Translate this page into:
Antiurolithiatic effect of Fucoxanthin on ethylene glycol-induced renal calculus in experimental rats
⁎Corresponding author. 15609288650tcf@sina.com (Chenfei Tian)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The urolithiasis/nephrolithiasis are a major health problem among the peoples, which could lead to severe clinical complications and kidney failure and most men were affects than women. Fucoxanthin, is an active compound present in brown seaweeds, and has the numerous pharmacological benefits. Present investigation designed to assess the anti-urolithiatic role of Fucoxanthin against ethylene glycol (EG) stimulated calculi in experimental rats. Adult male albino rats were segregated evenly into five groups. Group I: Normal Vehicle. Group II: 0.75% ethylene glycol (EG) in potable water for 4 weeks. Group III: 0.75% EG in potable water + Fucoxanthin (40 mg/kg bodyweight) was administered through orally for 4 weeks. Group IV: 0.75% EG in potable water + Fucoxanthin (80 mg/kg body weight) was supplemented orally for 4 weeks. Group V: 0.75% EG in potable water + Standard drug Cystone (750 mg/kg bodyweight) was supplemented orally for 4 weeks. The rat urine was collected before the anaesthesia. The animals were generally anaesthetized and killed by the cervical dislocation. Kidneys and blood (Plasma) samples were collected and processed for further investigations. The biochemical (BC) markers, renal, stone markers, antioxidants levels and Lipid peroxidation were analysed using plasma and kidney tissue homogenate of both control and investigational animals. Our results revealed that the elevated levels of biochemical markers were observed in lithiatic rats. The renal and stone markers were abnormal in lithiatic rats. The antioxidants were markedly decreased and lipid peroxidation (LPO) was increased in lithiatic rats. The histopathological study confirmed that the Fucoxanthin treatment (80 mg/kg body weight) prevented the damage of tubular membrane and reduced the deposition of calculi in glomeruli of the kidney tissue. Fucoxanthin treatment normalized the biochemical and renal stone markers in the experimental rats. Fucoxanthin treatment restores the antioxidants and decreased the LPO in experimental rats by its anti-urolithiatic properties.
Keywords
Calcium oxalate
Creatinine
Urea
Kidney stone
Ethylene glycol
Fucoxanthin
Urolithiasis
1 Introduction
Renal stone/urinary calculi are a commonest condition, occurring 12 in 100 of the global population (Alelign and Petros, 2018). A incidences of calculi was amplified due to the western dietary habits, environment factors and poor behaviour/lifestyle of this age group (Gindi et al., 2013). The calcium stones which present inside the region of the kidney and/or urinary tract are also called as urolithiasis/nephrolithiasis. Nearly 80% of the calculi were formed with the mixture of both calcium oxalate and phosphate (Aggarwal et al., 2013). Urolithiasis develops in humans, but the occurring risk is significantly higher in men (12 in 100) than the women (6 in 100) and it was highly occurred between 20 and 40 aged group peoples (Khan et al., 2016). The calculi are removed and cleared by the surgical, lithotripsy and high-power laser techniques. It is quite expensive and recurrence is also common due to lack of preventive treatment (Durner et al., 2016). The recurrence rate of calculi is significantly increased by 10% (1 year), 33% (5 years) and 50% (10 years) due to lack of preventive treatment. It was reported that the urolithiasis was also associated with other clinical complications like abscess, uro-sepsis, ureteral scarring, urine extravasation and kidney atrophy in some cases (Leslie et al., 2018). Currently, suitable treatments in advanced medicine are not available for the urolithiasis management (Gouru et al., 2018). Allopathic treatment with thiazide diuretics and alkali citrate were given as a preventive therapy but it cannot able to dissolve the calculi (Zisman, 2017).
The urolithiasis/nephrolithiasis suffering patients were treated with surgical procedures which are invasive and expensive. The calculi can be broken down with the help of percutaneous nephrolithotomy (PCNL) and extracorporeal shock Wave Lithotripsy (ESWL) techniques (Aboelkher et al., 2017). These techniques are less comfortable to patients and it will cause adverse effects like haemorrhage, tubular necrosis and fibrosis to the kidney (Yousefi Ghale-Salimi et al., 2018). Those treatment procedures are expensive and the patients should have to follow up for long period. So there is an urgent need to invent some more clinically advanced anti-urolithiatic drugs that to halt the recurrences, avert side effects and cost effective (Liu et al., 2018). The world health organization (WHO) also showing interest towards the usage of herbal drugs/traditional medicines due to easy availability, minimal cost and very low side effects. Cystone, a polyherbal formulation was developed based on the reference found in the ancient Ayurvedic system of medicine and widely utilized for an era to treat the urinary/renal calculi (Hiremath and Jalalpure, 2016).
