Antitumour and anti-haematotoxic activity of Asparagus racemosus L total dissolved solids in co-administration with cyclophosphamide in mice
⁎Corresponding authors. binilbharathan@gmail.com (P.B. Benil), arokiyaraj16@korea.kr (Selvaraj Arokiyaraj)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cancer is the second largest killer in the world. Even though the treatment and diagnosis of cancers have increased manifold, the survival rate has not increased proportionately. This discrepancy is mainly due to the toxicity of the chemotherapeutic agents. Natural herbs possessing significant antitumour activity can be utilized to address this issue. Asparagus racemosus is a widely distributed medicinal plant having reported antitumor properties. A concomitant use of the aqueous extract of Asparagus racemous (AEAR) along with Cyclophosphamide (CTX) was studied for the reversal of myelosuppression and hemotoxicity induced by CTX and to test whether AEAR possess significant antitumor activity against Dalton’s Lymphoma Ascites (DLA) tumour in BALB/c mice model. The study consisted of preliminary physico-chemical character determination and phytochemical screening. AEAR was used at 216, 432 and 864 g/kg body weight doses designated as lower, medium and high doses respectively and CTX was used at 25 mg/kg body weight. Hemogram, total leucocyte count, differential leucocyte count, bone marrow cellularity and – esterase positive cells were assessed. Malondialdehyde (MDA), glutathione, superoxide dismutase (SOD) and catalase (CAT) levels were estimated as oxidative stress markers. Tumour volume, size, mean survival time, increase in life span and in vitro cytotoxicity assays involving trypan blue exclusion test and MTT assay were done for antitumor study. AEAR showed significant elevation in hemogram, total leucocyte count, differential count, bone marrow cellularity and -esterase positive cell activity. AEAR at medium and high dose levels showed significant elevation in anti-oxidant enzyme levels of MDA, Glutathione, SOD and CAT enzymes. They also reduced the tumour volume, size and significantly increased the life span and mean survival time of DLA bearing mice. In in vitro cytotoxicity tests also, the medium and high dose of AEAR showed significant cytotoxicity. AEAR at doses of 432 and 864 mg/kg body weight doses significantly reverses the myelosuppression and hemotoxicity. It enhances the levels of antioxidant enzymes and is also an antitumor agent and exerts significant cytotoxicity against DLA tumour cells. Asparagus racemosus provides a ray of hope in addressing the toxicity while using chemotherapeutic agents when used as an adjuvant therapy.
Keywords
Antitumor
Cyclophosphamide
Asparagus racemosus
DLA tumour
Antioxidant
1 Introduction
Cancer still reigns supreme due to its alarming spread and fatality even after great advances in its diagnosis and treatment are made (Murthy and Mathew, 2004). Cancers are preventable and by concentrating on the modifiable risk factors like chronic tobacco use, disturbances in energy balance, diet, infections, alcohol, obesity and exposure to exogenous hormones can increase the survivorship (Arem and Loftfield, 2018). Once a cancer is diagnosed, the treatment choices are either to undergo surgery, radiotherapy or chemotherapy or a combination of these (Pazdur et al., 2003). The advances in the armamentarium against cancer include the use of strong chemotherapeutic agents coupled with immunological and biological agents. Indeed, the survival rate attained by cancer patient’s world over has increased manifold, but with many side effects and toxicities. Toxicity related to chemotherapeutic agents unglorifies the advances made in the field of cancer therapeutics due to the deteriorated quality of life of the patients. Several chemotherapeutic agents provoke myelosuppression and related haemato-toxicities in the patients. The myelosuppression resulting from chemotherapy includes diversion from the normal accepted ranges for the haematological parameters and haematocrit values. This departure from normalcy in blood values is termed haemato-toxicity in general. Cyclophosphamide (CTX) is one such chemotherapeutic agent which is widely used in the treatment of solid tumours and is a strong alkylating agent belonging to the nitrogen mustard class. It has the disadvantage of myelosuppression coupled with immunosuppression (Mythili et al., 2004). Survival of the patient from the cancers subsequent to treatment with these agents may end up with the patient having severe immuno-depletion resulting from the loss of innate immune responses, viz, lymphocytes and neutrophils leading to much serious problem of opportunistic infections boomeranging the benefits of chemotherapy. The answer to this issue has been sought for from the rich natural medicinal herbs. The array of natural phyto-constituents has the capability to act in multiple levels against cancers. They have cytotoxic, anti-angiogenic, anti-metastatic and pro-apoptotic properties that makes them the most popular among the natural antitumor drugs (Desai et al., 2008). Asparagus racemosus is a widely used medicinal plant indicated for haematological dyscrasias in traditional medical systems like Ayurveda. Here we are investigating the capability of its aqueous extract as total dissolved solids (TDS) in reversing the myelosuppression and haematoxicity induced by CTX and also to study their antitumour activity against Dalton’s Lymphoma Ascites (DLA) in mice.
