Translate this page into:
Antiproliferative and antioxidant potential of Tridax procumbens extracts against various human cancer cell lines: An insight for medicines from weeds
⁎Corresponding authors. anniegupta0601@gmail.com (Annie Gupta), sunil.kumar@gmpgeo.com (Sunil Kumar Panda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
This study aimed to evaluate the anticancer and antioxidant potential of Tridax potential and to identify its main phenolic compounds using reverse-phase HPLC. The crude extract of T. procumbens was separated into different fractions using the liquid–liquid partition method. T. procumbens crude extracts and fractions were tested on human cancer cell lines (PC-3, COLO-205, A549, A431, MDA-MB-231, MDA-MB-468, and K562) as well as a control cell line (HEK-293). The antioxidant activities of the extracts and fractions were also assessed. The majority of the extracts appeared to inhibit the progression of human cancer cell lines based on IC50 values that varied from 23.41 to 90.76 µg/mL. However, the chloroform fraction of the stem (7) displays strong activity against A431 and MDA-MB-231, with IC50 values of 23.41 g/mL and 29.45 µg/mL, respectively. The antioxidant result shows that extracts and their fractions scavenge free radicals with an IC50 value between 14.70 and 93.80 µg/mL. However, 7 showed more efficacies with an IC50 of 14.70 µg/mL in the DPPH assay and 29.90 µg/mL in the nitric oxide assay. HPLC analysis revealed that the flower part of T. procumbens contains high levels pf ferulic acid (0.55 to 2.65 mg/g) and kaempferol (1.1 to 4.95 mg/g). A strong antioxidant activity observed in DPPH and NO assay which helps to measure the extract’s effectiveness in scavenging NO free radical, neutralizing other reactive oxygen species (ROS), often correlate with significant antiproliferative effects in various cancer cell lines. This study highlights the potential of plant-based compounds in cancer treatment.
Keywords
Antiproliferation
Cell line
Antioxidant
T. procumbens
- DMEM
-
Dulbecco’s minimal essential media
- DMSO
-
Di methyl sulphoxide
- FBS
-
Fetal bovine serum
- IC50
-
Half maximal inhibitory concentration
- MTT
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NaOH
-
Sodium hydroxide
- PBS
-
Phosphate buffer saline
- HO3P
-
Phosphoric acid
- FeSO4
-
Ferrous sulphate
- GAE
-
Gallic acid equivalent
- FSE
-
ferrous sulphate equivalent
- AA
-
Ascorbic acid
- DPPH
-
2,2-Diphenyl-1-picrylhydrazyl
- NO
-
Nitric Oxide
- TAC
-
Total antioxidant capacity
- TCA
-
Trichloro acetic acid
- TF
-
Total Flavonoid
- TPTZ
-
2, 4, 6-Tripyridyl-S-triazine
Abbreviations
1 Introduction
Tridax procumbens is a common herb of the Asteraceae family indigenous to America. It is extensively spread in India, tropical Africa, Asia and Australia. It is frequently recognized as a “coat-button”. Leaf extract of T. procumbens has traditionally been used to treat liver diseases. The Urash tribe of southern Bihar used a thin paste produced from the leaves to alleviate cystitis irritation. The herb is also used to cure epilepsy, prevent hair loss, treat haemorrhagic cuts, and treat fever, cough, backaches, stomach-aches, and bronchial catarrh (Saraf et al., 1991; Taddei and Rosas-Romero 2000; Tiwari et al., 2004). It also contains anticoagulant, antifungal, antibacterial, anti-inflammatory, immunomodulatory, and insect repellent activities (Ravikumar et al., 2005; Salahdeen et al., 2004; Syed et al., 2020). These attributes make it a valuable resource in traditional medicine, especially in undeveloped and underdeveloped countries where conventional healthcare may be less accessible. For instance, in Guatemala, T. procumbens leaf juice is used as a treatment for diarrhoea, stomach pains, vaginitis, and inflammation. The entire plant, including the leaves, was used by Nigerians to treat a wide range of illnesses, including fever, cough, stomach aches, etc. (Prajapati, 2008).
