Translate this page into:
Antiproliferative and apoptotic effect of tuna blood on human lung cancer A549 cells via p38 MAPKs and Akt pathway
⁎Corresponding author. supita.t@psu.ac.th (Supita Tanasawet)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tuna blood has been reported the anti-inflammatory properties. However, the anticancer effects of tuna blood remain unelucidated. Therefore, this study investigated the anticancer effects of tuna blood against human non-small cell lung cancer (NSCLC) A549 cells and its potential mechanism of action. The amino acid profile presented in tuna whole blood freeze-dried (TWBF) and tuna blood cell freeze-dried (TRBF) were analyzed, and the antiproliferative effect was assessed by MTT assay. The nuclear staining, reactive oxygen species (ROS) generation, and mitochondrial membrane potential (MMP) were examined by Hoechst/PI staining, DCFHDA assay, and JC-1 staining. The expression of apoptosis-related proteins and the involvement of p38 MAPKs and Akt signaling were evaluated by western blotting. TWBF and TRBF inhibited the proliferation of lung cancer A549 cells with IC50 values of 354.97, 141.31 µg/mL, respectively. TRBF revealed more efficacy of cell proliferation inhibition than that of TWBF. The percentage of apoptotic cell death after treatment with 100 µg/mL TRBF was 35.95%. The increase in the ROS production and the disruption of MMP were also detected. At a mechanistic level, the exposure of TRBF to A549 cells showed an increase of the Bax/Bcl2 ratio and subsequently activated the proteolytic activity of caspase-3. TRBF administration induced apoptosis through the downregulation of protein kinase B (Akt) and upregulation of p38 MAPK. These findings indicate TRBF inhibits lung cancer cell proliferation and mediates apoptosis signaling, suggesting a promising chemotherapeutic candidate target NSCLC.
Keywords
Tuna blood
Anti-cancer
Mechanism
A549
Apoptosis
1 Introduction
The socioeconomic burden of non-communicating diseases (NCDs) has been considered a large impact worldwide. Nearly 84% of death are attributed to four major NCDs including cardiovascular disease, cancer (such as lung, liver, and colorectal cancer), respiratory disease, and diabetes mellitus (Gowshall and Taylor-Robinson, 2018). With the rising burden of cancer-related death among males and females, lung cancer is still the most frequent. Non-small cell lung cancer (NSCLC) is the most common histological subtype of lung cancer (Zappa and Mousa, 2016). Despite advances in the treatment strategies, the 5-year survival rate remains low (Kanitkar et al., 2018). There is increasing evidence of dietary and food supplements provided a beneficial effect by decreased risk of lung cancer (Barta et al., 2019).
The increasing interest in natural-sourced products including marine origin is being carried out for drug development to target many types of cancer. Marine-derived anticancer drug, Eribulin mesylate (Halaven®) for example, has been approved for clinical use by FDA (Swami et al., 2017). To date, several investigations have demonstrated the pharmacological activity of blood extracts from crocodiles exhibited antioxidant, anti-inflammatory, and anti-cancer activity (Klaypradit et al., 2021, Ou and Ho, 2016, Theansungnoen et al., 2016; Patathananone et al., 2015). Crocodile blood cell extract was found to possess the apoptosis and metastasis inhibitory properties in several cancer cell lines (Phosri et al., 2018; Theansungnoen et al., 2016; Patathananone et al., 2015). The whole blood extract of crocodile was also found to promote lung cancer cells death through the apoptosis mechanism and downregulated the survival pathway PI3K/AKT (Ou and Ho, 2016).
Recent evidence demonstrated that tuna blood suppresses lipopolysaccharide (LPS) -induced inflammatory mediators in macrophage cells (Klaypradit et al., 2021). Additionally, a dietary supplementation from tuna extract was found to decrease cancer pathology in mice model (Masuda et al., 2018). Therefore, this study aimed to explore the anticancer potentials of tuna blood against human lung cancer A549 cells and explore its mechanism of action.
2 Methodology
2.1 The preparation of tuna whole blood and tuna blood cell
Tuna whole blood and its fractionated tuna blood cell were prepared as previously described (Klaypradit et al., 2021). After centrifugation at 2,500 rpm for 30 min, the tuna blood cell was separated from the whole blood and finally freeze-dried to achieve the tuna whole blood freeze-dried (TWBF), and tuna blood cell freeze-dried (TRBF).
2.2 Determination of amino acid contents of TWBF and TRBF
Determination of amino acid was conducted according to the Commission directive 98/64/EC (European Commission, 1998). Five grams of tuna blood powder (TWBF and TRBF) were extracted with extraction mixture containing 0.1 mol HCl and 2% thiodiglycol and shaken for 1 h. Ten milliliters of water phase were mixed with 5 mL of sulfosalicylic acid and shaken for 5 min. Subsequently, they were subjected to centrifugation to achieve the water phase. The hydrolysis mixture (HCl containing phenol) was added to the water phase. The amino acid profile was analyzed using High Performance Liquid Chromatography (HPLC; Agilent LC 1200, USA).