2 Materials and methods
2.1 Collection of plant material
Fresh tubers were collected from mature plants growing at Kottakkal (11.0001°N and 75.9988°E), Kerala, India. The tubers were washed to remove all the foreign matter and were mopped dry and split vertically to remove the central ‘rib’ and was then dried under shade. The dried tubers were pulverised and reduced to powder straining through a mesh of size 80. The powder was stored in air tight glass vials till the experiments begun.
2.2 Determination of physico-chemical characters
The physico-chemical parameters of the drug were determined following the standard procedures as explained in the Ayurvedic Pharmacopoeia of India (The Ayurvedic Pharmacopoeia of India, 2008).
2.3 Preparation of aqueous extract
Aqueous extract of Asparagus racemosus (AEAR) was made following the conventional method of preparing a decoction as explained in Ayurvedic Formulary of India. 96 g of the drug powder was boiled in 16 times quantity of water (1536 ml) and was reduced to 1/8th (192 ml). The decoction was strained through a muslin cloth to remove the entire drug from the decoction. The strained decoction was again concentrated under reduced temperature and pressure in a rotary evaporator to obtain a sticky concentrate. The concentrate was then dried on a water bath to obtain dried total dissolved solids (TDS) of the drug. The TDS was powdered and stored in glass vials and kept freezed at −70 °C till the study started.
2.4 Preliminary phytochemical screening
The AEAR was subjected to qualitative tests to determine the major groups of phytochemicals present in them as per the standard procedures (Harborne, 1984).
2.5 Experimental animals
Male BALB/c mice weighing 25 ± 2.3 g were utilized for the study. The animals were procured from the Small Animal Breeding Station, Kerala Veterinary and Animal Sciences University, Thrissur. The animals were housed in standard mice cages with rice husk bedding and were maintained at 25 ± 3.8 °C with a relative humidity of 65 ± 6.5%- and 12-hour dark and light cycle. The animals were fed with standard mouse feed (Lipton India Pvt. Ltd.) and were provided with tap water ad libitum. All experiments were carried out at Amala Cancer Research Centre, Thrissur after obtaining permission from the Institutional Animal Ethics Committee (No: ACRC/IAEC/2016[7]).
2.6 Determination of haemo-protective effect
Twenty-five adult male BALB/c mice weighing 25 ± 5.2 g were taken and divided in to five groups of 5 animals each. Group 1 served as the Normal animals receiving regular diet and Group 2 received Cyclophosphamide (CTX) at 25 mg/kg body weight as intra-peritoneal injection. Group 3 to 5 received AEAR at doses 216, 432 and 864 mg/kg body weight orally along with CTX and they were designated as the Low dose, medium dose and high dose groups respectively. AEAR was given orally through an oral gavage at their respective doses for 15 days. All animals were euthanized after 15 days using ether anaesthesia. Necropsy was performed on the animals and thymus, spleen, liver, bladder and kidney were taken and weighed. Blood was collected from heart puncture and was used for determination of Haemoglobin, Total RBC count, Total WBC count, Total platelet count, Differential count and packed cell volume. Bone marrow cellularity and α-esterase activity was assessed after collecting the bone marrow cells from the femur.
2.7 Determination of haematological parameters
2 ml of blood was collected by heart puncture into a heparinized tube and Total WBC count, Differential count, Total RBC count, Packed cell volume and Total platelet count were assessed using an automated analyser (Sysmex India Pvt. Ltd.).
2.8 Determination of haemoglobin concentration
Haemoglobin concentration was determined spectrophotometrically using Drabkin’s method (Drabkin and Austin, 1935). 20 µl of blood was added to 5 ml of Drabkin’s solution and the resultant colour of cyanmethemoglobin developed was read at 530 nm.
2.9 Determination of bone marrow cellularity
Bone marrow cellularity was estimated after collecting the bone marrow from the animals at the end of the study. Femur of the animals were collected after euthanizing the animals and the marrow cells were collected with a jet of Phosphate buffered saline (PBS) as described by (Sredni et al., 1992). Bone marrow cells were counted using a haemocytometer after making a single cell suspension.
2.10 Determination of α-esterase positive cells
A thin smear of bone marrow was prepared on a glass slide and treated with α-naphthyl acetate and pararosaniline hydrochloride for 45 min followed by Hematoxylin counter staining as described by Bancroft and Cook, 1994).
2.11 Determination of anti-tumour activity
36 adult male BALB/c mice weighing 24 ± 5.5 g were divided into six groups of 5 animals each and were inoculated with DLA tumour cells. Group 1 DLA tumour cells inoculated mice served as control. Group 2 received CTX at 25 mg/kg body weight intra-peritoneally. Group 3 to 5 received AEAR at 216, 432 and 864 mg/kg body weight along with CTX. Group 6 received only AEAR at 864 mg/kg body weight.
2.12 Determination of percentage increase in lifespan (PILS) and mean survival time (MST)
All animals were inoculated with 108 Dalton’s Lymphoma Ascites Tumour cells intra-peritoneally. CTX and AEAR were given at their respective doses to various groups of animals for a period of 10 days. Death of the animals per day was recorded and mean survival time and percentage increase in life span were calculated (Eluru et al., 2015).
