b
48
33.7 ± 2.3
26.5 ± 1.2
22.4 ± 2.5
20.4 ± 3.8
20.4 ± 3.8
72
63.5 ± 2.9
60.4 ± 2.7
58.3 ± 3.3
37.5 ± 7.4
37.5 ± 3.3
Ethanol
24
56.1 ± 3.8
52 ± 5.5
48.9 ± 7.2
42.9 ± 6.9
20.4 ± 3.8
61.8 a
48
73.5 ± 5.4
71.4 ± 5.9
69.4 ± 7.2
59.2 ± 4.6
38.8 ± 4.6
72
92.7 ± 3.5
89.6 ± 2.9
87.5 ± 3.9
72.9 ± 4.2
52.1 ± 2.6
Methanol
24
36.7 ± 2.6
33.7 ± 3.2
28.6 ± 3.2
28.6 ± 3.2
20.4 ± 3.8
63.1 a
48
85.7 ± 5.4
81.6 ± 5.9
79.6 ± 7.2
69.4 ± 3.2
69.4 ± 4.6
72
93.8 ± 2.6
92.7 ± 3.5
80 ± 3.8
76 ± 2.1
70.8 ± 8.9
Concentration Means
63 a
59.3 a
55 ab
47.5b
38c
W. somnifera
Acetone
24
34.7 ± 1.9
26.5 ± 2.6
22.4 ± 2.5
14.3 ± 2.5
8.9 ± 4.1
28.7 bc
30.3 e
48
42.9 ± 2.5
36.7 ± 2.6
32.7 ± 5.2
24.5 ± 6.9
16.7 ± 6.6
72
44.8 ± 2.7
41.7 ± 1.9
37.5 ± 3.3
27.1 ± 3.3
18.4 ± 3.2
Ethanol
24
24.5 ± 1.9
17.3 ± 2.5
12.2 ± 2.5
8.9 ± 4.1
8.9 ± 4.1
29.3b
48
34.7 ± 1.9
30.6 ± 4.1
28.6 ± 4.6
8.9 ± 4.1
8.9 ± 4.1
72
70.8 ± 8.9
64.6 ± 4.1
62.5 ± 7.2
41.7 ± 7.1
16.7 ± 3.3
Methanol
24
28.6 ± 1.6
24.5 ± 1.9
22.4 ± 2.5
12.2 ± 2.5
12.2 ± 2.5
32.8 a
48
36.7 ± 2.6
32.7 ± 2.5
28.6 ± 3.2
18.4 ± 4.6
18.4 ± 3.2
72
67.7 ± 8.5
63.5 ± 6.9
61.5 ± 5.4
37.5 ± 6.6
27.1 ± 4.7
Concentration Means
42.8 a
37.6 ab
34.2b
21.5c
15.1 d
The data in Table 3 reveal that all plant extracts had a highly significant effect on the mortality of M. rosae adults (p < 0.05) via residual assay test. The results show that the five plants extracts increased mortality of M. rosae adults (p < 0.05) within the three solvents, five concentrations and three exposure periods comparing with the control treatment, which demonstrated zero mortality at 24 h and 10 % mortality at 48 and 72 h. Among all tested plant extracts, results demonstrated that highest mortality (65.3 %) was significantly observed by T. erecta, followed by T. vulgaris (52.5 %) and R. officinalis (42.6 %), while C. colocynthis and W. somnifera induced the lowest mortality (34.9 %) and (30.3 %), respectively. Similarly, among the three tested solvent, methanol extract afforded higher M. rosae mortality than ethanol and acetone extracts. There were statistically significant differences among the treatments on each concentration of plant extract and day after application. On 1st, 2nd, and 3rd day 800 μg/mL produced significantly higher mortalities than 200 and 100 μg/mL plant extract concentrations (Table 3). At 72 h exposure; maximum mortality was elicited by methanol extract of T. erecta with mean mortality percentages of 97.8 %, 93.3 %, 90.6 %, 80 % and 70 % at 800, 600, 400, 200 and 100 μg/mL concentrations, respectively. However, ethanol extract caused aphid mortality rates ranging from 93.3 % to 62.2 %, whereas, percentage mortality dipped to range from 95.6 % to 48 % for acetone T. erecta extract at the same concentrations and exposure time, respectively. Analysis of variance revealed that methanol extract did not differ significantly than ethanol extract in T. erecta, R. officinalis and T. vulgaris plants but was significantly different from acetone extract for all three plants. Although ethanol W. somnifera extract at 800 μg/mL produced the highest mean percentage mortality (70.8 %) 72 h post treatment, it recorded the lower mortality (8.9 %) at 200 and 100 μg/mL concentrations after 24 and 48 h exposure.