Numerous phytochemicals, such as flavanoids sterols, β-amyron, β-amyrin, lupeol, arachidic acid, fucosterol, luteolin, palmitic acid, and lauric acid, were found in T. procumbens (Ali, 2001) in previous phytochemical research (Surendra et al., 2016a; Surendra et al., 2016b). A methanolic extract of T. procumbens' leaves, stems, and flowers has been shown to provide substantial liver protection against the drug-induced toxicity caused by isoniazid and rifampicin in male Wistaer rats in previous investigations (Sagheer et al.,2018). On the A549 human lung cancer and MCF-7 (A549, 250 µg/mL) human breast cancer cell lines, methanol extracts of T. procumbens leaves have been found to have antiproliferative properties (Syed et al., 2020; Mundada et al., 2010; Aniel, 2010).
Another study (Sagadevan et al., 2018) showed the cytotoxic efficacy of T. procumbens leaf extract in human breast cancer cell lines (MCF-7, 150–300 µg/mL). Although there is limited information on the anticancer activity of T. procumbens, and some studies suggested that aqueous leaf extract has not shown significant anticancer properties. However, acetone and ethanol extract of leaf has potent activity against human lung (A549) carcinoma cells and human liver carcinoma (Hep G2) cells (Lakshmipriya et al., 2019; Vishnu and Srinivasa, 2015). Other studies have suggested that the aerial parts of this plant, such as the stems and flowers, may have medicinal benefits, including potential antiproliferative effects (Niño et al., 2006; Priya et al., 2011a; Ali). Previously the cytotoxic activity of methanolic leaf extract of T. procumbens has been described against human lung cancer (A549) cells and breast cancer (MCF-7) cells. Methanolic extract of leaves (250 µg/mL) exhibited high activity (84 ± 2.8 %) against human lung (A549) cancer cells while only 68 ± 3.1 % cytotoxicity in breast cancer cells (Syed et al., 2020). Another literature has been reported the cytotoxic potential of acetone and aqueous extract from the flower and leaf extract of T. procumbens against prostate epithelial cancer (PC3) cell line, acetone extract (250 µg/mL) showed 82.28 % cell death (Priya et al., 2011a; Priya et al., 2011b). Despite the plant not being extensively researched for its anticancer properties, particularly in relation to organ-specific human cancer cell lines, this study seeks to explore the antiproliferative effects of the stems and flowers, parts of the plant that have not been previously studied. From studies, the leaves have been previously examined (Vishnu and Srinivasa, 2015; Syed et al., 2020). Another review article has been reported that the T. procumbens exhibit synergistic effect with doxorubicin against breast cancer (Mateusz et al., 2023). Therefore, our focus is on the other aerial parts that offers the potential to uncover new bioactive compounds with therapeutic value. The study also focused on the medicinal use of the plant by simultaneously detecting two major bioactive phenols using reverse-phase high-performance liquid chromatography coupled with photodiode array detection.
2 Materials and Methods
2.1 Chemicals
The solvents used were of analytical grade. Tri-pyridyl-s-atrazine (TPTZ), Roswell Park memorial institute medium (RPMI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye, phosphate-buffered saline (PBS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich, Bangalore, India. Dulbecco’s minimal essential media (DMEM) and fetal bovine serum (FBS) were purchased from Gibco, Therm Fisher Scientific India Pvt. Ltd., Mumbai, India. Dimethyl sulphoxide (DMSO), isopropanol, HPLC-grade methanol, acetonitrile, water and chemicals utilized in our study were purchased from Merck Ltd., Mumbai India. Gallic acid, ferrous sulphate (FeSO4), Na2[Fe (CN)5NO].2H2O, tris- [hydroxyl methyl] amino-methane N-(1-naphthyl) ethylenediamine dihydrochloride, ascorbic acid, quercetin and hydrogen phosphite (H3PO3) were purchased from Himedia Laboratories Mumbai, India. Reference standards of ferulic acid (percentage purity ≥ 99 %), kaempferol (Percentage purity ≥ 97.0 %) were purchased from Sigma-Aldrich, (Mumbai), India.
2.2 Collection and extraction of plant material
The plants were acquired in the months of April and May from the fields of CSIR-CIMAP Lucknow, India. The sample was submitted to the Department of Pharmacognosy and Phytochemistry, Integral University, Lucknow, for taxonomic identification (Specimen No. IU/PHAR/HRB/15/23). The preparation of methanolic crude extracts from the leaves, stems, and flowers of T. procumbens was carried out according to the method described in our earlier publication (Sagheer et al., 2018). The concentrated methanolic extract of leaves (180 g), stem (150 g), and flower (80 g) were then subjected to fractionation by following the liquid–liquid partitioning method. The fractions were completely dried under a vacuum evaporator, and the solvent fractions of T. procumbens in hexane, chloroform, n-butanol, and methanol were stored at −20 °C for further study.