Fucoxanthin is an active marine carotenoid which predominantly available in the macroalgae and microalgae (Kim et al., 2012). Fucoxanthin acts as a medicinal/nutritional compound to avert and treat chronic kidney disorder/diseases (Peng et al., 2011). Several studies confirmed that the fucoxanthin possesses medicinal properties but structure was relatively unstable in nature. Fucoxanthin could be a potential antioxidant due to exclusive chemical structure possesses an allenic bond, hydroxyl (OH) and epoxide group (Zhang et al., 2015). Fucoxanthin is metabolized to form fucoxanthinol and then the fucoxanthinol converts to form amarouciaxanthin a in stomach, but the unchanged fucoxanthin was seen in plasma and liver of the rodents (Asai et al., 2004). The numerous studies proved that the fucoxanthin possessed anti-obesity, anti-inflammatory, anti-diabetes, anti-cancer and hepatoprotective activities. Fucoxanthin is potential to prevent renal cell apoptosis (Zhang et al., 2015). There is no preceding research concerning the antiurolithic potency of fucoxanthin in vivo. Current investigation intended to examine the antiurolithic effectual of fucoxanthin in an ethylene glycol (EG)-stimulated urolithiasis model and the role of fucoxanthin in the prevention of renal stones. In our study we used cystone as a standard drug to compare with the fucoxanthin.
2 Materials and methods
2.1 Chemicals and reagents
Fucoxanthin (Cat#F6932) and ethylene glycol (EG) (Cat#324558) was obtained from Sigma chemicals, USA. All the Biochemical and diagnostic kits and were procured from ERBA Diagnostics, Germany. The standard drug herbal formulation cystone was bought from Himalaya Drug Company, India. The chemicals and reagents used were of diagnostic grade.
2.2 Animals
Adult male albino wistar rats were procured from institutional animal house facility and it were acclimatized for one week in a standard laboratory condition with 12 h dark and light cycles, 60% humidity and at a temperature of 25 ± 3 °C. The rats were housed in sterile plastic cages and fed ad libitum with sterile standard rat food pellets, reverse osmosis water. The animal handling and experiments were followed according to the Animal ethical guidelines. All the experimental animals were provided with pathogen free standard pellet diet and water ad libitum throughout the research study.
2.3 Experimental protocol
Thirty albino wistar rats were equally distributed into five groups. Six wistar rats were used in each experimental group. Group I: Control rats provided with pathogen free standard pellet diet. Group II: The experimental rats received ethylene glycol (0.75%) in potable water for 4 weeks to develop calculi in the kidney. Group III: The experimental rats received EG (0.75%) in drinking water along with the oral supplementation of fucoxanthin (40 mg/kg bodyweight) for 4 weeks. Group IV: The experimental rats received EG (0.75%) in potable water along with the oral supplementation of fucoxanthin (80 mg/kg body weight for 4 weeks. Group V: The experimental rats received EG (0.75%) in potable water along with the oral administration of standard drug cystone, (750 mg/kg body weight) for 4 weeks. The urine samples aseptically collected in the metabolic cages on 28th day of experimental period, before killing the experimental rodents. Then the animals were anesthetized and killed by dislocating the cervical spine. The blood samples and kidneys were collected for the analysis.
2.4 Collection and analysis of urine
The urine samples of each rat were gathered using metabolic cages on the 28th day of the experimental period. The urine were subjected to spinning at 3000 rpm for 5 min to eliminate the debris particles and the supernatants were stored at −20 °C until further investigations. The urine crystal deposition score was investigated in the supernatant sample of control and experimental rats using light microscope at the 40× magnification.