As shown in Table 4, probit analysis exposed the LC50, slope value, intercept, Chi-square, probability and confidence interval limits at 95 %. The data indicate that T. erecta extract was significantly more efficient against M. rosae individuals than other four plant extracts under laboratory conditions. The T. erecta extract exhibited lower LC50 values of 195.7, 84.6 and 34.7 μg/mL for contact assay, whereas, it recorded 375.1, 98.7 and 81.6 μg/mL for residual efficacy at 24, 48 and 72 h exposure, respectively. For the other four plants, LC50 values at 72 h post application ranged from lowest to highest as follows: 77.5 and 87.6; 121.5 and 176.7; 126 and 283.4; and 249.4 and 417.5 μg/mL for T. vulgaris, R. officinalis, C. colocynthis and W. somnifera via contact and residual assay, respectively. Table 4 shows that the highest degree of homogeneity in the M. rosae individual response was observed after exposure to the T. vulgaris extract via contact assay, with a slope value of 0.058 at 48 h post treatment. On the contrary, the other tested plant extract concentrations exhibited low slope values (Table 4), which recognizes the uniformity in the response of M. rosae individuals to these concentrations and assay methods. LC50—lethal concentration that kills 50% of insects; X2—chi-square value; SE standard error; p-value—probability.
Plant extract
Bioassay
Exposure time (h)
LC50
(μg/mL)95 % confidence limits
Slope ± SE
Intercept
X2
p-Value
Lower
Upper
C. colocynthis
Contact
24
609.5
480.2
858
0.034 ± 0.008
29.2
2.81
0.023
48
332.9
128.6
471.1
0.032 ± 0.004
39.3
0.72
0.005
72
126
44.2
227.3
0.049 ± 0.011
44.3
4.22
0.022
Residual
24
4213.7
1792.8
7476.7
0.022 ± 0.003
11.3
0.26
0.008
48
1366.6
813.3
4911.5
0.032 ± 0.006
18.5
0.27
0.014
72
283.4
199
382.4
0.042 ± 0.007
34.5
0.09
0.008
T. erecta
Contact
24
195.7
71.8
306.6
0.042 ± 0.003
23.7
0.60
0.001
48
84.6
35.4
130.6
0.039 ± 0.008
53.6
2.40
0.019
72
34.7
11.5
60.5
0.032 ± 0.006
74.3
1.14
0.011
Residual
24
375.1
414.9
894.9
0.029 ± 0.004
42.9
0.90
0.006
48
98.7
15.6
175.1
0.028 ± 0.005
50.1
0.52
0.011
72
81.6
50.5
110.4
0.053 ± 0.007
57.2
3.78
0.006
R. officinalis
Contact
24
558.5
268.4
701.8
0.034 ± 0.005
33.4
2.29
0.006
48
254.6
153.2
306.8
0.044 ± 0.005
36.7
0.95
0.003
72
121.5
60.1
246.7
0.056 ± 0.006
45.1
10.7
0.002
Residual
24
2640.7
1257.1
4307.2
0.028 ± 0.004
13.9
0.15
0.007
48
550.2
418.2
832
0.045 ± 0.007
22.8
0.22
0.009
72
176.7
114.8
233.3
0.045 ± 0.013
41.3
1.08
0.040
T. vulgaris
Contact
24
403.4
301.9
474.5
0.057 ± 0.011
23.8
2.40
0.015
48
230
199.3
312.3
0.058 ± 0.015
30.9
1.94
0.028
72
77.5
28.5
124.3
0.038 ± 0.006
54.4
0.14
0.008
Residual
24
1639.5
884.3
3728.9
0.029 ± 0.007
19.9
1.35
0.030
48
204.1
82.6
315.6
0.029 ± 0.004
42.7
0.07
0.007
72
87.6
41.4
130.4
0.042 ± 0.008
53.2
0.30
0.013
W. somnifera
Contact
24
614
446.5
1061.3
0.043 ± 0.006
24.3
0.15
0.005
48
360.8
265
506.1
0.044 ± 0.008
30.2
3.6
0.012
72
249.4
179.1
323.1
0.049 ± 0.008
33.9
2.54
0.007
Residual
24
6617
2071.8
9478.7
0.028 ± 0.001
7
0.73
0.001
48
1788.6
1034.4
3440.5
0.035 ± 0.005
12.1
0.76
0.006
72
417.5
337.2
535.6
0.055 ± 0.013
22.6
1.36
0.026
3.2 GC–MS analysis of T. erecta extract
The chemical compounds of the methanol extract of T. erecta flowers with their retention time, molecular formula, and peak area percentage are presented in Table 5. A total of 31 components were identified in the extract of T. erecta flowers. The GC–MS analysis results revealed the presence of Hydrogen isocyanate, which is the most abundant compound (26.47 %); followed by Tetrahydro-4H-pyran-4-ol (21.99 %); Glycine, ethyl ester (15.83 %); 1H-Pyrazole, 4,5-dihydro-3-methyl-1-propyl- (8.95 %); 1,3-Dioxolane-4-methanol, 2-pentadecyl-, acetate (5.69 %); l-Norvaline, N-ethoxycarbonyl-, pentyl ester (5.59 %); 4-Methoxycarbonyl-4-butanolide (4.05); [(2-Ethyl-5-methylfuran-3,4- (1.67 %); Propanethioic acid, S-propyl ester (1.21 %); beta-Amyrin (1.19 %); and a small quantity of other components. R.T., Retention time (min).