2.3 Culture of the cell lines
One normal cell line and seven cancer cell lines specific to human organs have been selected for this study. The following cancer cell lines were purchased from the cell repository at the National Centre for Cell Science (NCCS), Pune, India: prostate cancer (PC3) cells, lung cancer (A549) cells, squamous carcinoma (A431), breast carcinoma (MDA-MB-231), breast adenocarcinoma (MDA-MB 468), colon carcinoma (COLO-205), erythroleukemia (K562). The normal kidney cell line (HEK-293) was also included. All human cancer cell lines were cultivated in Dulbecco's minimum essential medium (DMEM) or RPMI, depending on the type of cell, and incubated at 37 °C with 5 % CO2 in a humid environment. The media were supplemented with 10 % fetal bovine serum (FBS) and 1 % antibiotic–antimycotic (Ab/Am).
2.4 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)assay
The protocol describes by Pathak et al. (2019) was adapted to a 96-well plate format. The cells were trypsinized after attaining 80–90 % confluence and seeded in 96-well plates (∼2 × 103 cells/well). The plate was kept in the dark for 4 h at 37 °C. The next day, cells were incubated with various concentrations (2, 10, 50 and 100 µg/mL,) of extracts and fractions (dissolved in DMSO) of T. procumbens for 24 h. Then, 10 μL of MTT (500 µg/mL) dye was added to each well of 96 well plates. After incubation, formazan crystal was solubilized in 100 μL dimethyl sulphate (DMSO), and their optical density was recorded at 570 nm. The results were expressed as percent cytotoxicity.
2.5 Antioxidant assays
2.5.1 DPPH free radical scavenging assay
The antioxidant or free radical-scavenging properties of methanolic extracts and fractions of tested plant were investigated in-vitro (Luqman et al., 2009). Various concentrations (2, 10, 25, and 50 µg/mL) of extract and fractions were combined with 100 mMTris-HCl buffer (pH 7.4) and 500 mM 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and then incubated for 30 min at room temperature to estimate radical scavenging activity. A spectrophotometer was used to measure the optical density at 517 nm, and the findings were compared to the control (Ahmad et al., 2014).
2.5.2 Reducing potential assay
The reduction potential of extracts of T. procumbens was assessed using a method described by (Yen and Chen, 1995). For the reducing potential assay, different concentrations of the extracts and fraction were mixed in 200 mM phosphate buffer (pH 6.6) along with 1 % ferricyanide and incubated in a water bath at 50 °C for 20 min. After incubation, 10 % trichloroacetic acid (TCA, 250 µL) was mixed to precipitate, and the blend was centrifuged at 5000 rpm for 5 min. In the supernatant, the same amount of distilled water was mixed. The absorbance was recorded in the reagent blank after adding 0.10 % ferric chloride (FeCl3) at 700 nm.
2.5.3 Nitric oxide scavenging assay
The scavenging potential of T. procumbens extract was assessed using a method previously reported by Marcocci et al. (1994). Different concentrations of the extracts were mixed with sodium nitroprusside in buffered saline (10 mM) and incubated for 30 min at room temperature. After incubation, 100 µL of Griess reagent was mixed with 50 µL of the reaction mixture. The absorbance was measured at 546 nm, and the scavenging potential of the extract and fractions was calculated as percent change compared to the control.
2.6 Total phenolic contents estimation
Total phenolic contents (TPC) of the extract of T. procumbens were determined using the Folin-Ciocalteu reagent in terms of gallic acid equivalents (GAE) (Singleton et al., 1999; Luqman, et al., 2012). The extracts were mixed with Folin-Ciocalteu reagent at a 1:9 dilution and 7.5 % sodium carbonate solution. The mixtures were incubated at 37 °C for 90 min. The absorbance was measured at 765 nm, and the results were estimated relative to the control. The phenolic content was quantified in terms of GAE using a gallic acid standard curve.