2.5 Collection and analysis of plasma
The blood was gathered from retro-orbital sinus of each experimental rat under anaesthetic condition. The blood was heparinized and the plasma was separated via spinning the tube at 3000 rpm for 10 min. The plasma was used to quantify the aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT) and (lactate dehydrogenase) LDH using commercial kits (Erba Diagnostics Mannheim, GmbH, Germany).
2.6 Kidney tissue collection and sample preparation
The kidneys were excised and rinsed with ice cold 0.15 M potassium chloride. Then the kidneys were dried and weighed. Kidney homogenate was prepared using 0.15 M KCl using homogenizer. The homogenate was centrifuged at 3000 rpm for 15 min. The supernatant was collected and stored at −80 °C for further investigations.
2.7 Determination of biochemical parameters
The kidney tissue supernatant and urine supernatant were used to estimate the biochemical (BC) parameters such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT) and (lactate dehydrogenase) LDH using commercial kits (Erba Diagnostics Mannheim, GmbH, Germany).
2.8 Quantification of renal stone markers
The kidney tissue supernatant was used to quantify the renal stone markers like calcium, oxalate, uric acid, magnesium, phosphorous, urea and creatinine via commercial kits (Erba Diagnostics Mannheim, GmbH, Germany).
2.9 Determination of antioxidant and lipid peroxidation
The kidney tissue supernatant was used to quantify the antioxidants such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), reduced glutathione (GSH) and lipid peroxidation (LPO) via commercial kits (Erba Diagnostics, Germany).
2.10 Histopathology
After formalin (10%) fixation and dehydration process, paraffin-embedded kidney tissue sections (4 µm) were stained with haematoxylin and eosin. The stained kidney tissues were examined using light microscope at 400× magnification.
2.11 Statistical analysis
All the values were portrayed as mean ± SEM (standard error mean). The data were investigated using one-way ANOVA Duncan method using SPSS 22 version software. The values of p ≤ 0.05 were considered as statistically significant.
3 Results
3.1 Fucoxanthin reduced the urine crystal deposition score in experimental rats
Fig. 1 represents the microscopic evaluation of urine crystal deposition in control and investigational rats. The crystal was not noted in the urine sample of control rats (Group I). Numerous crystals were noticed in the urine sample of lithiatic rats (Group II). The crystal formation was reduced in the 40 mg/kg fucoxanthin treated groups (Group III). The crystal formation was significantly reduced in the 80 mg/kg fucoxanthin treated groups (Group IV). The crystal formation was also significantly reduced in the 750 mg/kg standard approved drug cystone treated groups. In this study 80 mg/kg fucoxanthin treatment reduced the 70% of crystal formation and deposition.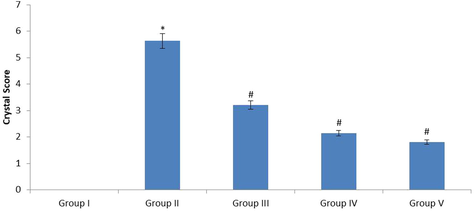
The urine crystal deposition score was analysed in the urine sample of control and experimental rats. The severity grade of crystal formation was scored as 0 = <1 crystal, 1 = ≥1–10, 2 = ≥11–30, 3 = ≥31–50, 4 = ≥51–75 and 5 = >75 crystals. The values were expressed as Mean ± SEM. *p < 0.05, #p < 0.01. Group I: Healthy Control, Group II: 0.75% EG induced renal lithiasis, Group III: 0.75% EG + 40 mg/kg Fucoxanthin, Group IV: 0.75% EG + 80 mg/kg Fucoxanthin, Group V: 0.75% EG + 750 mg/kg standard drug Cystone.