No.
R.T.
Compound Name
Molecular Formula
Area (%)
1
6.1012
Tetrahydro-4H-pyran-4-ol
C5H10O2
21.99
2
6.6189
4-Methoxycarbonyl-4-butanolide
C6H8O4
4.05
3
6.7734
1H-Pyrazole, 4,5-dihydro-3-methyl-1-propyl-
C7H14N2
8.95
4
7.0905
Hydrogen isocyanate
CHNO
26.47
5
7.1326
Glycine, ethyl ester
C4H9NO2
15.83
6
7.2164
4-Benzyloxy-3-methoxy-2-nitrobenzoic acid
C15H13NO6
0.48
7
8.8763
1,3-Dioxolane-4-methanol, 2-pentadecyl-, acetate,
C21H40O4
5.69
8
8.9501
l-Norvaline, N-ethoxycarbonyl-, pentyl ester
C13H25NO4
5.59
9
9.0027
Propanethioic acid, S-propyl ester
C6H12OS
1.21
10
9.1526
Benzene, 1,2-difluoro-
C6H4F2
0.37
11
12.0871
Dioxoethylenebis (iminosulfur pentafluoride)
C2H2F10N2O2S2
0.08
12
12.5619
2-Thiobarbituric acid, tris(tert-butyldimethylsilyl) deriv
C22H46N2O2SSi
0.77
13
12.6616
Cyclohexasiloxane, dodecamethyl-
C12H36O6Si6
0.97
14
12.8194
3-Trifluoromethylbenzylamine, N,N-diundecyl
C30H52F3N
0.04
15
13.3310
Cholestan-3-one, dimethylhydrazone, (5alpha)-
C29H52N2
0.01
16
13.5587
Ethylphosphonic acid
C2H7O3P
0.63
17
15.4391
Heptyl methyl ethylphosphonate
C10H23O3P
0.24
18
15.8932
Pentanedioic acid, (2,4-di-t-butylphenyl) mono-ester
C19H28O4
0.15
19
16.0149
Furan, 2,5-dibutyl-
C12H20O
0.19
20
16.9014
Trioxide, bis(trifluoromethyl)
C2F6O3
0.45
21
24.1026
Trimethylphosphine oxide
C3H9OP
0.01
22
24.1507
Syringylacetone
C11H14O4
0.85
23
24.1527
(Phenylthio)acetic acid, propyl ester
C11H14O2S
0.27
24
28.0613
Hexadecanoic acid, methyl ester
C17H34O2
0.68
25
29.0088
2-Butynenitrile, 4,4,4-trifluoro-
C4F3N
0.01
26
30.1970
d-Alanine, N-propargyloxycarbonyl-, dodecyl ester
C19H33NO4
0.13
27
32.1660
5-(Pent-3-en-1-yn-1-yl)-2,2′-bithiophene
C13H10S2
0.42
28
41.9315
[(2-Ethyl-5-methylfuran-3,4-
C13H26O3Si2
1.67
29
48.6880
2-(2′,4′-Dimethoxyphenyl)-6-methoxy-benzofuran
C17H16O4
0.11
30
56.0952
alpha-Tocopherol-.beta.-d-mannoside
C35H60O7
0.51
31
60.0878
beta-Amyrin
C30H50O
1.19
3.3 Virulence of EPB
The data in Fig. 1 represent the toxicity of entomopathogenic bacteria X. budapestensis DSM 16,342 at six concentrations against M. rosae adults via contact and residual assay at three exposure times under laboratory conditions. The EPB cells significantly affected aphid mortality (p < 0.05) in the two method of evaluation. The percentage of adult mortality increased significantly with bacterial cell concentration and exposure duration (p < 0.05). The interactive effect of bacterial cell suspension concentrations, and exposure time on individual infection via contact assay was not significant (p = 0.9765). The highest mortality percentage (97.9 %) was recorded in the plates where the adults were exposed to 108 CFU/mL distilled water after 72 h of application, followed by 107 CFU/mL (93.7 %) without significant differences between these two concentrations. On the other hand, the lowest (44.9 %) was recorded when the individuals were exposed to 103 CFU/mL of X. budapestensis 24 h post treatment (Fig. 1A). Similarly, the residual efficacy of EPB on M. rosae adults recorded the same results. The data indicate that the mortality rate had a direct relationship with the exposure time and bacterial CFU concentration (p < 0.05). The regression analysis of the data shows that the mortality of M. rosae adults significantly increased with increasing bacterial concentration (R2 = 0.9852; p < 0.05). Maximum (92.2 %) and minimum (42 %) mortality rates were achieved when the adults were treated with 108 CFU/mL at 72 h exposure and 103 CFU/mL at 24 h, respectively (Fig. 1B).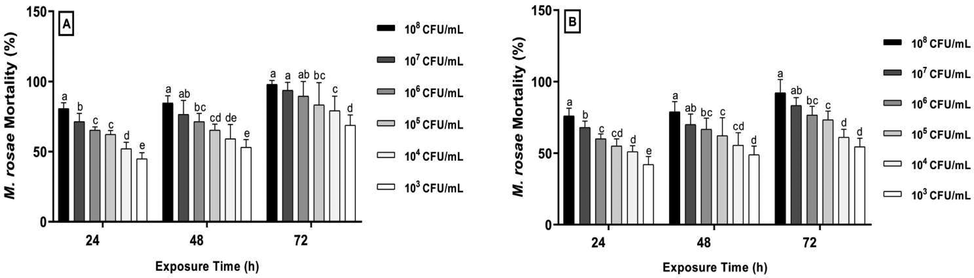
Corrected mortality percentage (mean ± SE) of the rose aphid.