2.3 Cell lines and culture
MRC-5 (normal lung cell lines) and A549 (cancerous lung cell lines) cells were received from the CLS cell lines service (Eppelheim, Germany) and maintained in DMEM medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 µg/mL streptomycin, and 2 mM L-glutamine, at 37 ˚C with 5% CO2 in a humidified incubator.
2.4 Cell viability assay
The MTT assay was performed to examine the cellular metabolic activity indicated cell proliferation. The cells were treated with TWBF and TRBF (0–1000 µg/mL). After 24 h. of treatment, the cells were incubated with MTT solution (0.5 mg/mL) for 4 h. The DMSO was added to dissolve the formazan product. The optical density of each well was then measured at 570 nm with a microplate reader (Bio-Tex, U.S.A.).
2.5 Nuclear staining assay
The cells were treated with TWBF and TRBF at different concentrations (0–100 µg/mL) for 24 h. Cells were washed and stained with Hoechst 33,342 (10 µg/mL) and PI (2 µg/mL) for 30 min. The apoptotic cells, which have chromatin condensation and DNA fragmentation were detected with the fluorescent microscope (Olympus IX73; 20 × ) supplied with a DP73 digital camera system (Olympus, Tokyo, Japan). The data was presented the percentage of apoptotic cells. Apoptosis cells (%) = (total apoptosis cells / total cells) × 100.
2.6 Measurement of intracellular ROS production
The cells were treated with TWBF and TRBF (0–100 µg/mL) for 2 h. The generation of ROS was measured as previously described (Kongprasom et al., 2019). Briefly, cells were washed and then incubated with a working solution of 50 µM DCFH-DA for 1 h. The fluorescence intensity was detected at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a fluorescence microplate reader (BioTex, U.S.A.).
2.7 Mitochondrial membrane potential (MMP)
Cells were incubated with TWBF and TRBF (0–100 µg/mL) for 24 h. After the addition of 1 µg/mL of JC-1 (Merck, Germany) for 10 mins at 37 ˚C in the dark, cells were assessed by fluorescence spectrophotometry with an excitation/emission at 490 and 525 nm wavelengths.
2.8 Western blot analysis
The proteins were collected from A549 cells treated with TWBF and TRBF for 24 h, then loaded on 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to the nitrocellulose membrane and blocked. Primary antibodies against Akt, pAkt, ERK, pERK, p38, p‐p38, Bcl-2, Bax, procaspase-3, active caspase-3, and β‐actin were probed on the membranes at 4 °C overnight. Blots were then washed with TBST, followed by incubation with HRP‐ conjugated secondary antibody at room temperature for 2 h. Protein bands were visualized by an enhanced chemiluminescence (ECL) western blotting substrate and analyzed using Image J software (NIH, Maryland).
2.9 Statistical analysis
The data were presented as mean ± standard error of the mean (SEM). One-way Analysis of variance (ANOVA) with Tukey's test was used to analyze statistical differences between groups. P < 0.05 was considered significant.
3 Results
3.1 Amino acid contents of TWBF and TRBF
Tuna blood containing tuna whole blood freeze-dried (TWBF) and tuna blood cell-freeze dried (TRBF) were determined for the amino acid contents (mg/g) (Table 1). The amino acid profile demonstrated both essential and nonessential amino acids. Aspartic acid (66.86 mg/g) and glutamic acid (66.07 mg/g) had the highest content in TWBF. Whereas aspartic acid (60.27 mg/g) and tyrosine (60 mg/g) had the highest content in TRBF. The presence of tyrosine (60 mg/g) and phenylalanine (38.15 mg/g) content in TRBF were higher than that of TWBF. ND: not detected.