2.7 Total flavonoid content estimation
The colorimetric approach was used to determine the total flavonoid contents (Ahmad et al., 2014; Meda et al., 2005). The extracts were diluted in 50 µL of distilled water, 150 µL of methanol, 10 μL of 1 M potassium acetate (CH3COOK), 10 μL of 10 % aluminium chloride (AlCl3), and 280 μL of distilled water. After 30 min of incubation, 200 µL of the reaction mixture was transferred to the well plate, where the absorbance at 415 nm was measured. The total flavonoid content was expressed as mg/g of dried extract or percentage of quercetin equivalent (QE).
2.8 Total antioxidant capacity (TAC) estimation
Using a conventional procedure, the total antioxidant capacity of the T. procumbens extract was determined (Prieto et al., 1999). Different concentrations of the extract and fraction were combined with 1 mL of the total antioxidant capacity (TAC) reagent, which is a mixture of 635 µM ammonium molybdate, 25 mM sodium phosphate monobasic, and 607 mM H2SO4, respectively. The mixture was heated to 95 °C in a boiling water bath for 90 min, and the optical density was measured at 695 nm. The total antioxidant capacity (TAC) was expressed as ascorbic acid equivalents (AAE) using a standard curve (Negi et al., 2011).
2.9 Ferric ion reducing antioxidant potential (FRAP) determination
The FRAP assay determines the antioxidant potential of extracts through the reduction of the ferric tripyridyltriazine (Fe (III)-TPTZ) complex to the ferrous tripyridyltriazine (Fe (II)-TPTZ) (Benzie et al., 1996). In the FRAP assay, different concentrations of extracts were mixed into a freshly prepared ferric ion-reducing antioxidant potential (FRAP) reagent (10 mM TPTZ, 20 mM FeCl3, and 300 mM acetate buffer pH 3.6 in a ratio of 1:1:10). After 5 min of incubation at 37 °C, the absorbance of the complex was recorded at 593 nm. The ferric reducing potential of extracts was expressed as ferrous sulphate equivalent (FSE) reduced by the standard curve of ferrous sulphate (Luqman et al., 2012).
2.10 HPLC analysis
Preparation of standards: Ferulic acid and kaempferol standard stock solution (1 mg/mL) were prepared in HPLC-grade methanol and kept at 4 °C. The stock solutions were properly diluted in methanol in order to generate a working solution with a lower concentration of 0.1 mg/mL.
HPLC conditions: Methanolic extracts of stem, leaves, flower of T. procumbens was performed on using a Waters HPLC-PDA-2996 (Waters Corporation, Milford, MA, USA) outfitted with a Waters 600 controller, a Waters Delta 600 solvent delivery system with in-line degasser AF, a Rheodyne 7125 sample injector fitted with a 20 µL loop, and a Waters 2996 photodiode array detector (PDA, Waters Corporation, Milford, MA, USA), with Waters Empowered 2.154 software. A gradient mode with mobile phase A (consisting of 0.1 % formic acid in water) and B (pure acetonitrile) at a flow rate of 1.0 mL min−1 was used to carry out the chromatographic separation. The column used was a Waters µ Bondapak C18 (3.9 x 300 mm) column with a Waters µ Bondapak C18 guard column. The injection volume for the sample and the standard was 20 µL. Before being delivered to the column for separation, mobile phases were properly filtered through a 0.22-m Millipore filter and ultrasonically degassed for 15 min. The run time was 20 min. Extract with traces of compound 1, 2, and other substances were found at 254 nm. Well-resolved peaks were identified and quantified using external standards.
2.11 Data analysis
All tests were carried out in triplicate. Data were expressed as mean ± SD and calculations were performed using MS-Excel. The median inhibitory concentration (IC50) was estimated using Windows version 4.07 (Systat Software Inc., Chicago, USA). The graphs were designed with using GraphPad Prism version 5.1 software.
3 Results
3.1 Extraction and fractionation of T. procumbens
The choice of solvent plays a crucial role in determining the effectiveness of the extract and fractions. The active components consistently extracted by n-hexane and chloroform compared to n-butanol. This difference in effectiveness can be attributed to the polarity of the solvents and their ability to dissolve different types of phytochemicals. For instance, n-hexane is a non-polar solvent, which is more suitable in extracting non-polar compounds like lipids, fatty acids, and some terpenoids. Also, these compounds own significant antiproliferative properties, mostly against human cancer cells. However, chloroform, is moderately polar solvent therefore it can extract a wider range of bioactive compounds, including both non-polar and slightly polar molecules such as alkaloids, flavonoids, and some glycosides. But, n-butanol is a more polar solvent and is commonly better at extracting polar constituents like acids and sugars. The yielded dried fractions obtained from crude extracts have been summarized in (Table 1).