2.13 Determination of tumour volume
The capability of AEAR to act as an anti-tumour agent was determined by the reduction in volume of the tumour with drug administration (Nalini et al., 2011). The greater (a) and lesser (b) diameters of the tumour was measured using Vernier callipers and the volume was computed using the formula:
2.14 Determination of percentage increase in size of tumour
The solid tumour measurements at every fifth day starting from day 15 of tumour cell implant were taken to calculate the percentage increase in tumour size according to the formula:
Similarly, percentage decrease in size of tumour (PDST) was determined by the formula;
2.15 Determination of percentage increase in body weight
Weight of the animals was recorded every third day and percentage increase was calculated by the formula:
2.16 Determination of relative organ weight
At the end of the study, animals were sacrificed and Spleen, thymus, kidney, liver and bladder were harvested from each group and washed with ice cold PBS and blotted dry. The weights of the organs were recorded accurately using a sensitive weighing balance.
2.17 Determination of antioxidant enzyme levels
By the end of the study, a 10% homogenate of liver was made with 10% KCl solution and total protein (Lowry et al., 1951) and level of lipid peroxidation (Ohkawa et al., 1979) was estimated. A portion of the homogenate was used for glutathione estimation (Beutler and Kelly, 1963). The remaining homogenate was used for estimation of SOD (Kakkar et al., 1998) and Catalase (Maehly and Chance, 1954).
2.18 Determination of short-term in vitro cytotoxicity
Trypan blue exclusion assay was utilized to test short term in vitro cytotoxicity (Shrivastava and Ganesh, 2010). Cells were aspirated from the tumour bearing mice from their peritoneal cavity and washed with PBS for three times and the suspension of cells were added to tubes previously added with different concentrations of AEAR and the final volume of the tubes were made up to 1 ml using PBS. A control tube was also maintained containing only the cell suspension. The tubes were incubated for 3 h at 37 °C and trypan blue was added at the end of the incubation and the non-viable cells were counted using a haemocytometer and the percentage cytotoxicity was calculated using the equation:
2.19 Determination of Long-term in vitro cytotoxicity
DLA cells were aspirated from tumour bearing mice and adjusted the concentration of the cells to 1 × 105 cells/mL using sterile PBS. Cytotoxic effect of AEAR was assessed using MTT dye reduction assay (Ramnath et al., 2009).
2.20 Statistical analysis
All data were represented as mean ± SEM. The difference in means between different groups were tested using one-way ANOVA and multiple comparisons between various groups with the control animals were done using Dunnett’s multiple comparison tests. Percentage increase in life span and percentage decrease in tumour size were compared using Kruskal-Wallis test and multiple comparisons with Mann-Whitney U test. 5% was fixed as the level of statistical significance. All statistical tests were performed using statistical software SPSS 16.0.
3 Results
3.1 Determination of physico-chemical characters
The physico-chemical parameters of the powdered tubers of Asparagus racemosus are tabulated in Table 1.
| Parameter | Results |
|---|---|
| Total ash | 11.93 ± 0.2 |
| Acid insoluble ash | 1.43 ± 0.3 |
| Water soluble ash | 2.3 ± 0.2 |
| Sulphated ash | 5.4 ± 0.4 |
| Water soluble extractive | 32.55 ± 0.3 |
| Alcohol soluble extractive | 13.05 ± 1.3 |
| Crude fibre content | 10.05 ± 1.1 |
| Loss on drying | 10.7 ± 0.9 |
| pH | 5.14 ± 0.2 at 24 ± 2 °C |
3.2 Preliminary phytochemical screening
The results of the phytochemical screening of AEAR is as shown in Table 2.
| Test | Reaction | |
|---|---|---|
| Carbohydrates | ||
| Fehling’s test | + | |
| Molish’s test | + | |
| Flavonoids | ||
| Lead acetate test | + | |
| Shinoda’s test | + | |
| Alkaline Reagent test | + | |
| Protein | ||
| Xanthoproteic test | + | |
| Biuret test | + | |
| Amino acids | ||
| Ninhydrin test | + | |
| Phenols | ||
| Ferric chloride test | + | |
| Phytosterols | ||
| Salkowski’s test | + | |
| Liberman-Burchard’s test | + | |
| Saponins | ||
| Froth test | + | |
| Foam test | + | |
| Fixed oils & fats | ||
| Stain test | − | |
| Resins | ||
| Acetone water test | − | |
| Tannins | ||
| Braemer’s test | + | |
| Glycosides | ||
| Modified Borntrager’s test | + | |
| Legal’s test | + | |
| Keller-Killiani test | + | |
| Alkaloids | ||
| Mayer’s test | − | |
| Wagner’s test | + | |
| Dragendorff’s test | − | |
| Hager’s test | + | |
| Froehde’s test | + | |
| Marquis test | − | |
3.3 Determination of haematological parameters
On treating the BALB/c mice induced with hemo-toxicity by treating intra-peritoneally with Cyclophosphamide and subsequent treatment with AEAR at three doses showed statistically significant normalization of the hemogram with that of the control animals. Haemoglobin, haematocrit, total RBC count, MCV, MCH, MCHC, total platelet count, lymphocyte and neutrophils showed equivalent values as compared to the control animals. Only total WBC count showed slight reduction statistically significant at 5% (p < 0.05) as compared to the control animals (Table 3).