Amino acid
TWBF
TRBF
Content (mg/g)
Content (mg/g)
Alanine
48.95
44.26
Arginine
24.77
19.53
Aspartic acid
66.86
60.27
Cystine
7.93
7.32
Glutamic acid
66.07
50.76
Glycine
29.44
21.96
Histidine
28.34
20.89
Hydroxylysine
ND
ND
Hydroxyproline
ND
ND
Isoleucine
29.17
26.26
Leucine
59.27
56.29
Lysine
58.91
47.19
Methionine
17.85
15.51
Phenylalanine
32.37
38.15
Proline
24.43
23.03
Serine
33.28
24.68
Threonine
31.11
27.62
Tryptophan
10.11
7.01
Tyrosine
28.84
60
Valine
41.19
35.81
3.2 Antiproliferative effect of TWBF and TRBF
To determine the effect of tuna blood (TWBF and TRBF) on lung cancer A549 cells, we firstly evaluated cell viability using the metabolic MTT assay. The cancerous A549 cells and non-cancerous MRC-5 cells were treated with different concentrations of TWBF and TRBF for 24 h. We observed that TWBF at the concentration of 500 and 1000 µg/mL significantly inhibited the viability of A549 cells with its IC50 value at 354.97 µg/mL. The treatment with 250, 500, and 1000 µg/mL TWBF to the normal lung fibroblast MRC-5 cells demonstrates the percentage of cell survival at 41.57, 26.66, and 25.29, respectively, and its IC50 value is 225.94 µg/mL (Fig. 1a and c). Moreover, TRBF has been employed and found that 75, 100, 250, 500 and 1000 µg/mL of TRBF significantly inhibit A549 cell viability and its determined IC50 value was 141.31 µg/mL. A high concentration of TRBF (250, 500, and 1000 µg/mL) was found to decrease the viability of normal MRC-5 cells to 51.93, 46.81, and 38.51, and its IC50 value is 478.94 µg/mL (Fig. 1b and c). The selectivity index (SI) of TWBF and TRBF from this present study was 0.64, and 3.39, respectively (Fig. 1c). The percentage of inhibition of cell proliferation exposed to 25, 50, 100 µg/mL of TRBF was 15.76, 25.22, and 46.35, respectively as shown in Fig. 1d. Therefore, TRBF is more potent than TWBF (Fig. 1a-d), and it requires less concentration to cause growth inhibition to A549 cells.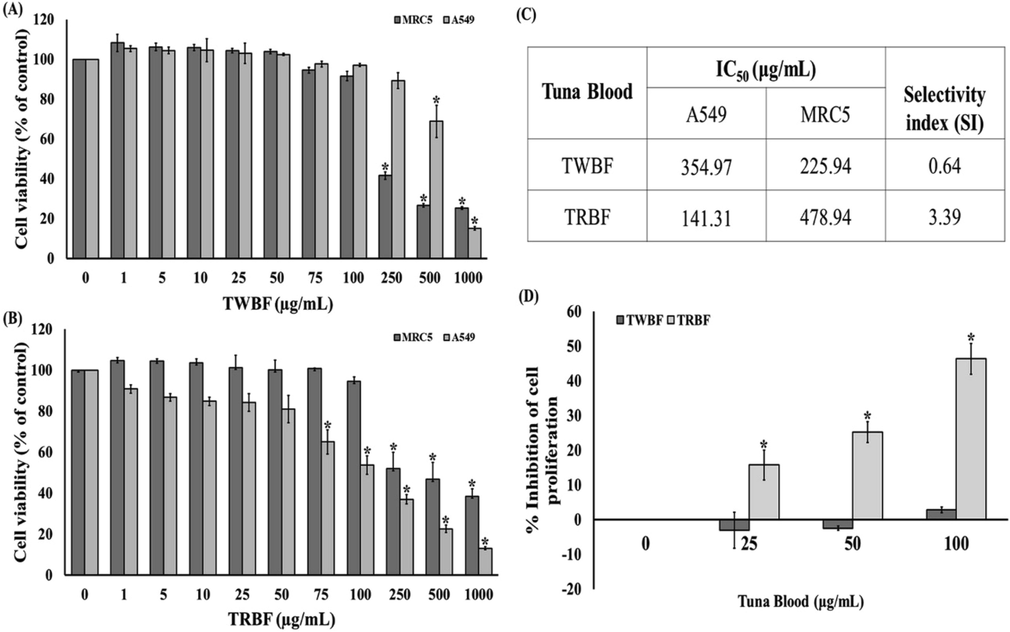
Cytotoxic effect of tuna blood (TWBF and TRBF) on normal lung MRC-5 cells and lung cancer A549 cells was assessed by MTT assay. (a) Treatment with TWBF different concentrations on MRC-5 and A549 cells for 24 h; (b) Treatment with TRBF different concentrations on MRC-5 and A549 cells for 24 h. (c) IC50 and selectivity index (SI) of TWBF and TRBF. (d) The percent growth inhibition of A549 cells after treatment with TWBF and TRBF. Data are presented as the mean ± SEM. *P < 0.05 vs. the control group (0 µg/mL).