Crude extracts
Fraction
% yields (w/w)
Methanolic extract of Leaves (1)
n-Hexane (2)
Chloroform (3)
n-Butanol (4)28
19.5
15.3
Methanolic extract of Stem (5)
n-Hexane (6)
Chloroform (7)
n- Butanol (8)25.25
18.11
13.2
Methanolic extract of Flower (9)
n-Hexane (10)
Chloroform (11)
n-Butanol (12)22.21
15.33
11.14
3.2 Anti-proliferative activity of T. procumbens extracts
Antiproliferative activity of crude methanolic extracts and their fractions (hexane, CHCl3, and n-butanol) of T. procumbens were examined on organ-specific human cancer cell lines (prostate cancer (PC3) cells are androgen-independent, lung cancer (A549) cells are categorized by their epithelial morphology and their ability to produce lecithin with a high percentage of unsaturated fatty acids, squamous carcinoma (A431) cells express high levels epidermal growth factor receptor (EGFR), breast carcinoma (MDA-MB-231) is triple-negative (lacking estrogen receptor, progesterone receptor, and HER2 expression), breast adenocarcinoma (MDA-MB 468) has overexpression of the EGFR and are also triple-negative, colon carcinoma (COLO-205) is adherent with an epithelial morphology, erythroleukemia (K562) has genetic abnormality in chromosome 22 and HEK −293 is normal cells.) by employing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Fig. 1). The results indicated that the extracts and fractions inhibited different human cancer cell lines.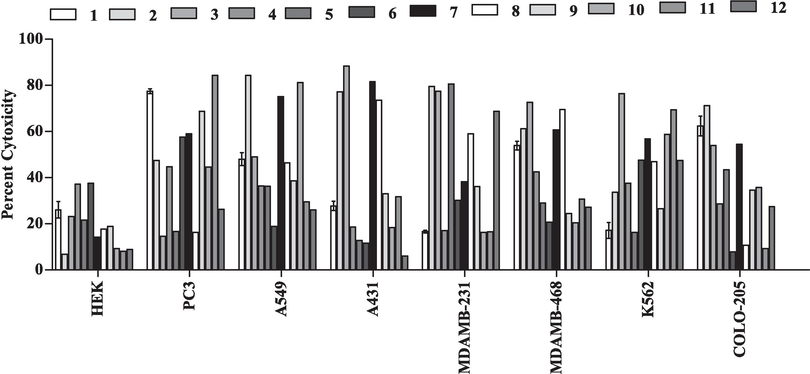
Antiproliferative activity of extract and fraction of T. procumbens crude methanol leaves extract (1); hexane (2); chloroform (3); butanol (4) fractions of leaves, crude methanol stems extract (5); hexane (6); chloroform (7); butanol (8) fraction of stem and crude methanol flowers extract (9); hexane (10); chloroform (11) and butanol (12) flower fractions against tested organ specific human cancer cell lines at higher concentration (100 µg/mL). Data are presented in mean ± SD.