| CONTROL | CTx | CTX + Low DOSE | CTX + Medium Dose | CTX + High dose | |
|---|---|---|---|---|---|
| Haemoglobin (g/dL) | 15.81 ± 0.92 | 7.40 ± 0.40c | 8.75 ± 0.40c | 10.24 ± 0.38c | 14.95 ± 1.11 ns |
| Haematocrit (%) | 48.32 ± 3.84 | 23.07 ± 1.81c | 28.74 ± 2.99c | 33.71 ± 3.43a | 45.40 ± 3.64 ns |
| Total RBC (×106/mm3) | 9.23 ± 0.70 | 6.13 ± 0.96a | 7.18 ± 0.59 ns | 7.57 ± 0.89 ns | 8.57 ± 2.21 ns |
| MCV (fL) | 53.41 ± 5.61 | 41.83 ± 7.54 ns | 42.00 ± 6.91 ns | 45.20 ± 5.15 ns | 55.54 ± 7.29 ns |
| MCH (pg) | 17.46 ± 1.47 | 13.25 ± 1.92 ns | 12.46 ± 1.01 ns | 13.65 ± 0.79 ns | 18.71 ± 2.84 ns |
| MCHC (%) | 33.04 ± 1.44 | 32.49 ± 1.98 ns | 31.90 ± 3.67 ns | 32.24 ± 4.71 ns | 33.99 ± 4.17 ns |
| Total WBC (×103/mm3) | 3.09 ± 0.39 | 1.81 ± 0.23a | 1.95 ± 0.11a | 2.10 ± 0.12 ns | 2.39 ± 0.39 ns |
| Platelet Count (×103/mm3) | 601.81 ± 54.2 | 358.46 ± 24.74b | 425.05 ± 36.23 ns | 420.18 ± 54.90a | 560.04 ± 64.48 ns |
| Lymphocyte (%) | 61.61 ± 0.43 | 45.72 ± 0.21c | 50.44 ± 0.23c | 51.18 ± 0.38c | 58.86 ± 0.34c |
| Neutrophil (%) | 55.96 ± 0.39 | 72.40 ± 0.82c | 63.16 ± 0.46c | 51.80 ± 0.11c | 54.84 ± 0.27 ns |
| Bone Marrow Cellularity (×106 cells/femur) | 18.43 ± 2.4 | 8.57 ± 0.16c | 19.62 ± 0.13c | 20.53 ± 0.18c | 21.49 ± 0.20c |
| α-esterase activity (esterase positive cells per 4000 cells) | 976.8 ± 23.6 | 635.58 ± 41.6c | 781.25 ± 56.6c | 810.32 ± 57.8c | 1027.75 ± 35.7c |
(a – significant at 5% level (p < 0.05), b – significant at p < 0.01, c – significant at p < 0.001 and ns – not significant (p > 0.05)).
3.4 Determination of anti-tumour activity
Anti-tumour activity of AEAR against Dalton’s Lymphoma Ascites (DLA) tumour bearing BALB/c mice was performed by assessing the change in organ weight, body weight (Fig. 1), mean survival time (MST) (Fig. 2), percentage increase in life span (PILS), tumour volume, tumour size (PITS), percentage decrease in tumour size (PDTS), tumour cell growth and non-viable cells. Results are shown in Table 4, Table 5 and Table 7 respectively.
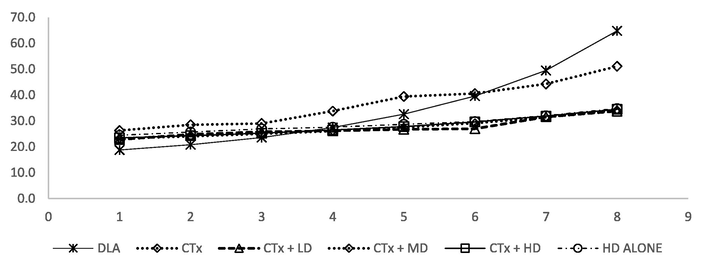
- Body weight of BALB/c mice treated with Cyclophosphamide and AEAR.