3.3 Morphological changes of A549 cells
The morphological alteration of A549 cells after exposure to TWBF and TRBF demonstrated a marked reduction in the number of A549 cells as well as the loss of cellular process and spindle-shaped structure observed by bright-field microscope (Fig. 2a and c). We further determined whether the death of cells after exposure with tuna blood through the apoptosis signaling by fluorescently labeling the cells with the apoptotic Hoechst 33,342 and necrotic propidium iodide (PI) dye. The cells incubated with 100 µg/mL TWBF were significantly undergoes apoptosis which revealed by the bright blue nuclear staining with Hoechst 33,342 indicating nuclear condensation or fragmentation (Fig. 2a). The percentage of apoptotic nuclei of 100 µg/mL TWBF was 5.30 (Fig. 2b). As depicted in Fig. 2c, treatment with TRBF at 50 and 100 µg/mL evidently caused condensed chromatin and apoptotic body. In addition, the percentage of apoptotic nuclei was 31.10 and 35.95, respectively, and showed higher potency than that of TWBF. (Fig. 2b and d).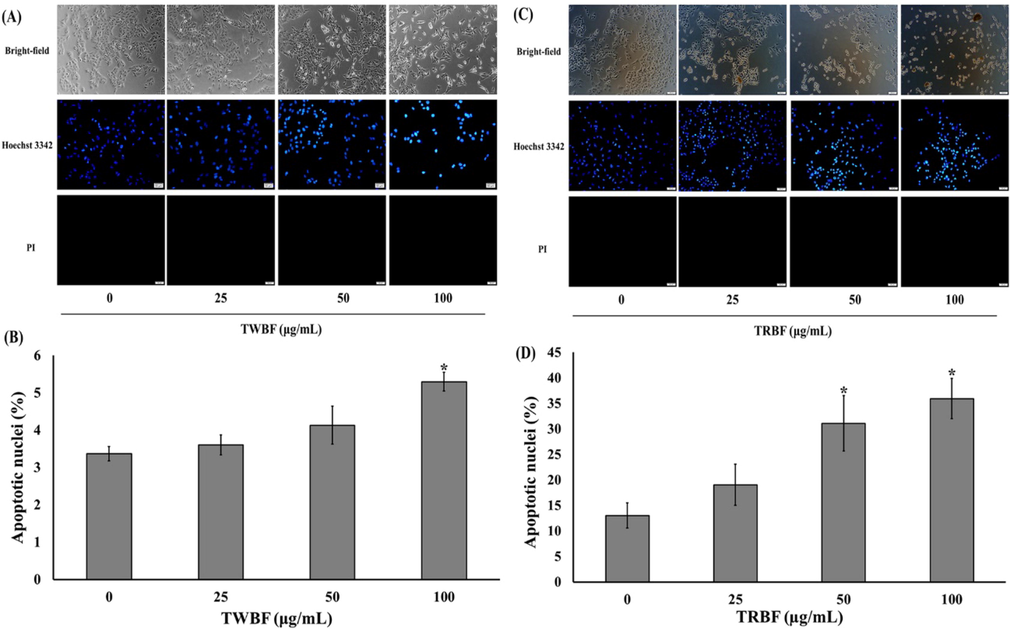
Effect of tuna blood (TWBF and TRBF) on cell morphology of lung cancer cells (a) Representative immunofluorescence images of Hoechst 33342/propidium iodide staining on A549 cells after exposure to TWBF. (b) Quantitative data represents the percentage of apoptotic nuclei on A549 cells after exposure to TWBF. (c) Representative immunofluorescence images of Hoechst 33342/propidium iodide staining on A549 cells after exposure to TRBF. (d) Quantitative data represents the percentage of apoptotic nuclei on A549 cells after exposure to TRBF. Scale bar = 50 µM. Data are presented as the mean ± SEM. *P < 0.05 vs. the control group (0 µg/mL).
3.4 ROS generation and MMP in A549 cells
We explore the participation of ROS-induced apoptosis by treatment with tuna blood. Fig. 3a demonstrated that TWBF and TRBF at the dosage of 100 µg/mL significantly caused the elevation of ROS levels to 109.54 and 113.19, respectively in comparison to the control. Since high ROS production might lead to mitochondrial membrane damage, the mitochondrial membrane potential (MMP) was further examined by JC-1. Fig. 3b showed that only 100 µg/mL TWBF significantly reduced the MMP, while TRBF at dosage of 50 and 100 µg/mL significantly decreased the membrane potential to 95.08, and 94.27, respectively.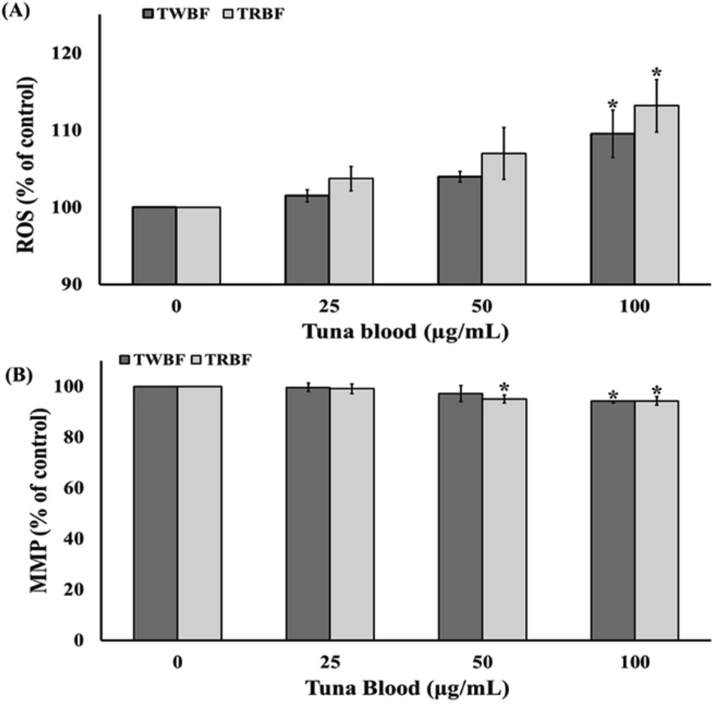
Effect of tuna blood (TWBF and TRBF) on ROS generation and mitochondrial membrane potential on A549 cells. (a) Relative quantification of TWBF and TRBF-induced intracellular ROS production probed by DCFH-DA assay. (b) Relative quantification of TWBF and TRBF-induced mitochondrial membrane potential depolarization probed by JC-1 assay. Data are presented as the mean ± SEM. *P < 0.05 vs. the control group (0 µg/mL).