Likewise, the data in Table 6 reveal that the contact assay was more effective against M. rosae adults than the residual one, with LC50 values at 24, 48 and 72 h after treatment of 3.98 × 103, 9.33 × 102 and 1.41 × 102 CFU/mL, respectively, for the former method and 1 × 104, 2 × 103 and 7.94 × 102 CFU/mL, respectively, for the latter. LC50—lethal concentration that kills 50% of insects; X2—chi-square value; SE standard error; p-value—probability.
Bioassay
Exposure time (h)
LC50
(CFU/mL)95 % confidence limits
Slope ± SE
Intercept
X2
p-Value
Lower
Upper
Contact
24
3.98 × 103
7.94 × 102
1.26 × 104
6.9 ± 0.43
25.1
1.50
0.001
48
9.33 × 102
1.26 × 102
3.47 × 103
6.2 ± 0.17
34.4
1.80
0.001
72
1.41 × 102
3.31 × 101
4.27 × 102
5.6 ± 0.48
54.7
2.45
0.001
Residual
24
1 × 104
2 × 103
3.16 × 104
6.5 ± 0.37
23.2
1.66
0.001
48
2 × 103
2 × 102
7.94 × 103
5.6 ± 0.34
32.7
1.06
0.001
72
7.94 × 102
2 × 102
2.51 × 103
7.4 ± 0.48
32.8
3.31
0.001
3.4 Compatible activity of plant extracts and EPB
Fig. 2 show the corrected mortality rates after 72 h of treatment for both individual and combination applications. Control groups 1 and 2 had a mortality rate of 4 % after 3 days, as stated in the “Methods” section. The mortality rates produced by T. erecta methanol extract treatment (82.3 %) were clearly different from the other four plant extracts, but not significantly different from X. budapestensis (83.3 %). After 72 h of application, however, the combination of each plant extract and X. budapestensis was significantly different from the individual treatments for either of them. Maximum mortality (97.9 %) was recorded by concomitant application of T. erecta extract and X. budapestensis cell suspension, whereas, minimum mortality was recorded via single application of W. somnifera extract which was 20.8 %.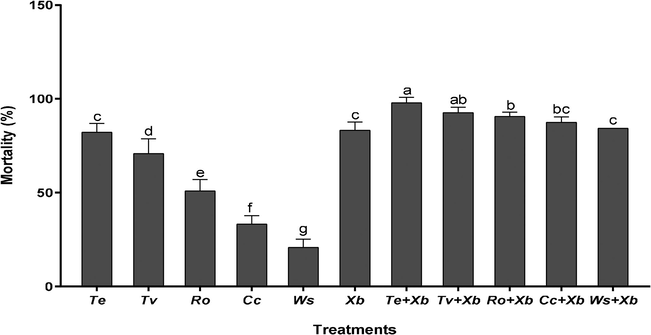
Corrected mortality rates (% ± SE) of M. rosae as affected by five plant extracts and EPB alone and in combination after 72 h of application.