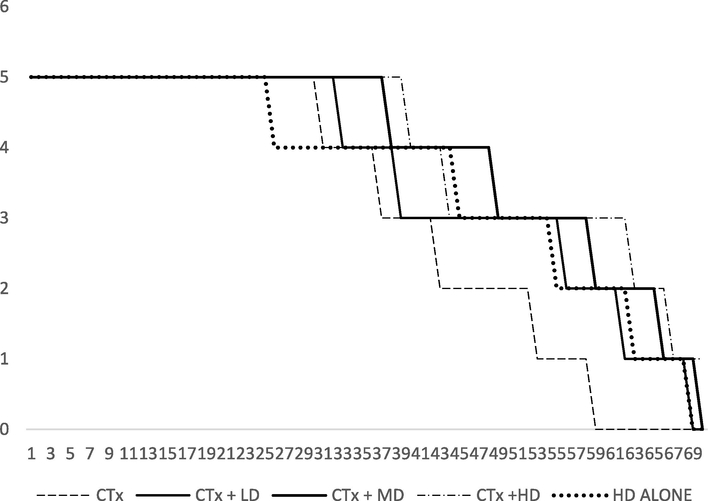
- Survival of BALB/c mice treated with Cyclophosphamide and AEAR.
| Spleen | Thymus | Kidney | Liver | Bladder | |
|---|---|---|---|---|---|
| Control | 0.34 ± 0.02 | 0.22 ± 0.01 | 1.09 ± 0.01 | 3.21 ± 0.03 | 0.07 ± 0.005 |
| CTX treated | 0.30 ± 0.01 ns | 0.18 ± 0.01a | 1.4 ± 0.01c | 3.42 ± 0.04 ns | 0.10 ± 0.004 ns |
| CTX + LD | 0.38 ± 0.02 ns | 0.17 ± 0.01b | 1.37 ± 0.01c | 3.29 ± 0.07 ns | 0.09 ± 0.008 ns |
| CTX + MD | 0.40 ± 0.01b | 0.19 ± 0.01 ns | 1.28 ± 0.03c | 3.24 ± 0.06 ns | 0.11 ± 0.009a |
| CTX + HD | 0.41 ± 0.02b | 0.22 ± 0.02 ns | 1.12 ± 0.03 ns | 3.27 ± 0.06 ns | 0.08 ± 0.004 ns |
| HD alone | 0.39 ± 0.01 ns | 0.19 ± 0.01 ns | 1.29 ± 0.03 ns | 3.23 ± 0.06 ns | 0.10 ± 0.01 ns |
(a – significant at 5% level (p < 0.05), b – significant at p < 0.01, c – significant at p < 0.001 and ns – not significant (p > 0.05)).
| Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | Day 15 | |
|---|---|---|---|---|---|---|---|---|
| DLA | 18.8 ± 1.3 | 20.8 ± 1.3 ns | 23.6 ± 1.1 ns | 27.5 ± 0.95c | 32.6 ± 1.3c | 39.6 ± 1.02c | 49.5 ± 1.5c | 64.8 ± 1.6c |
| CTX treated | 23.3 ± 0.63 | 24.0 ± 0.63 ns | 25.0 ± 0.53 ns | 26.1 ± 0.51 ns | 27.5 ± 0.59NS | 29.2 ± 0.86 ns | 31.4 ± 0.68b | 34.2 ± 0.66c |
| CTX + LD | 22.8 ± 0.85 | 24.8 ± 0.58 ns | 25.8 ± 0.86 ns | 26.2 ± 2.22c | 26.8 ± 3.09c | 27.0 ± 1.00c | 31.6 ± 0.51c | 33.8 ± 1.71c |
| CTX + MD | 24.2 ± 0.83 | 24.9 ± 0.79 ns | 26.1 ± 0.80 ns | 27.9 ± 2.01 ns | 28.9 ± 2.51 ns | 31.0 ± 1.0 ns | 32.6 ± 1.96a | 33.4 ± 1.78b |
| CTX + HD | 23.45 ± 0.79 | 24.3 ± 1.03 ns | 25.3 ± 0.99 ns | 26.5 ± 1.05 ns | 27.8 ± 1.1 ns | 29.6 ± 1.2b | 31.8 ± 1.3c | 34.5 ± 1.4c |
| HD alone | 24.5 ± 0.99 | 25.72 ± 1.5 ns | 26.9 ± 0.97 ns | 27.6 ± 1.04 ns | 28.7 ± 0.75 ns | 29.8 ± 1.12a | 32.1 ± 1.31c | 34.8 ± 1.26c |
(a – significant at 5% level (p < 0.05), b – significant at p < 0.01, c – significant at p < 0.001 and ns – not significant (p > 0.05) when compared to the value at Day 1).
| DLA | CTX | CTX + LD | CTX + MD | CTX + HD | HD | |
|---|---|---|---|---|---|---|
| TBARS (nmol MDA/gm tissue) | 2.46 ± 0.35 | 4.87 ± 0.12a | 4.02 ± 0.79b | 3.44 ± 0.27b | 2.01 ± 0.28 ns | 2.46 ± 0.62 ns |
| GSH (uMol/mg protein) | 2.21 + 0.34 | 1.4 ± 1.5a | 1.5 ± 0.7b | 2.1 ± 0.3 ns | 2.5 ± 0.9 ns | 1.9 ± 0.8 ns |
| SOD(U/mg protein) | 1.6 ± 0.11 | 0.9 ± 0.3c | 2.3 ± 0.25a | 3.1 ± 0.45b | 4.8 ± 0.5c | 2.42 ± 0.4a |
| Catalase (uMol H2O2/gm of tissue) | 6.48 ± 0.20 | 4.54 ± 0.3b | 7.44 ± 0.6 ns | 8.63 ± 0.7b | 13.6 ± 0.9c | 10.8 ± 0.29a |
(a – significant at 5% level (p < 0.05), b – significant at p < 0.01, c – significant at p < 0.001 and ns – not significant (p > 0.05) when compared to DLA treated group).