3.5 Bax, Bcl-2, cleaved and uncleaved caspase-3 protein expression in A549 cells
The alteration of Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) expression, as well as crucial enzyme caspase-3, are recognized to be important to mediate apoptosis signaling networks. We attempt to elucidate these proteins to confirm the role of tuna blood on the induction of apoptosis by western blot analysis. After exposure of 50 and 100 µg/mL of TWBF to A549 cells, the Bcl-2 protein expression was significantly up-regulated when compared to the control. However, all concentrations of TWBF showed no significant differences in the changes of Bax, cleaved, and uncleaved caspase-3 expression (Fig. 4a and b). Additionally, treatment with TRBF (100 µg/mL) significantly downregulated Bcl-2 protein expression compared to the control. TRBF at 50 and 100 µg/mL also dramatically increased Bax expression. Likewise, TRBF at 50 and 100 µg/mL significantly increased cleaved caspase-3 expression levels while decreased uncleaved caspase-3 levels as demonstrated in Fig. 4c and d. Therefore, only TRBF was selected to further elucidate deeper mechanisms due to its potency in apoptosis induction.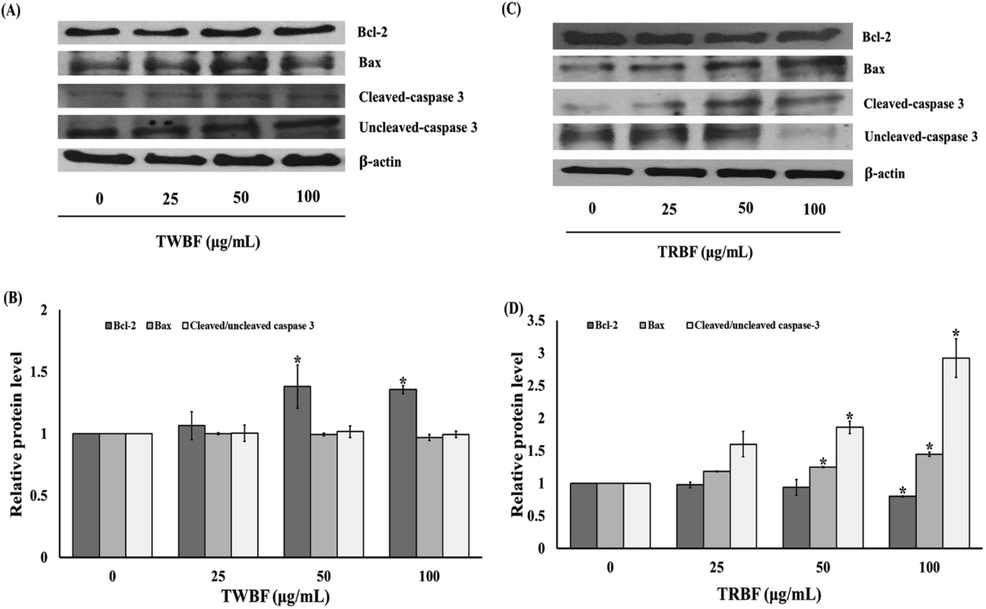
Effect of tuna blood (TWBF and TRBF) on apoptosis-related protein expression on A549 cells. (a) Western blot analysis of Bcl-2, Bax, Cleaved/Uncleaved caspase-3 protein expression in A549 cells treated with TWBF. (b) Quantification of Bcl-2, Bax, Cleaved/Uncleaved caspase-3 protein level in A549 cells treated with TWBF. (c) Western blot analysis of Bcl-2, Bax, Cleaved/Uncleaved caspase-3 protein expression in A549 cells treated with TRBF. (d) Quantification of Bcl-2, Bax, Cleaved/Uncleaved caspase-3 protein level in A549 cells treated with TRBF. Data are presented as the mean ± SEM. *P < 0.05 vs. the control group (0 µg/mL).