3.2 Effect of fucoxanthin on biochemical parameters in the plasma of experimental rats
Fig. 2 represents the positive outcome of fucoxanthin on biochemical (BC) parameters in plasma of control and investigational groups. The AST, ALT, ALP, GGT and LDH was noticeably (p ≤ 0.05) elevated in the EG treated lithiatic rats (Group II) when correlated with normal control rats (Group I). The fucoxanthin 40 mg/kg treatment appreciably (p ≤ 0.01) declined the augmentation of BC markers when correlated with lithiatic rats (Group II). The fucoxanthin 80 mg/kg treatment significantly (p ≤ 0.01) declined the elevation of BC markers when correlated with lithiatic rats (Group II). The standard approved drug cystone 750 mg/kg treatment significantly (p ≤ 0.01) declined the elevation of BC markers when correlated with lithiatic rats (Group II). In this study 80 mg/kg fucoxanthin treatment showed more effective against lithiatic rats.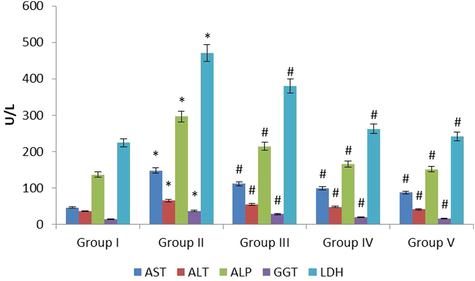
The anti-urolithiatic effect of fucoxanthin on plasma biochemical markers in control and experimental rats. The values were expressed as Mean ± SEM. *p < 0.05, #p < 0.01.Group I: Healthy Control, Group II: 0.75% EG induced renal lithiasis, Group III: 0.75% EG + 40 mg/kg Fucoxanthin, Group IV: 0.75% EG + 80 mg/kg Fucoxanthin, Group V: 0.75% EG + 750 mg/kg standard drug Cystone.
3.3 Effect of fucoxanthin on biochemical parameters in the kidney tissues of experimental rats
Fig. 3 represents the effect of fucoxanthin on biochemical (BC) parameters in renal tissue homogenate of control and investigational rodents. The AST, ALT, ALP, GGT and LDH were drastically (p ≤ 0.05) escalated in the EG treated lithiatic rats (Group II) when correlated with normal control rats (Group I). The fucoxanthin 40 mg/kg (Group III) treatment significantly (p ≤ 0.01) declined the elevation of BC markers when correlated with lithiatic rats (Group II). The fucoxanthin 80 mg/kg (Group IV) treatment remarkably (p ≤ 0.01) declined the elevation of BC markers when correlated with lithiatic rats (Group II). The standard approved drug cystone 750 mg/kg (Group V) treatment significantly (p ≤ 0.01) declined the elevation of BC markers when correlated with lithiatic rats (Group II). In this study 80 mg/kg fucoxanthin treatment showed more effective on BC parameters on experimental models.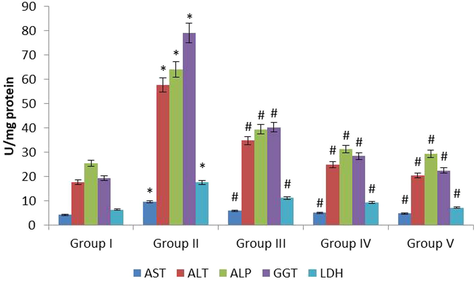
The anti-urolithiatic effect of fucoxanthin on renal biochemical markers in control and experimental rats. The values were expressed as Mean ± SEM. *p < 0.05, #p < 0.01.Group I: Healthy Control, Group II: 0.75% EG induced renal lithiasis, Group III: 0.75% EG + 40 mg/kg Fucoxanthin, Group IV: 0.75% EG + 80 mg/kg Fucoxanthin, Group V: 0.75% EG + 750 mg/kg standard drug Cystone.