In PC-3 cell line, the fractions (11, 1, 9, 7 and 6) inhibit the growth of cell line above 50 % and their IC50 were 37.44, 54.47, 59.93 and 83.57 µg/mL respectively. But, fraction 11 demonstrated the most potent activity against PC-3 cell line with an IC50 of 37.44 µg/mL (Fig. 2A). In contrast, the remaining tested extracts exhibited less than 50 % inhibition of PC-3 cell line growth, with inhibition rates ranging from 14.54 % to 47.48 % (Fig. 1).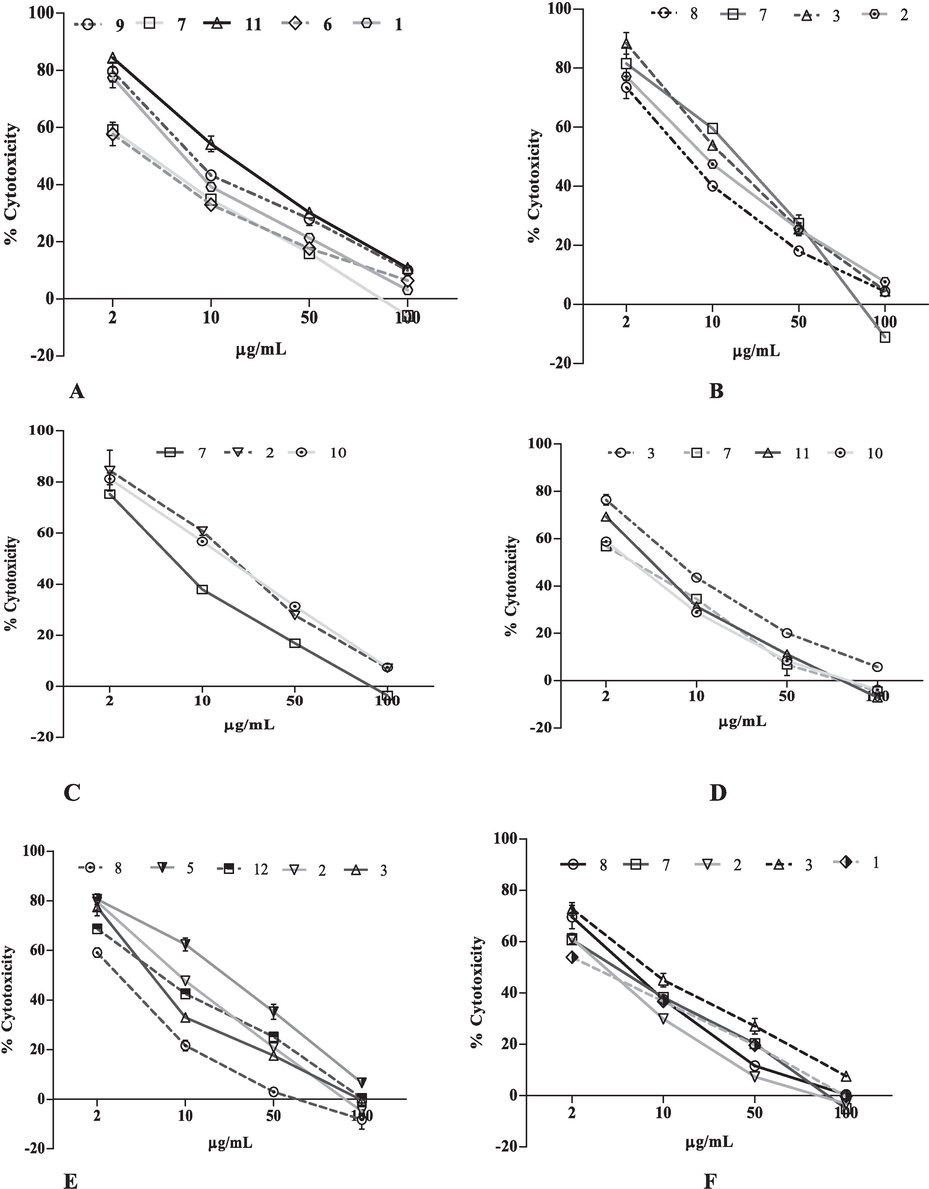
The effect of extract and fraction of T. procumbens in dose-dependent manner, crude methanol leaves extract (1); hexane (2); chloroform (3);butanol (4) fractions of leaves, crude methanol stems extract (5); hexane (6); chloroform (7); butanol (8) fraction of stem and crude methanol flowers extract (9); hexane (10); chloroform (11) and butanol (12) flower fractions against organ specific human cancer cell lines: (A) PC-3; (B) A549; (C) A431; (D) MDA-MB-231; (E) MDA-MB-468; (F) K-562 and (G) COLO-205. Data are presented in mean ± SD.
The effect of extract and fraction of T. procumbens in dose-dependent manner, crude methanol leaves extract (1); hexane (2); chloroform (3);butanol (4) fractions of leaves, crude methanol stems extract (5); hexane (6); chloroform (7); butanol (8) fraction of stem and crude methanol flowers extract (9); hexane (10); chloroform (11) and butanol (12) flower fractions against organ specific human cancer cell lines: (A) PC-3; (B) A549; (C) A431; (D) MDA-MB-231; (E) MDA-MB-468; (F) K-562 and (G) COLO-205. Data are presented in mean ± SD.