4 Discussion
Because of the environmental impacts associated with the use of synthetic insecticides for pest control and the development of resistant aphid strains, natural products, particularly those derived from plants, have been developed as a potential alternative to chemical pesticides. Extracts of plant origin are often utilized for plant protection measures because of their efficiency against various life stages of insect pests (Chermenskaya et al., 2012). Botanical pesticides have the distinct benefit of being easily accessible, environmentally acceptable, and cost-effective, with minimum residual effects, and widespread public recognition (Yousuf et al., 2021). The present investigation established that extracts of five different medicinal plants were toxic to the rose aphid, M. rosae. On their immediate application, all treatment concentrations effectively killed rose aphids, although the three solvent extracts had different toxicity effects. The toxicity produced by studied plant extracts against M. rosae can be sorted in the following descending order based on LC50 values: T. erecta > T. vulgaris > R. officinalis > C. colocynthis > W. somnifera. The phytochemical analysis of T. erecta revealed the presence of 41 compounds including hexadecanoic acid; phenylthio acetic acid; alpha-tocopherol-beta-d-mannoside; Furan, 2,5-dibutyl; and 5-(Pent-3-en-1-yn-1-yl)-2,2′-bithiophene in addition to a variety of other metabolites were responsible for the toxicity of this plant. In confirming this result, several species of marigold (Tagetes spp.) have been found to contain phytochemicals with pesticidal potential. Numerous investigations reveald that T. erecta L. has been proven to have insecticidal efficacy against aphids (Ravikumar, 2010), mosquitoes (Sharma and Saxena, 1994), sand flies (Dinesh et al., 2014), termites (Elango et al., 2012), and several caterpillars (Aldana-Llanos et al., 2012). The main biocidal compounds of volatile oils extracted from Tagetes spp. aerial plant parts include monoterpenoids, carotenoids, and flavonoids (Ravikumar, 2010). Thiophenes, which are mostly found in roots and flowers, are biologically active against a wide range of insect species (Gil et al., 2002). Our results is in accordance with previous findings (Najafabadi et al., 2018), where T. vulgaris and R. officinalis essential oils had contact toxicity toward the rose aphid and T. vulgaris essential oil had higher insecticidal activity than R. officinalis essential oil. The toxic effect of plant extracts is attributed to a variety of complex chemical components found in plants, including fatty acids, flavonoids, phenols, saponins, terpenoids, and alkaloids (Yaniv et al., 1999). These phyto components either prevent or disrupt insect feeding by making treated leaf surfaces unattractive or disagreeable to insects (Rajashekar et al., 2012; Talukder, 2006). These plant extracts may also modify insect feeding behavior or disturb hormonal balance, turning their food indigestible. In addition, several plant-derived essential oils, such as thyme, rosemary, lavandula, and ziziphora, have insecticidal characteristics that act against a broad range of soft-bodied arthropod pests (Alexenizer and Dorn, 2007). This is due to a variety of mechanisms of action, including antifeedant and repellent activities, molting and respiration inhibition, growth and fecundity suppression, cuticle disruption, and central nervous system activation on the octopamine pathway (Chaubey, 2007). The toxicity of the methanol plant extracts was higher than that of the acetone and ethanol counterparts, which is consistent with the findings of (Shehawy et al., 2019), who found that the methanolic extract of C. colocynthis caused the maximum mortality in Aphis craccivora, followed by ethyl acetate and petroleum ether extract. The stronger aphicidal action of methanol plant extracts could be attributed to the higher solubility of many volatile metabolites/secondary components of plants in this solvent, and thus the extracts' potency. This observation is also similar to the findings of (Maqsood et al., 2020), who found that methanol extracts exceeded ethanol and chloroform extracts in regards of cabbage aphid, Brevicoryne brassicae L. mortality. It was obvious from our investigation that as the concentration of plant extracts increased, the lethal activity increased as well. Thus, the most effective toxic concentration of plant extracts was 800 μg/mL, followed by 600 μg/mL, while the least efficient toxic concentration was 100 μg/mL. This is somewhat in agreement with Hori (1998) who evaluated essential oils of five plants (rosemary, thyme, peppermint, lavender and spearmint) against Myzus persicae and concluded that the rosemary oil at a dose of 10 μl and the thyme oil at a dose of 1 μl, caused 70 % mortality in the population of the aphid. In this study, the toxicity of the EPB, X. budapestensis via contact or residual assay increased with the concentration and post application periods, where the highest mortality was achieved with the higher concentration (108 CFU/mL) at 72 h post treatment. This finding is in line with previous investigation by our team (Alotaibi et al., 2021), who tested the three bacterial species on the carob moth, Ectomyelois ceratoniae and found that X. budapestensis had the highest significant virulence. Moreover, chemical composition of this bacterium revealed the presence of 21 compounds. Several secondary metabolites with effective bioactivities such as benzylideneacetone (antibacterial molecule), iodinine, phenethylamides, indole analogues, xenorhabdins, xenorxides, and xenocoumacins (antibiotics), as well as primary metabolites like alkaline protease have all been reported to be produced and secreted by Xenorhabdus sp. (Morgan et al., 2001; Caldas et al., 2002; Ji et al., 2004; Mohamed, 2007; Bode, 2009), therefore, all of which are optional to play roles as insecticidal and immunosuppressive compounds. Our results also showed that contact assay of EPB was more effective than residual one on rose aphid. This investigation was confirmed by (Iqbal et al., 2020) who recorded the high mortality rate of cotton aphids (Aphis gossypii) induced by crude cell extract, bacterial culture, and methanol extract of EPB, Xenorhabdus spp. and demonstrate that toxic metabolites can pass horizontally (most likely through direct contact) between infected and uninfected aphids. In the present study, EPB cell suspension required 3 days only to kill 97.9 % of M. rosae, whereas, Mahar et al. (Mahar et al., 2005), reported that X. nematophila cell suspension needed up to 6 days to kill 93 % G. mellonella larvae. The only disadvantage of EPBs from this genus is their slow action, however this can be overcome by combining them with other compounds in various methods. Recent approaches have shown that using two different components together can result in higher pest mortality than using them separately (Reddy and Chowdary, 2021). Synergism is the term used to describe how combining two products can enhance pest control effectiveness. The use of a plant extract with insecticidal property in combination with an entomopathogen is an unique way to reduce pollution risks while also minimizing the amount of pesticide used and the development of pest resistance (Srivastava et al., 2011). These botanical biopesticide combinations provide effective control, equivalent to synthetic insecticides. For the first time, we attempted to assess the compatible toxicity of selected plant extracts with EPB against M. rosae. Thus, the current findings revealed that combining each plant extract with EPB resulted in much higher rose aphid mortality than separate treatments after 72 h of application. It was also clear that the combination between T. erecta extract at low concentration and X. budapestensis caused a greater M. rosae adults mortality than separate treatments of both. This suggests that such plants may be able to sustain EPB's virulence, when they are combined together. Previous reports on the successful synergistic interactions between botanicals and EPB have verified our findings (Mhalla et al., 2018; Konecka et al., 2019; Noureldeen et al., 2019; Konecka et al., 2020). On the contrary, Halder et al. (Halder et al., 2017) claimed that Bt combined with neem oil had an incompatible interaction in managing Epilachna deodecastigma beetle.