3.4 Effect of fucoxanthin on renal and stone makers of kidney in the experimental rats
Fig. 4 represents the effect of fucoxanthin on renal and stone markers in renal tissue homogenate of preclinical models. The calcium, oxalate, creatinine, urea were raised (p ≤ 0.05) and the uric acid, magnesium and inorganic phosphorous were (p ≤ 0.05) declined in the EG treated lithiatic rats (Groups II) when correlated with normal control rats (Group I). The concentration of calcium, oxalate, creatinine, urea were (p ≤ 0.01) declined and the concentration of uric acid, magnesium and inorganic phosphorous were (p ≤ 0.01) exalted in the fucoxanthin (40 mg/kg) treated rats (Group III) when correlated with EG treated lithiatic rats (Group II). The concentration of calcium, oxalate, creatinine, urea were (p ≤ 0.01) decreased and the concentration of uric acid, magnesium and inorganic phosphorous were (p ≤ 0.01) increased in the fucoxanthin (80 mg/kg) treated rats (Group IV) when correlated with EG treated lithiatic rats (Group II). The concentration of calcium, oxalate, creatinine, urea were (p ≤ 0.01) decreased and the concentration of uric acid, magnesium and inorganic phosphorous were (p ≤ 0.01) increased in the fucoxanthin (80 mg/kg) treated rats (Group IV) when correlated with EG treated lithiatic rats (Group II). The concentration of calcium, oxalate, creatinine, urea were (p ≤ 0.01) decreased and the concentration of uric acid, magnesium and inorganic phosphorous (p ≤ 0.01) increased in the standard cystone (80 mg/kg) treated rats (Group V) when correlated with EG treated lithiatic rats (Group II). The 80 mg/kg fucoxanthin treatment potentially regulates the renal stone markers in the experimental models.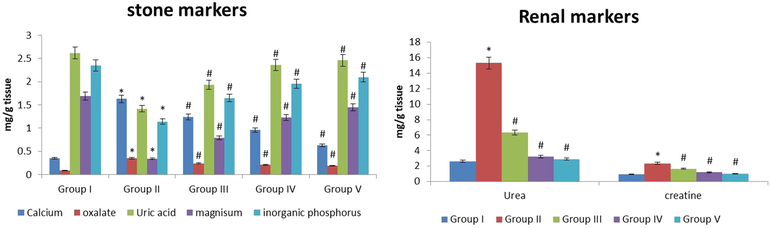
The anti-urolithiatic effect of fucoxanthin on renal stone markers in control and experimental rats. The values were expressed as Mean ± SEM. *p < 0.05, #p < 0.01. Group I: Healthy Control, Group II: 0.75% EG induced renal lithiasis, Group III: 0.75% EG + 40 mg/kg Fucoxanthin, Group IV: 0.75% EG + 80 mg/kg Fucoxanthin, Group V: 0.75% EG + 750 mg/kg standard drug Cystone.
3.5 Effect of fucoxanthin on antioxidants and lipid peroxidation level in the experimental rats
Fig. 5 represents the effect of fucoxanthin on antioxidants in kidney tissue homogenate of control and experimental models. The superoxide dismutase, catalase, glutathione peroxidase, reduced glutathione were (p ≤ 0.05) lowered and lipid peroxidation was (p ≤ 0.05) inclined in the EG treated lithiatic rats (Group II) when correlated with normal control rats (Group I). The levels of anti-oxidants were (p ≤ 0.01) increased and LPO was (p ≤ 0.01) declined in the fucoxanthin (40 mg/kg) treated rats (Group III) when correlated with EG treated lithiatic rats (Group II). The anti-oxidants were (p ≤ 0.01) inclined and LPO was (p ≤ 0.01) declined in fucoxanthin (80 mg/kg) treated rats (Group IV) when correlated with EG treated lithiatic rats (Group II). The anti-oxidants were (p ≤ 0.01) inclined and Lipid peroxidation was (p ≤ 0.01) lowered in the standard cystone (80 mg/kg) treated rats (Group V) when correlated with EG treated lithiatic rats (Group II). The 80 mg/kg fucoxanthin treatment increased the activities of antioxidants and reduced the lipid peroxidation in the experimental models.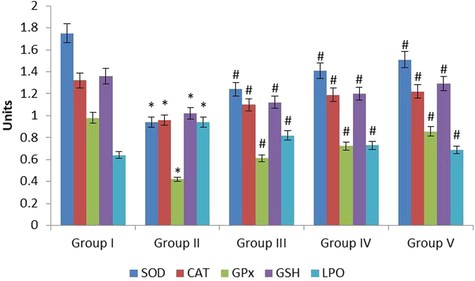
The anti-urolithiatic effect of fucoxanthin on antioxidants and LPO in control and experimental rats. The values were expressed as Mean ± SEM. *p < 0.05, #p < 0.01. Group I: Healthy Control, Group II: 0.75% EG induced renal lithiasis, Group III: 0.75% EG + 40 mg/kg Fucoxanthin, Group IV: 0.75% EG + 80 mg/kg Fucoxanthin, Group V: 0.75% EG + 750 mg/kg standard drug Cystone.