| DLA | CTX | CTX + LD | CTX + MD | CTX + HD | HD | |
|---|---|---|---|---|---|---|
| Mean survival time | 19.32 ± 0.14 | 22.98 ± 0.32a | 22.32 ± 0.99 ns | 23.40 ± 0.39b | 24.33 ± 0.15c | 23.1 ± 0.42 ns |
| PILS | 0 | 18.9 ± 1.5b | 15.7 ± 5.8 ns | 21.1 ± 2.3b | 25.9 ± 0.7b | 13.3 ± 7.8 ns |
| Tumour Volume | 7.23 ± 0.11 | 2.19 ± 0.03c | 2.33 ± 0.35c | 2.16 ± 0.65c | 1.78 ± 0.48c | 3.42 ± 0.39c |
| PIST | 8.36 ± 0.10 | 4.14 ± 0.02c | 3.94 ± 0.88c | 3.13 ± 0.69c | 2.82 ± 0.99c | 4.81 ± 0.29b |
| PDST | 0 | 50.42 ± 0.49b | 53.1 ± 10.45b | 62.42 ± 8.51b | 66.1 ± 12.0b | 42.54 ± 3.13b |
| Trypan blue exclusion Assay | 0.90 ± 0.06 | 63.97 ± 9.16c | 67.9 ± 1.21c | 72.27 ± 1.41c | 78.07 ± 0.73c | 53.30 ± 2.79c |
| MTT Cytotoxicity Assay | 0.46 ± 0.07 | 72.3 ± 5.24c | 74.53 ± 2.98c | 80.9 ± 1.08c | 82.37 ± 4.37c | 61.9 ± 2.38c |
(a – significant at 5% level (p < 0.05), b – significant at p < 0.01, c – significant at p < 0.001 and ns – not significant (p > 0.05) when compared to the DLA treated group).
3.5 Determination of mean survival time (MST)
The DLA tumour bearing mice survived on an average to 19.32 ± 0.14 days. Treating with CTX had elevated the survival of the animals to 22.98 ± 0.32 days. The lower dose of AEAR treated animals survived for 22.32 ± 0.99 days while those in Medium dose treated and High dose treated survived up to 23.40 ± 0.39 and 24.33 + 0.15 days respectively. The group of animals treated alone with the high dose of AEAR survived for 23.1 ± 0.42 days. The increase in MSL was significant (p < 0.05) for Medium dose treated and high dose treated animals.
3.6 Determination of percentage increase in life span (PILS)
Considering the life span of animals in tumour control group as zero, the CTX treated animals showed an increase in life span to 18.9 ± 1.5%. Animals treated with Lower dose of AEAR showed an increase in life span of 15.7 ± 5.8% only which was found statistically insignificant. Medium dose of AEAR on co-administration with CTX showed an increase in life span of 21.1 ± 2.3% and higher dose showed an increase of 25.9 ± 0.7% which were found to be statistically significant at p < 0.001. Animals treated with AEAR at high dose alone showed an increase in life span of 13.3 ± 7.8% only which was statistically insignificant.
3.7 Determination of body weight
DLA tumour bearing animals showed significant increase in the body weight after tumour initiation. The maximum weight gain reported was 30.8 ± 1.6%. CTX administration produced a weight gain of 8.9 ± 21.7%. The lower dose of AEAR produced a maximum weight gain of 6.9 ± 1.7% and the medium dose of AEAR produced 2.45 ± 1.78% weight gain. The high dose of AEAR attained a maximum weight gain of 8.22 ± 1.4% and animals received AEAR at high dose alone showed a weight gain of 8.54 ± 1.3%. The results on the body weight of the animals are shown in Table 5 and Fig. 1.
3.8 Determination of antioxidant enzymes
AEAR on administration along with CTX has significantly enhanced the levels of antioxidant enzymes in the animal tissues. AEAR could reduce lipid peroxidation significantly in a dose dependent manner. Levels of glutathione, SOD and CAT were significantly increased by the administration of AEAR (p < 0.001). The results are shown in Table 6.
3.9 Determination of short-term in vitro cytotoxicity by trypan blue dye exclusion method
Cytotoxicity of AEAR on DLA cells was evaluated by Trypan blue dye exclusion method. The viable cells unstained by trypan blue was counted and the percentage cytotoxicity of the tumour cells at different concentrations are shown in Table 7. The co-administration of AEAR along with cyclophosphamide significantly increased the cytotoxicity AT P < 0.001.