5 Conclusions
Interestingly, our results indicate that the studied extracts, especially T. erecta, had strong negative effects on the rose aphid M. rosae through its contact and residual toxicity. However, all solvents extract caused significant mortality of M. rosae. In addition, we verified the aphicidal contact or residual activity of EPB X. budapestensis cells against M. rosae adults. Based on our findings, the combination of EPB with each plant extract resulted in a significantly higher rate of aphid mortality than either EPB or plant extracts alone. This indicated that all of the five tested plant extracts were compatible with EPB but with varying degrees. Moreover, these findings are promising for future research as they have the potential to be validated on a commercial scale and considered an important alternative to chemical pesticides to control aphids. Further studies are currently required on these plant extracts to assess their compatibility with other biocontrol agents on insect pests and associated beneficial insects in the field for IPM program.
6 Institutional Review Board Statement
Not applicable.
7 Informed Consent Statement
Not applicable.
Funding
This research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through the project number 1–441-123.
CRediT authorship contribution statement
Ahmed Noureldeen: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. Uttam Kumar: Writing – original draft. Muhammad Asad: Writing – review & editing. Hadeer Darwish: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. Sarah Alharthi: Data curation, Resources. Mustafa A. Fawzy: Formal analysis, Project administration. Amal M. Al-Barty: Formal analysis, Methodology. Saqer S. Alotaibi: Formal analysis, Resources. Ahmed Fallatah: Methodology. Akram Alghamdi: Investigation, Writing – review & editing. Bander Albogami: Resources, Validation. Najla Alkashgry: Investigation, Methodology, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A method of computing the effectiveness of an insecticide. J. Econ. Entomol.. 1925;18:265-267.
- [Google Scholar]
- Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.) Sci. Rep.. 2020;10(1)
- [Google Scholar]
- Neoaplectana species: Specificity of association with bacteria of the genus Xenorhabdus. Exp. Parasitol.. 1983;55:258-263.
- [Google Scholar]
- Natural Occurrence of insect pathogenic nematodes (Steinernematidae and Heterorhabditidae) in soil in Australia. J. Austr. Entomol. Soc.. 1986;25:241-244.
- [Google Scholar]
- Biological activity of dose extracts of Tagetes erecta L. on Spodoptera frugiperda (J. E. Smith) Southwest Entomol.. 2012;37(1):31-38.
- [Google Scholar]
- Screening of medicinal and ornamental plants for insecticidal and growth regulating activity. J. Pest Sci.. 2007;80(4):205-215.
- [Google Scholar]
- Potential of three entomopathogenic bacterial isolates for management of the carob moth, Ectomyelois ceratoniae (Lepidoptera: Pyralidae) Agriculture. 2021;11:1256.
- [Google Scholar]
- Gas chromatographic investigation of rose concrete, absolute and solid residue. Flavour Fragrance J.. 2005;20(5):481-486.
- [Google Scholar]
- Allium sativum, Rosmarinus officinalis, and Salvia officinalis essential oils: a spiced shield against blowflies. Insects. 2020;11(3):143.
- [Google Scholar]
- Entomopathogenic bacteria as a source of secondary metabolites. Curr. Opin. Chem. Biol.. 2009;13:224-230.
- [Google Scholar]
- DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int. J. Syst. Bacteriol.. 1993;43:249-255.
- [Google Scholar]
- Purification and characterization of an extracellular protease from Xenorhabdus nematophila involved in insect immunosuppression. Appl. Environ. Microbiol.. 2002;68:1297-1304.
- [Google Scholar]
- Biological control of aphids on ornamental crops. In: Heinz K.M., van Driesche R.G., Parrella M.P., eds. Biocontrol in Protected Culture. Batavia: Ball publishing; 2004. p. :277-295.