Fractions of extracts (10, 2 and 7) significantly decreased the proliferation of human lung carcinoma cell lines (A549) more than 50 % with IC50 values of 31.01 µg/mL, 31.99 µg/mL and 60.92 µg/mL respectively in which fraction 10 (hexane flowers fraction) exhibited strongest activity against A549 cell line with IC50 31.01 µg/mL (Fig. 2B). Conversely, the other tested extracts inhibit the growth of A549 cell line. However, the inhibition of A549 cell line was not more than 50 % (18.19 % to 48.97 %, Fig. 1).
Furthermore, twelve extracts were also tested against skin cancer cell lines (A431). Fraction (7, 3, 2 and 8) inhibited the growth of A431 cell line more than 50 %with IC50 29.45 µg/mL, 38.31 µg/mL, 48.12 µg/mL and 61.82 µg/mL respectively. On the contrary, the fraction 7showed very strong activity against A431 cell line with IC50 29.45 µg/mL (Fig. 2C). The remaining extracts inhibit the growth of A431 cell line less than 50 % (6.04 % to 33.03 %, Fig. 1).
All the crude extracts and their fractions were examined for activity against human cancer cell line. After 24 h incubation, extracts (5, 2, 12, 3 and 8) more than 50 % with their IC50 23.41 µg/mL, 45.80 µg/mL, 57.33 µg/mL, 69.88 µg/mL and 88.76 µg/mL respectively (Table 3). Moreover, the extract 5 (methanol extract of stems) exhibits more potent activity with IC50 23.41 µg/mL against MDAMB-231 amongst all the tested extracts (Fig. 2D). However, the remaining tested extracts (1, 4, 6, 7, 9, 10, 11 and 12) delayed the cell growth less than 50 % (16.19 % to38.19 %, Fig. 1).
Extracts/
fractionsHEK-293
PC-3
A 549
A 431
MDAMB-231
MDAMB-468
K-562
COLO-205
1
−
59.93
−
−
−
93.02
−
78.28
2
−
−
31. 99
48.12
45.80
81.34
−
61.09
3
−
−
−
38.31
69.88
47.83
36.86
90.76
4
−
−
−
−
−
−
−
−
5
−
−
−
−
23.41
−
−
−
6
−
83.57-
−
−
−
−
−
7
−
77.72
60.92
29.45
−
69.18
83.05
86.80
8
−
−
61.82
88.76
71.03
9
−
54.47
−
−
−
−
−
−
10
−
−
31.01
−
−
−
84.51
−
11
−
37.44
−
−
−
−
55.78
−
12
−
−
−
−
57.33
−
−
−
In MDAMB-468 (human breast adenocarcinoma), the extracts (3, 7, 8, 2, and 1) among all tested extracts were shown the inhibition of cell growth more than 50 % (Fig. 2E) respectively. In contrast, 3 (chloroform leaves fraction) out of five has more potent cytotoxic activity against MDA MB-468 with IC50 47.83 µg/mL.
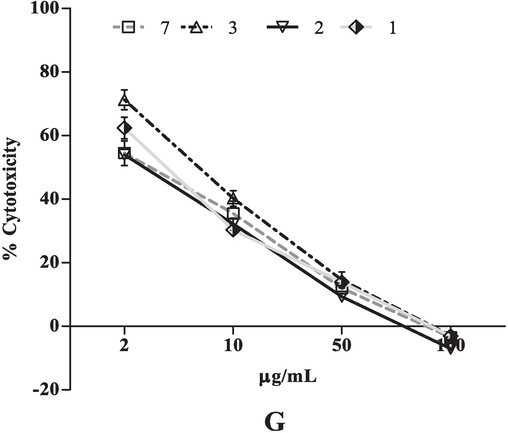
All the extracts tested against K562 (leukemia cancer) cell line in which fractions (3, 11, 7 and 10) showed cytotoxic activity 50 % respectively. Interestingly, among five the fraction 3has significant cytotoxicity effect with IC50 36.86 µg/mL (Fig. 2F).