3.10 Determination of long-term in vitro toxicity using MTT assay
Cytotoxic effect of AEAR on co-administration with CTX was investigated using MTT assay and the results are shown in Table 7. Cells were treated with different concentrations of AEAR along with CTX and the percentage of cell viability was computed. AEAR significantly inhibited the proliferation of DLA cells which was found to be statistically significant at p < 0.001.
4 Discussion
Cancer is an umbrella term used to define diseases occurring at different sites of the body and it encompasses >100 diseases including malignant tumours. The decade of 1940s saw the rise of chemotherapy against cancers by the introduction of nitrogen mustards and antifolate drugs which later paved way to adjuvant chemotherapy and hormonal therapy that helped in the dramatic cure rate in cancers (Shumay et al., 2001). Chemotherapeutic agents are usually administered according to body weight of the patients and even then, they are capable of producing toxicities and they poses a hurdle of inconsistent plasma concentration and therapeutic/toxic effect (Harrold and Parker, 2009). CTX is a member of nitrogen mustard subfamily of DNA alkylators that interferes with the nucleic acid synthesis of cancer cells. Immunosuppression from bone marrow suppression and hindering hemopoiesis are the most evident toxicity profile of CTX. Several mechanisms involving the reactive oxygen species generation, DNA damage, defect in cell signalling etc. are attributed to carcinogenesis (Sahai, 2005). This has created curiosity to turn to more natural products in treating cancers. Many plants have been investigated for their chemoprotective effect to use in conjunction with chemotherapeutic agents to potentiate anti-cancerous properties (Jain et al., 2016). Asparagus racemosus is a widely growing medicinal herb of the tropics rich in phyto-active ingredients used widely in the traditional healing system of Ayurveda. The genus Asparagus is medicinally important owing to the presence of steroid saponins and sapogenins (Kawale et al., 2014). The present study aimed at investigating the role of Asparagus racemosus aqueous extract’s role in reversing the hemotoxicity and bone marrow suppression along withs its anticancerous effect against DLA cells in in vivo and in vitro models. The physico-chemical and phytochemical profile study of the dried roots showed values in accordance with the standards of Ayurvedic Pharmacopoeia of India. Here hemogram along with differential count was performed on BALB/c mice treated with CTX. The co-administration of AEAR has significantly increased the values of hemogram to normal. CTX is activated initially by the liver microsomal oxidation system to a cytotoxic metabolite 4-hydroxy-CTX which is later converted to acrolein, which causes myelosuppression. Hemogram along with total leucocyte counts, bone marrow cellularity and α-esterase activity were studied. AEAR showed dose dependant increase in the values of hemogram as wells a leucocyte counts, bone marrow cellularity and α - esterase positive cells indicating that the drug stimulates the hemopoiesis and immunosuppression from CTX treatment. Also, the reduction in the weight of the organs like spleen, thymus, liver, kidney and bladder also showed significant increase. The concomitant increase in the weight of organs of immunological importance and other organs in extract alone treated group also shows the immunostimulant activity of the drug. Present study also evaluated the role AEAR as an anticancer drug against DLA cells in in vivo and in vitro models. The study shows significant anticancer activity of AEAR against DLA in mice as shown by significant reduction in tumour size, volume, percentage decrease in tumour size and concomitant increase in mean survival time and percentage increase in lifespan.
The mechanism of toxicity related to CTX administration is pointed to the lipid peroxidation induced by free radical generation. Malondialdehyde (MDA), the intermediate formed during oxidative degeneration is a target molecule to quantify lipid peroxidation (Rajneesh et al., 2008). The elevated levels of MDA were reduced as evidenced by the decreasing level od TBARS levels in DLA tumour bearing mice. Glutathione is a strong antioxidant defence mechanism and a strong inhibitor of the neoplastic process (Estrela et al., 2006). In the present study, AEAR significantly increased the levels of glutathione in mice treated with CTX in a dose dependant manner. Superoxide dismutase (SOD) and Catalase (CAT) are responsible for scavenging superoxide and hydrogen peroxide respectively. They are immensely linked with tumour growth. AEAR significantly increased SOD levels in DLA tumour bearing mice. The depletion in SOD levels is attributed to the loss of Mn2 + related SOD levels and to the loss of mitochondrial function in tumour cells (Veni et al., 2011). AEAR regaining the normal cellular function can be inferred from this finding. Catalase levels were also depleted in the tumour bearing mice. AEAR could also significantly elevate the levels of CAT at medium and higher doses.
The phytochemical screening of the AEAR showed strong presence of flavonoids, phytosterols, saponins and phenols (Ahmad et al., 2017). Flavonoids are a diverse group of phytoconstituents chiefly attributed with antioxidant and anti-cancerous properties. There are reported chemo-preventive activity of flavonoids by signal transduction preventing cell proliferation and angiogenesis (Romagnolo and Selmin, 2012). Several researches point to the prevention of cell growth, multiplication, inducing apoptosis and delaying angiogenesis and metastasis by phytosterols (Woyengo et al., 2009). Saponins are attributed with immunomodulatory potential by modulating cytokine interplay and have tremendous cytostatic and cytotoxic effects on malignant tumour cells (Yıldırım and Kutlu, 2015). Phenolics are strong scavengers of free radicals and are capable of inducing enzymes for xenobiotic metabolism and regulation of gene expression and modulation of cellular signalling pathways leading to decrease in cell proliferation and induce apoptosis and invasion (Rosa et al., 2016). The presence of these phyto-constituents in Asparagus racemosus may be responsible for the activity exhibited by it. The cytotoxic and antitumour properties coupled with the capability to reverse the myelosuppression and immunosuppression induced by CTX makes Asparagus racemosus an important drug for co-administration in cancer therapy.