3.6 The Akt, ERK1/2, and p38 MAPK pathway in A549 cells
The MAPK and Akt (serine/threonine kinase) are recognized in multiple cellular functions i.e., cellular proliferation, growth, migration, survival, and apoptosis. Next, we characterize the effect of TRBF on the induction of the kinase signaling pathway by western blotting to understand the regulation of TRBF induced A549 cells apoptosis. TRBF treatment (50 and 100 µg/mL) significantly decreased the expression of p-Akt to 0.32 ± 0.08 and 0.21 ± 0.06, respectively compared with untreated cells. The phosphorylation status of p38 was significantly increased in the presence of 50 and 100 µg/mL TRBF to 1.13 ± 0.03 and 1.33 ± 0.04, respectively compared to the control group. However, there were no significant changes in ERK1/2 expression during TRBF at all concentrations (Fig. 5a and b).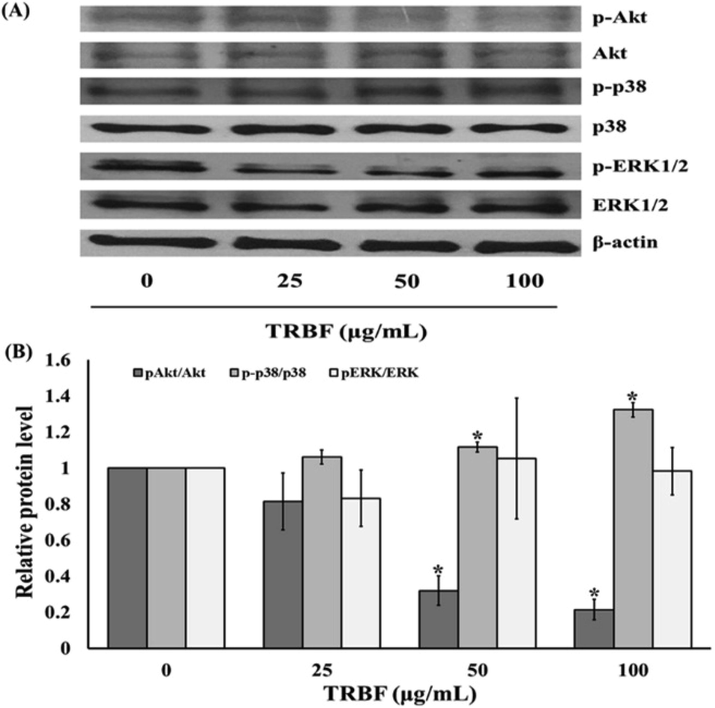
Effect of Tuna blood cell freeze dried (TRBF) on the Akt, p38 MAPK, and ERK1/2 signaling pathway on A549 cells. (a) Western blot analysis of pAkt/Akt, p-p38/p38, pERK/ERK protein expression in A549 cells treated with TRBF. (b) Quantification of pAkt/Akt, p-p38/p38, pERK/ERK protein level in A549 cells treated with TRBF. Data are presented as the mean ± SEM. *P < 0.05 vs. the control group (0 µg/mL).
4 Discussion
Previous study revealed that dietary supplementation from tuna extract inhibited the progression of cancer in vivo (Masuda et al., 2018). The role of tuna blood on anti-inflammatory activity has been recently reported (Klaypradit et al., 2021). Since inflammation plays a key role in cancer development and shares some common signaling pathways (Zhang et al., 2017), this study therefore aimed to explore whether the tuna blood possesses anticancer potential against human lung cancer cells and elucidate its mechanisms of apoptosis induction. Tuna whole blood freeze-dried (TWBF) and its fractionated tuna blood cell freeze-dried (TRBF) were applied to determine their effect on A549 lung cancer cells. Our results demonstrated that both TWBF and TRBF inhibit the proliferation of A549 cells; however, TRBF has superior inhibitory ability than TWBF as shown by its lesser IC50 value and percent of inhibition of cell proliferation (Fig. 1). One possible reason might be due to the high content of tyrosine and phenylalanine presented in TRBF than TWBF (Table 1). Tyrosine and phenylalanine are the amino acids involved in amino acid metabolism and protein structuring (Parthasarathy et al., 2018; Choi and Coloff, 2019). Phosphorylation of tyrosine hydroxyl group could modulate several cellular functions, which allow for druggable targets in the development of anticancer drugs such as a tyrosine-mimetic drug SM-88 (Choi and Coloff, 2019; Nguyeni et al., 2020). As TRBF contains a high content of Fe2+, Fe2+ can interact with hydrogen peroxide and generate the oxygen radicals, therefore capable to react with several biomolecules such as lipid, protein, and DNA causing cytotoxicity (Klaypradit et al., 2021). Selectivity index (SI) was used to assess the cytotoxic selectivity or safety effect of the chemotherapeutic agents. SI was calculated from the ratio of IC50 value of the treatments on normal cells to the cancerous cells. It has been considered that SI greater than three is high selectivity and is highlighted as the promising anticancer compound for further development of chemotherapeutic agents; therefore, only TRBF was considered selective to the A549 lung cancer cells (Fig. 1c) (Bézivin et al., 2003).
Several attempts have been made to target the uncontrolled growth of cancer by activating the programmed cell death or apoptosis signaling; thus shred a highlight in cancer therapy (Theansungnoen et al., 2016; Ou and Ho, 2016; Pfeffer and Singh, 2018). Cells that undergo apoptosis have distinct morphological and biochemical characteristics. As demonstrated by the microscopic study, A549 cells underwent apoptosis after exposure to TWBF and TRBF (Fig. 2). TRBF treatment caused typical apoptotic features rather than TWBF. The morphological features of apoptosis were cellular shrinkage, plasma membrane blebbing, and chromatin condensation (Pfeffer and Singh, 2018). The apoptosis characteristic of treated A549 cells might be triggered by ROS which is an important second messenger involved in a variety of events in signal transduction pathway such as mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/Akt, and p53 tumor suppressor; ultimately control cell proliferation, survival, and apoptosis (Redza-Dutordoir and Averill-Bates, 2016).