3.6 Effect of fucoxanthin on renal histopathology of experimental rats
Fig. 6 represents the effect of fucoxanthin on histopathological analysis of kidney tissue in control and investigational groups. The normal renal arrangement and absence of calcium crystal deposition was noticed in the control rats (Fig. 6A). The large crystals were noticed in lithiatic rats treated with EG alone. The damaged renal parenchyma, degeneration of glomeruli, dilation of renal tubules and clogged blood vessels were noticed in the kidney tissue of EG treated lithiatic rats (Fig. 6B). The fucoxanthin (40 mg/kg) treated rats kidney tissue showed mild calcification (glomeruli) but calcification were not spotted in tubular structures (Fig. 6C). The fucoxanthin (80 mg/kg) treated rats kidney tissue showed complete prevention of calculi generation in glomeruli and tubular structures of both the kidneys. The blood vessels and connective tissues were visibly normal (Fig. 6D). The renal tissue of standard approved drug cystone (750 mg/kg) treated rats showed absence of calcification in many tubules and glomeruli (Fig. 6E). The effects of fucoxanthin 80 mg/kg were equally analogous with the standard drug treatment.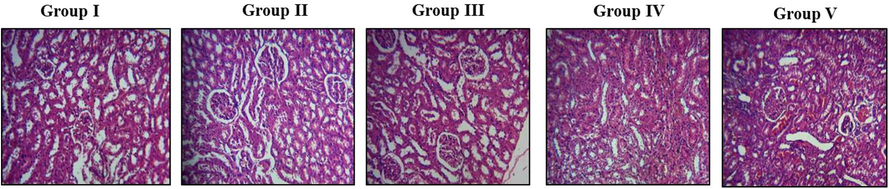
The anti-urolithiatic effect of fucoxanthin on histopathological alterations in control and experimental rats. Group I: Healthy Control, Group II: 0.75% EG induced renal lithiasis, Group III: 0.75% EG + 40 mg/kg Fucoxanthin, Group IV: 0.75% EG + 80 mg/kg Fucoxanthin, Group V: 0.75% EG + 750 mg/kg standard drug Cystone.
4 Discussion
Urolithiasis is the 3rd most and common disorder of the urologic system, which occurs in the both male and female (Suman et al., 2017). Almost 80% of the calculi are the mixture of calcium, phosphates and oxalates which locates in the region of the renal and urinary tract (Courbebaisse et al., 2017). The calculi occur in the humans at the age between 30 and 50 years due to improper diet, lack of water intake and poor lifestyle (Kishore et al., 2013). Natural products based medications have been extensively used to dissolve the calculi in the past decades. Fucoxanthin is a bioactive compound present in the marine seaweed. It used as a classical medicine in ancient times to prevent and to treat the chronic disorders in humans (Kim et al., 2012). So, we used study the antiurolithiatic effect of fucoxanthin on EG induced calculi in rodent models. In our research study, we planned to develop urolithiasis because the urologic patterns of male wistar rats are identical to humans.
The ethylene glycol is absorbed by the experimental rat which easily develops urolithiasis/nephrolithiasis by the activity of liver and kidney enzyme LDH (Goyal et al., 2017). The levels of biochemical markers were highly elevated in the EG induced lithiatic rats (Vasanthi et al., 2017). The fucoxanthin (80 mg/kg) treatment potentially reduced the activities of plasma and kidney biochemical markers in the lithiatic rats. It proved that the fucoxanthin treatment prevent the kidney damage by normalized the biochemical markers. Our study confirmed that the EG toxicity forms the calculi in the kidneys of the investigational rats. The oxalates and calcium precipitates to form calculi which seriously injure the outer lining of renal tubules which directs to the deposition of crystals (Cunningham et al., 2016).