Acknowledgments
Authors would like to extend their sincere gratitude to The Research Director, Dr. Ramadasan Kuttan, Amala Cancer Research Centre, Thrissur and The Principal, Vaidyaratnam Ayurveda College, Kottakkal, Kerala for facilitating the smooth conduct of the study. The authors acknowledge King Saud University, Riyadh, Saudi Arabia, for funding this work through Researchers Supporting Project number (RSP-2019/11).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pharmacognostical and phytochemical evaluation of root of Asparagus racemosus Willd. J. Drug Delivery Therapeut.. 2017;7(6):76-80.
- [Google Scholar]
- Cancer epidemiology: a survey of modifiable risk factors for prevention and survivorship. Am. J. Lifestyle Med.. 2018;12(3):200-210.
- [Google Scholar]
- Manual of Histologic Techniques and Their Diagnostic Applications. Edinburgh: Churchill Livingstone; 1994.
- Spectrophotometric studies. II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J. Biol. Chem.. 1935;112:51.
- [Google Scholar]
- Anti-tumour activity of Limonia acidissima L. methanolic extract in mice model of Dalton’s ascitic lymphoma. Int. J. Pharmacogn. Phytochem. Res.. 2015;7(6):1094-1100.
- [Google Scholar]
- Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci.. 2006;43(2):143-181.
- [Google Scholar]
- Methods of plant analysis. In: Phytochemical Methods. Dordrecht: Springer; 1984. p. :1-36.
- [Google Scholar]
- Clinically relevant cancer chemotherapy dose scheduling via mixed-integer optimization. Comput. Chem. Eng.. 2009;33(12):2042-2054.
- [Google Scholar]
- Medicinal plants for treatment of cancer: a brief review. Pharmacogn. J.. 2016;8(2):87-102.
- [Google Scholar]
- Increased oxidative stress in rat liver and pancreas during progression of streptozotocin induced diabetes. Clin. Sci.. 1998;94:623-632.
- [Google Scholar]
- Pharmacognostical and physicochemical analysis of Asparagus adscendens Buch. Ham. ex Roxb. (Shweta musali) J. Pharmacogn. Phytochem.. 2014;3(4):131-139.
- [Google Scholar]
- Maehly, A.C., Chance, B., 1954. The assay of catalase and peroxide. In: Methods of Biochemical Analysis, vol. 1, New York, pp. 357–424.
- Effect of cyclophosphamide pretreatment on hematological indices of Indian Bonnet Monkeys. Ind. J. Pharmacol.. 2004;36(3):175.
- [Google Scholar]
- Evaluation of anti-tumour activity of Cissus quadrangularis Linn. against Dalton’s Ascitic Lymphoma and Erlich Ascitic Induced Carcinoma in Mice. Int. J. Pharm. Sci. Rev. Res.. 2011;8(1):75-79.
- [Google Scholar]
- Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Cancer Management: A Multidisciplinary Approach. F.A Davis Company; 2003.
- Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med. J.. 2008;49(8):640.
- [Google Scholar]
- Regulation of Caspase-3 and Bcl-2 expression in Dalton's Lymphoma Ascites cells by Abrin. Evid. Based Complement. Alternat. Med.. 2009;6:233-238.
- [Google Scholar]
- Flavonoids and cancer prevention: a review of the evidence. J. Nutr. Gerontol. Geriatr.. 2012;31(3):206-238.
- [Google Scholar]
- Anticancer properties of phenolic acids in colon cancer–a review. J. Nutr. Food. Sci.. 2016;6(468)
- [CrossRef] [Google Scholar]
- Tumor inhibition and cytotoxicity assay by aqueous extract of onion (Allium cepa) & Garlic (Allium sativum): an in-vitro analysis. Int. J. Phyto Med.. 2010;2:80-84.
- [CrossRef] [Google Scholar]
- Why some cancer patients choose complementary and alternative medicine instead of conventional treatment. J. Family Pract.. 2001;50(12):1067.
- [Google Scholar]
- The immunomodulator administered orally as a radioprotective agent. Int. J. Immunopharmacol.. 1992;14:619.
- [Google Scholar]
- The Ayurvedic Pharmacopoeia of India, 2008, first ed., part I, vol. IV, Ministry of Health and Family Welfare, India.
- Alterations in serum SOD and CAT levels in patients with breast cancer. J. Experiment. Sci.. 2011;2(2):58-60.
- [Google Scholar]