The elevated intracellular ROS levels (Fig. 3a) activate the opening of mitochondrial permeability transition pore (mPTP). Subsequently, the MMP was decreased (Fig. 3b) which led to the release of cytochrome C from the inner mitochondrial membrane. The cytosolic cytochrome C binds to apoptotic protease activating factor-1 (Apaf1) and procaspase-9. The apoptosome then activates the downstream proteolytic enzymes caspases. Among them, caspase-3 cleaves several cellular protein organelles in the cytosol and nucleus (Pfeffer and Singh, 2018; Redza-Dutordoir and Averill-Bates, 2016; Zhang et al., 2016). Moreover, the anti-apoptosis Bcl-2 protein was downregulated whereas the pro-apoptosis Bax protein was upregulated in response to TRBF administration. Also, cleaved caspase-3 was increased. Conversely, TWBF exposure was found to increase Bcl-2 expression; however, no changes on Bax and caspase-3 protein expression (Fig. 4). Studies have shown that Bcl-2 family proteins are associated with ROS and apoptosis. Excessive ROS generation in the apoptotic cells increase pro-apoptosis Bax and decrease anti-apoptosis Bcl-2 thereby trigger mitochondrial-dependent pathway (Pfeffer and Singh, 2018; Redza-Dutordoir and Averill-Bates, 2016; Zhang et al., 2016; Wang et al., 2018). This study reported that TRBF induces intrinsic apoptosis on lung cancer cells rather than that of TWBF administration. This result was in well agreement with the immunofluorescence staining (Fig. 2). Consistent with our finding, a fraction extracted from crocodile whole blood reduced cell viability and ultimately promote cancer cells apoptosis including lung cancer A549 cells, cervical cancer Hela cells, prostate cancer PC-3 cells, colorectal cancer CaCo-2 cells (Chook et al., 2021).
It remains to be further elucidated the involved molecular mechanisms by which TRBF mediates lung cancer cell apoptosis. The signal transduction of the MAPKs and Akt pathway plays an important role in the coordinated balance between cell survival and cell apoptosis in the decision of cell fate. Akt was found to be essential in the cell survival pathway. We demonstrated the suppression of Akt on A549 cells upon treatment with TRBF in a dose-dependent manner (Fig. 5). Similar to this study, the induction of apoptosis from crocodile blood extract was found to inhibit the PI3K/Akt survival pathway by increased phosphatase and tensin homolog deleted on chromosome TEN (PTEN) expression (Ou and Ho, 2016). It has been reported that ROS can directly activate PI3K and its downstream components. Moreover, ROS can inactive PTEN by oxidizing cysteine residues; therefore, dephosphorylate phosphatidylinositol (3,4,5)-triphosphate (PIP3) synthesis and eventually inactivate Akt which is required for cell survival (Pfeffer and Singh, 2018). The mitogen-activated protein kinase (MAPK) consists of its subfamily: the ERK1/2 and p38 which are known to play a key role in several cellular processes such as proliferation and apoptosis (Guo et al., 2020). ERK1/2 is activated upon the stimulation from mitogen or growth factor and is crucial for cell proliferation, migration, invasion, and angiogenesis. However, p38 MAPK has been shown to participate in cell proliferation and apoptosis which are triggered by cellular stress or inflammatory mediators (Swami et al., 2017; Guo et al., 2020; Deacon et al., 2003; Lee et al., 2006). In our experiment, TRBF caused the activation of p38 MAPK whereas no obvious changes on ERK1/2 expression in A549 lung cancer cells (Fig. 5). The ability of TRBF to modulate apoptotic activity would fit well with the activation of p38 MAPK signaling which might be regulated from the cellular stress caused by the increased intracellular ROS level. Consequently, the regulation of Bax/Bcl-2 decrease MMP, thereby releasing cytochrome C to the cytosol and stimulate caspases inducing apoptotic cell death (Zhang et al., 2016; Guo et al., 2020; Deacon et al., 2003; Lee et al., 2006).
5 Conclusion
Taken together, the present findings suggested the possible role of TRBF inhibited cell proliferation and induced A549 lung cancer cells apoptosis and the mechanism were associated, at least in part, with ROS-dependent p38 MAPK as well as downregulate Akt signaling in an ERK1/2 independent pathway (Fig. 6).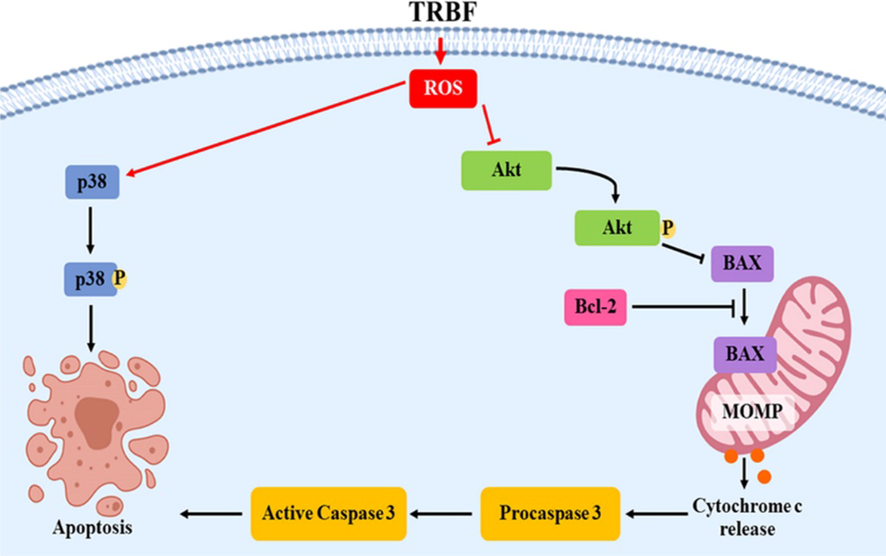
Scheme of apoptosis promoted by TRBF in A549 cells. →: stimulate; --|: inhibit.