The calculi caused damage to the renal tubules, reduced the glomerular filtration, block the flow of urine and accumulate the nitrogenous waste in the blood stream (Pareta et al., 2011; Rathod et al., 2012). Likewise, in our study histological slides revealed that the calcification was observed in the glomeruli and tubules of the kidney. Fucoxanthin (80 mg/kg) treatment inhibits the calcification in the glomeruli and tubules of the kidney. Moreover it prevents the damage to blood vessels and glomeruli of the kidneys. Previous studies revealed that the EG administration increased the levels of urea, creatinine and uric acid in the renal tissue of rodent models (Ahmed et al., 2016). Our study proved that the EG toxicity significantly elevated the renal markers such as urea and creatinine in the renal tissue of the rats. In contrast, the level of uric acid was decreased in the EG treated rat models. The fucoxanthin (80 mg/kg) treatment regulates the renal markers such as uric acid, urea and creatinine in the lithiatic rats. The phosphorous and calcium act as an initiating factor while magnesium restricts the formation of calculi in the renal tubules (Penniston and Nakada, 2018). Our study report confirmed that the calcium was markedly increased in the renal tissue of EG induced lithiatic rats, but declined inorganic phosphorous and magnesium was observed. Fucoxanthin (80 mg/kg) treatment restricts the accumulation of calcium and oxalate (calculi) by inhibiting the activity of LDH in the lithiatic rats. The elevated levels of phosphorus was seen the urolithiatic patients (Evan et al., 2015).
In contrast, our study revealed that the inorganic phosphorus was declined in the experimental rat models. Fucoxanthin (80 mg/kg) treatment restores the statuses of phosphorus and magnesium in the lithiatic rats. Numerous studies stated that the levels of superoxide dismutase, catalase, reduced glutathione and glutathione peroxidase were diminished in the EG induced lithiatic rats. The levels of LPO were markedly increased in the EG induced lithiatic rats (Abhirama and Shanmuga Sundaram, 2018; Jagannath et al., 2012; Li et al., 2015; Panigrahi et al., 2017; Shirfule et al., 2013). Our research lined with the previous studies, fucoxanthin (80 mg/kg) treatment markedly augmented the activity of SOD, Catalase, GSH, GPx and declined the levels of LPO in the EG induced lithiatic rats. The fucoxanthin treatment prevents the calcification in the glomeruli and tubules of the kidney by suppressing the LDH, which has the antiurolithiatic potential against EG induced calculi in the rodent models. Most of the previous studies done in the medicinal plant extracts (Pawar and Vyawahare, 2017; Ingale et al., 2012; Bano et al., 2018) and there are very limited reports claims the antiurolithiatic efficacy of the bioactive compounds (Grases et al., 2015; Ghodasara et al., 2010). In the present study, it was evidently proved that the fucoxanthin was appreciably attenuated the ethylene glycol induced kidney stone formation in experimental animals, which highlighting the therapeutic supremacy of the fucoxanthin.
5 Conclusion
We concluded that the present research study explored that the fucoxanthin treatment exhibited more significant antiurolithiatic properties against Ethylene glycol induced calculi in rodent models. Fucoxanthin (80 mg/kg) treatment restored the antioxidants levels in the EG induced calculi in lithiatic rats. It also regulates the stone and renal markers in the lithiatic rats. Fucoxanthin treatment prevents the damage of glomeruli and tubules of kidney in the lithiatic rats. However, further molecular study is required to explore the anti-urolithiatic potential of fucoxanthin in preclinical models.
Acknowledgements
This work was supported by Researchers Supporting Project (RSP-2019/36), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antiurothilic and antioxidant activity of ethanol extract of whole-plant Biophytum sensitivum (Linn.) DC in ethylene-glycol-induced urolithiasis in rats. Phcog. Res.. 2018;10:181-187.
- [Google Scholar]
- Percutaneous nephrolithotomy versus extracorporeal shock wave lithotripsy for moderate-sized kidney stones. Menoufia Med. J.. 2017;30:372-377.
- [Google Scholar]
- Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed. Res. Int. 2013
- [Google Scholar]
- In vitro urolithiasis models: an evaluation of prophylactic management against kidney stones. J. Pharmacog. Phytochem.. 2016;5(3):28-35.
- [Google Scholar]
- Biotransformation of fucoxanthinol into amarouciaxanthin a in mice and HepG2 cells: formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Disposition. 2004;32(2):205-211.
- [Google Scholar]
- Effect of Piper cubeba L. fruit on ethylene glycol and ammonium chloride induced urolithiasis in male Sprague Dawley rats. Integrative Med. Res.. 2018;7(4):358-365.
- [Google Scholar]
- Nephrolithiasis of adult: from mechanisms to preventive medical treatment. Rev. Med. Int.. 2017;38(1):44-52.
- [Google Scholar]
- Kidney stones: pathophysiology, diagnosis and management. British J. Nurs.. 2016;26(20):1112-1116.