- [Google Scholar]
- Toxicity of essential oils from Cuminum cyminum (Umbelliferae), Piper nigrum (Piperaceae) and Foeniculum vulgare (Umbelliferae) against stored-product beetle Tribolium castaneum Herbst (Cole: Tenebrionidae) Electron. J. Environ. Agric. Food Chem.. 2007;34:1719-1727.
- [Google Scholar]
- Insecticidal effects of Ungernia severtzovii bulb extracts against the grain aphid Schizaphis graminum (Rondani) Ind. Crop. Prod.. 2012;36:122-126.
- [Google Scholar]
- Ornamental plants and role of mutation. Delhi: Daya Publishing House; 1997. p. :220.
- The potentiality of botanicals and their products as an alternative to chemical insecticides to sandflies (Diptera: Psychodidae): a review. J. Vector Borne Dis.. 2014;51(1):1-7.
- [Google Scholar]
- Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability. 2015;7(2):2189-2212.
- [Google Scholar]
- Efficacy of medicinal plant extracts against Formosan subterranean termite, Coptotermes formosanus. Ind. Crops Prod.. 2012;36(1):524-530.
- [Google Scholar]
- Xenorhabdus and Photorhabdus spp. Bugs that kill bugs. Annu. Rev. Microbiol.. 1997;51:47-72.
- [Google Scholar]
- Integration of planting time and insecticide to manage aphid infestations in wheat for better crop productivity. Pak. J. Zool.. 2017;49:1343-1351.
- [Google Scholar]
- Fabclavines: Bioactive peptide polyketide-polyamino hybrids from Xenorhabdus. Chembiochem. 2014;15:512-516.
- [Google Scholar]
- Isolation and in vitro identification of proteinase inhibitors from soybean seeds inhibiting helicoverpa gut proteases. J. Plant Interact. 2013;8:170-178.
- [Google Scholar]
- Root thiophenes in Tagetes minuta L. accessions from Argentina: genetic and environmental contribution to changes in concentration and composition. Biochem. Syst. Ecol.. 2002;30(1):1-13.
- [Google Scholar]
- Potential of entomopathogens and neem oil against two emerging insect pests of vegetables. Indian J. Agric. Sci.. 2017;87(2):220-224.
- [Google Scholar]
- CpxRA influences Xenorhabdus nematophila colonization initiation and outgrowth in Steinernema carpocapsae nematodes through regulation of the nil locus. App. Environ. Microbiol.. 2009;75:4007-4014.
- [Google Scholar]
- Friend and foe: the two faces of Xenorhabdus nematophila. Nat. Rev. Microbiol.. 2007;5:634-646.
- [Google Scholar]
- Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol.. 1998;24(9):1425-1432.
- [Google Scholar]
- Bioremedy of cotton aphid (Aphis gossypii glov.) (Hemiptera: Aphididae) by the application of different fractions of entomopathogenic bacteria (Xenorhabdus spp.). Pakistan. J. Zool.. 2020;52:875-884.
- [Google Scholar]
- Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol.. 2006;51:45-66.
- [Google Scholar]
- Jalalizand, A.Z.; Karimi, A.; Modaresi, M.E.; Mahamoodi, E. Determing morphological traits and genetic diversity of rose aphids using RAPD and RFLP-PCR molecular markers. In Proceedings of the International Conference on Applied Life Sciences (ICALS 12), Konya, Turkey, September 2012; p. 324.
- Macrosiphum euphorbiae (Thomas). Honolulu: Hawaii; Department of Entomology; 1992. p. :118-121.
- Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. FEMS Microbiol. Lett.. 2004;239:241-248.
- [Google Scholar]
- Safety of Chromolaena odorata (Asteraceae) leaf extracts against Trichogramma japonicum Ashmead. Ann. Pl Protec. Sci.. 2018;26(2):276-280.
- [Google Scholar]
- Integrated pest management (IPM) for roses. In: Encyclopedia of Rose Science. Amsterdam: Elsevier Science; 2003. p. :466-473.
- [Google Scholar]
- Insecticidal activity of mixtures of Bacillus thuringiensis crystals with plant oils of Sinapis alba and Azadirachta indica. Ann. Appl. Biol.. 2019;174(3):364-371.
- [Google Scholar]
- Synergistic interaction between carvacrol and Bacillus thuringiensis crystalline proteins against Cydia pomonella and Spodoptera exigua. Biocontrol. 2020;65:447-460.
- [Google Scholar]
- Pathogenicity of bacterium, Xenorhabdus nematophila isolated from entomopathogenic nematode (Steinernema carpocapsae) and its secretion against Galleria mellonella larvae. J. Zhejiang Univ. Sci. B.. 2005;6:457-463.
- [Google Scholar]
- 22,23-Dihydrospinasterol and Fernenol from Citrullus Colocynthis L. with aphicidal activity against cabbage aphid Brevicoryne brassicae L. Molecules. 2020;25:2184.