Acknowledgements
We thank the publication clinic, Research and Development Office, Prince of Songkla University for English revision. SS was supported as a research assistant from Faculty of Science Research Fund (Contract no.1-2561-02-012), Prince of Songkla University, Songkhla, Thailand.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine.. 2003;10:499-503.
- [Google Scholar]
- The diverse functions of non-essential amino acids in cancer. Cancers (Basel). 2019;11(5):675.
- [CrossRef] [Google Scholar]
- Potential of crocodile blood as a medication and dietary supplement: A systematic review. Clin. Exp. Pharmacol. Physiol.. 2021;48(8):1043-1058.
- [Google Scholar]
- p38 mitogen-activated protein kinase mediates cell death and p21-activatedd kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol Biol Cell.. 2003;14:2071-2087.
- [Google Scholar]
- European Commission. Commission Directive 98/64/EC of 3 September 1998. 1998. Establishing community methods of analysis for the determination of amino-acids, crude oils and fats, and olaquindox in feeding stuffs, and amending Directive 71/393/EEC. Off J Eur Communities.257, 14–28.
- The increasing prevalence of non-communicable diseases in low-middle income countries: The view from Malawi. Int J Gen Med.. 2018;11:255-264.
- [Google Scholar]
- Causes of death in long-term survivors of non-small cell lung cancer: A regional surveillance, epidemiology, and end results study. Ann Thorac Med.. 2018;13(2):76-81.
- [Google Scholar]
- Tuna blood inhibits lipopolysaccharide-induced inflammatory mediators in RAW264.7 macrophages. Funct. Foods Health Dis.. 2021;11(4):201-212.
- [Google Scholar]
- Pseuderanthemum palatiferum (Nees) Radlk extract induces apoptosis via reactive oxygen species-mediated mitochondria-dependent pathway in A549 human lung cancer cells. Trop J Pharm Res.. 2019;18(2):287-294.
- [Google Scholar]
- Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta - Mol Cell Res.. 2006;1763(9):958-968.
- [Google Scholar]
- Dietary supplementation of selenoneine-containing tuna dark muscle extract effectively reduces pathology of experimental colorectal cancers in mice. Nutrients.. 2018;10(10):1380.
- [CrossRef] [Google Scholar]
- Unveiling prognostics biomarkers of tyrosine metabolism reprogramming in liver cancer by cross-platform gene expression analyses. PLoS One.. 2020;15:e0229276.
- [Google Scholar]
- Crocodile blood extract induces the apoptosis of lung cancer cells through PTEN activity. Oncol Rep.. 2016;36(3):1457-1466.
- [Google Scholar]
- A Three-Ring circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Front Mol Biosci.. 2018;5:29.
- [Google Scholar]
- Inhibition of HeLa cells metastasis by bioactive compounds in Crocodile (Crocodylus siamensis) white blood cells extract. Environ Toxicol.. 2015;31(11):1329-1336.
- [Google Scholar]
- Siamese crocodile white blood cell extract inhibits cell proliferation and promotes autophagy in multiple cancer cell lines. J Microbiol Biotechnol.. 2018;28(6):1007-1021.
- [Google Scholar]
- Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta - Molecular Cell Research.. 2016;1863(12):2977-2992.
- [Google Scholar]
- Eribulin in non-small cell lung cancer: challenges and potential strategies. Expert Opin Investig Drugs.. 2017;26(4):495-508.
- [Google Scholar]
- Cationic Antimicrobial Peptides Derived from Crocodylus siamensis Leukocyte Extract, Revealing Anticancer Activity and Apoptotic Induction on Human Cervical Cancer Cells. Protein J.. 2016;35(3):202-211.
- [Google Scholar]
- Sotetsuflavone inhibits proliferation and induces apoptosis of A549 cells through ROS-mediated mitochondrial-dependent pathway. BMC Complement Altern Med.. 2018;18(1):235.
- [CrossRef] [Google Scholar]
- Non-small cell lung cancer: Current treatment and future advances. Transl Lung Cancer Res.. 2016;5(3):288-300.
- [Google Scholar]
- ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev.. 2016;2016:4350965.
- [CrossRef] [Google Scholar]
- Resolution of cancer-promoting inflammation: a new approach for anticancer therapy. Front Immunol.. 2017;8:71.
- [Google Scholar]