- [Google Scholar]
- Current trends in urolithiasis treatment in various European health systems. Urol. Int.. 2016;96:125-131.
- [Google Scholar]
- Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacogn. Res.. 2010;2:388-392.
- [Google Scholar]
- Antiurolithiatic and invitro anti-oxidant activity of leaves of Ageratum conyzoides in rat. World J. Pharm. Pharm. Sci.. 2013;2:636-649.
- [Google Scholar]
- Antilithiatic potential of Vernonia cinerea against calcium oxalate calculi in experimental rats. J. Phytopharmacol.. 2017;6(2):149-155.
- [Google Scholar]
- Effects of polyphenols from grape seeds on renal lithiasis. Oxid. Med. Cell Longev.. 2015;2015:813737
- [Google Scholar]
- Effect of hydro-alcoholic extract of Vernonia cinerea Less. Against ethylene glycol-induced urolithiasis in rats. Indian J. Pharmacol.. 2016;48:434-440.
- [Google Scholar]
- Effect of Hygrophila spinosa in ethylene glycol induced nephrolithiasis in rats. Indian J. Pharmacol.. 2012;44:639-642.
- [Google Scholar]
- Study of antiurolithiatic activity of Asparagus racemosus on albino rats. Indian J. Pharmacol.. 2012;44:576-579.
- [Google Scholar]
- A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol.. 2012;166(7):1843-1855.
- [Google Scholar]
- Effect of ethanolic extract of Portulaca oleracea Linn. On ethylene glycol and ammonium chloride induced urolithiasis. Int. J. Pharm. Pharmaceut. Sci.. 2013;5(2):134-140.
- [Google Scholar]
- Leslie, S.W., Sajjad, H., Murphy, P.B., 2018. Renal Calculi. Books (NCBI), 1–7.
- Potential mechanisms responsible for the antinephrolithic effects of an aqueous extract of Fructus aurantii. Evid. Based Complement Alternat. Med.. 2015;2015:491409
- [Google Scholar]
- Inhibition of autophagy attenuated ethylene glycol induced crystals deposition and renal injury in a rat model of nephrolithiasis. Kidney Blood Press Res.. 2018;43:246-255.
- [Google Scholar]
- Antiurolithiatic and antioxidant efficacy of Musaparadisiaca pseudostem on ethylene glycol-induced nephrolithiasis in rat. Indian J. Pharmacol.. 2017;49:77-83.
- [Google Scholar]
- Aqueous extract of Boerhaavia diffusa root ameliorates ethylene glycol-induced hyperoxaluric oxidative stress and renal injury in rat kidney. Pharm. Biol.. 2011;49(12):1224-1233.
- [Google Scholar]
- Protective effect of ethyl acetate fraction of Biophytum sensitivum extract against sodium oxalate-induced urolithiasis in rats. J. Tradit. Complement Med.. 2017;7(4):476-486.
- [Google Scholar]
- Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar. Drugs. 2011;9(10):1806-1828.
- [Google Scholar]
- Updates in the metabolic management of calcium stones. Curr. Urol. Rep.. 2018;19(6):41.
- [Google Scholar]
- Anti-urolithiatic effects of Punica granatum in male rats. J. Ethnopharmacol.. 2012;140(2):234-238.
- [Google Scholar]
- Exploring antiurolithic effects of gokshuradi polyherbal ayurvedic formulation in ethylene-glycol-induced urolithic rats. Evid. Based Complement Alternat. Med.. 2013;2013:763720
- [Google Scholar]
- Antiurolithiatic activity of nerium oleanderon ethylene glycol induced nephrolithiasis in rats. IJCMPR. 2017;3:1317-1321.
- [Google Scholar]
- Antiurolithiatic effect of Sirupeelai Samoola Kudineer. A polyherb siddha decoction on ethylene glycol-induced renal calculus in experimental rats. Phcog. Mag.. 2017;13:273-279.
- [Google Scholar]
- Urolithiasis. 2018;46:419.
- Fucoxanthin: a promising medicinal and nutritional ingredient. Evid. Based Complement Alternat. Med.. 2015;2015:723515
- [Google Scholar]
- Effectiveness of treatment modalities on kidney stone recurrence. CJASN. 2017;12(10):1699-1708.
- [Google Scholar]