- [Google Scholar]
- Changing role of insecticides in crop protection. Annu. Rev. Entomol.. 1980;25(1):219-256.
- [Google Scholar]
- Combinational effect of Rumex tingitanus (Polygonaceae) hexane extract and Bacillus thuringiensis endotoxin against Spodoptera littoralis (Lepidoptera: Noctuidae) BioMed Res. Int. 2018:1-7.
- [Google Scholar]
- Purification and characterization of an alkaline protease produced by the bacterium Xenorhabdus nematophila BA2, a symbiont of entomopathogenic nematode Steinernema carpocapsae. Res. J. Agric. Biol. Sci.. 2007;3:510-521.
- [Google Scholar]
- Sequence analysis of insecticidal gene from Xenorhabdus nematophila PME1296. Appl. Environ. Microbiol.. 2001;67:2062-2069.
- [Google Scholar]
- Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol.. 2006;51:45-66.
- [Google Scholar]
- Effects of thyme and rosemary essential oils on population growth parameters of Macrosiphum rosae (Hemiptera: Aphididae) on cut flower rose. J. Crop Prot.. 2018;7(1):51-63.
- [Google Scholar]
- Entomopathogenic bacteria, Xenorhabdus: an alternative biocontrol agent for integrated management of root-knot nematode on grapevine. J. Pure Appl. Microbiol.. 2019;13(3):1499-1508.
- [Google Scholar]
- Scope of botanical pesticides in integrated pest management. J. Insect Sci.. 1993;6:15-20.
- [Google Scholar]
- Chemical examination and insecticidal properties of Tagetes erecta and Tagetes patula. Asian J. Bio Sci.. 2010;5(1):29-31.
- [Google Scholar]
- Botanical biopesticide combination concept—a viable option for pest management in organic farming. Egypt. J. Biol. Pest Control. 2021;31(23):1-10.
- [Google Scholar]
- Insecticidal properties of Ocimum basilicum and Cymbopogon winterianus against Acanthoscelides obtectus, insect pest of the common bean (Phaseolus vulgaris L.) Insects. 2019;10(5):151.
- [Google Scholar]
- Evaluation of the susceptibility of the pea aphid, Acyrthosiphon pisum, to a selection of novel biorational insecticides using an artificial diet. J. Insect Sci.. 2009;9:65.
- [Google Scholar]
- Field evaluation of two indigenous coccinellids species released for controlling the rose aphid, Macrosiphum rosae (L.) on rose plants. Egypt. J. Biol. Pest Control. 2017;27(2):217-221.
- [Google Scholar]
- Preliminary molecular characterization and utilization of native Orius sp. (Hemiptera: Anthocoridae) for controlling aphids infesting Taif's rose. Arch. Phytopathol. Plant Protec.. 2012;45(3):373-380.
- [Google Scholar]
- Phytotoxicological evaluation of Tagetes erectes on aquatic stages of Anopheles stephensi. Indian J. Malariol.. 1994;31(1):21-26.
- [Google Scholar]
- Toxicity effects and biochemical changes of insecticide alternatives on cowpea aphid (Aphis craccivora)(Homoptera: Aphididae) Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control. 2019;11:129-138.
- [Google Scholar]
- When mutualists are pathogens: an experimental study of the symbioses between Steinernema (entomopathogenic nematodes) and Xenorhabdus (bacteria) J. Evol. Biol.. 2004;17:985-993.
- [Google Scholar]
- Experimental investigation on nutritional variation in plant foliage of rose (Rosa damascene): effect of pest infestation. Int. J. Sci. Res. Publication.. 2014;4(5):1-12.
- [Google Scholar]
- Toxicity of solvent extracts of some plant species from north- western Himalayan region against the cabbage aphid, Brevicoryne brassicae Linn. Pest Manage. Econ. Zool.. 2005;13:131-134.
- [Google Scholar]
- SPSS. IBM SPSS Statistics for Windows (Version 23.0); IBM Corp.: Armonk, NY, USA; Chicago, IL, USA, 2015.
- A review on prospective of synergistic approach in insect pest management. J. Entomol. Res.. 2011;35:255-266.
- [Google Scholar]
- Plant products as potential stored product insect management agents- A mini review. Emirates J. Food and Agri.. 2006;18(1):17-32.
- [Google Scholar]
- Bioefficacy of certain insecticidal formulation against bean aphid, Aphis craccivora Koch. Ann. Plant Prot. Sci.. 2002;10:136-137.
- [Google Scholar]
- Origin and evolution of carnivorism in the Ascomycota (fungi) Proce. Natl. Acad. Sci.. 2012;109:10960-10965.
- [Google Scholar]
- Colocynth: Potential arid land oilseed from an ancient cucurbit. Perspect. New Crop. New Uses. 1999;3:257-261.
- [Google Scholar]
- Repellent activities of some plant extracts against rose aphid, Macrosiphum rosae (Hemiptera: Aphididae) Munis Entomol. Zool.. 2021;16(2):1045-1055.
- [Google Scholar]