Moreover, the cytotoxic activities of all the extracts were also tested against the COLO-205 cell line. The four extracts (2, 1, 7 and 3, Fig. 2G) showed cytotoxic activity more than 50 %. The results revealed, all the tested crude extracts were inhibited only 37 ± 1.87 % of HEK-293 (human embryonic kidney) cells at the tested higher concentration (100 μg/mL; Fig. 1).
3.3 Scavenging of NO and DPPH by extract and fractions of T. procumbens
Though, the various extracts of T. procumbens exhibit activity against organ specific human cancer cell lines. The scavenging potential of 12 extracts of T. procumbens were tested in which 10 extracts showed scavenging activity through IC50 values of 14.70 µg/mL to 93.40 µg/mL respectively (Table 2). However, obtained data of free radicals showed that inhibition of testing extracts was in descending order: chloroform fraction of stem (7) > crude methanol leaves extract (1) > crude methanol stem extracts (5) > chloroform fraction of flower (11) > crude methanol flower extract (9) > butanol fraction of flower (12) > butanol fraction of leaves (4) > chloroform fraction of leaves (3) > hexane fraction of flower (10) > butanol fraction of stem (8) (Fig. 3A).Table 2
Extract and fractions
DPPH
NO
Methanolic crude extract of leaves (1)
40.03
82.39
Hexane fraction of leaves (2)
−
−
Chloroform fraction of leaves (3)
90.81
96.80
n-Butanol fraction of leaves (4)
89.82
−
Methanolic crude extract of stem (5)
50.35
−
Hexane fraction of stem (6)
−
−
Chloroform fraction of stem (7)
14.70
29.90
n-Butanol fraction of stem (8)
93.40
−
Methanolic crude extract of flower (9)
80.48
34.26
Hexane fraction of flower (10)
91.40
−
Chloroform fraction of flower (11)
80.32
−
n-Butanol fraction of flower (12)
85.47
−
Ascorbic Acid (AA)
33.04
27.26
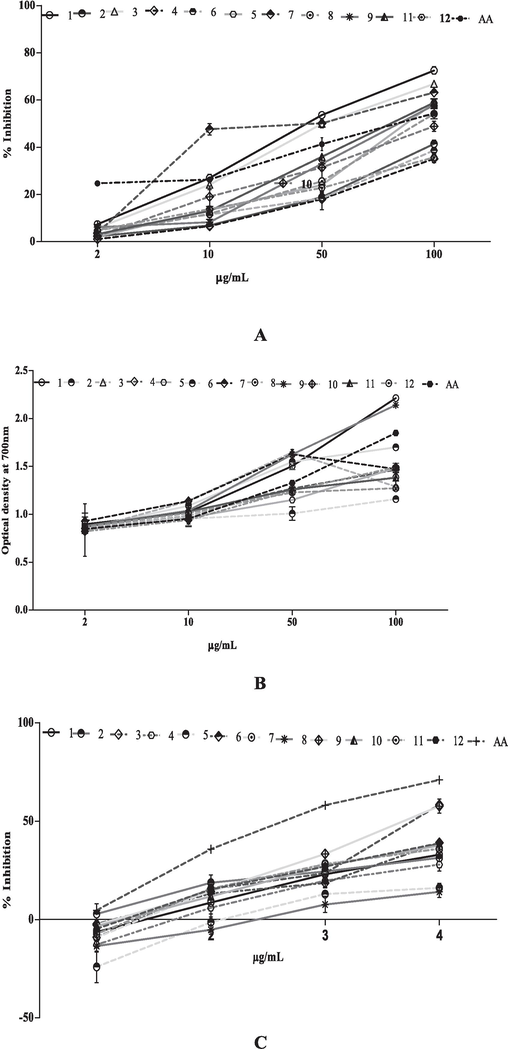
Antioxidant activity of extract and fractions of T. procumbens crude methanol leaves extract (1); hexane (2); chloroform (3);butanol (4) fractions of leaves, crude methanol stems extract (5); hexane (6); chloroform (7); butanol (8) fraction of stem and crude methanol flowers extract (9); hexane (10); chloroform (11) and butanol (12) flower fractions in concentration dependent manner: (A) DPPH free radical inhibition; (B) Reducing power assay; (C) Nitric oxide inhibition; (D) Total Phenolic activity; (E) Total Flavonoid content; (F) Total Antioxidant capacity and (G) Ferric reducing antioxidant power. Values are presented in mean ± SD.
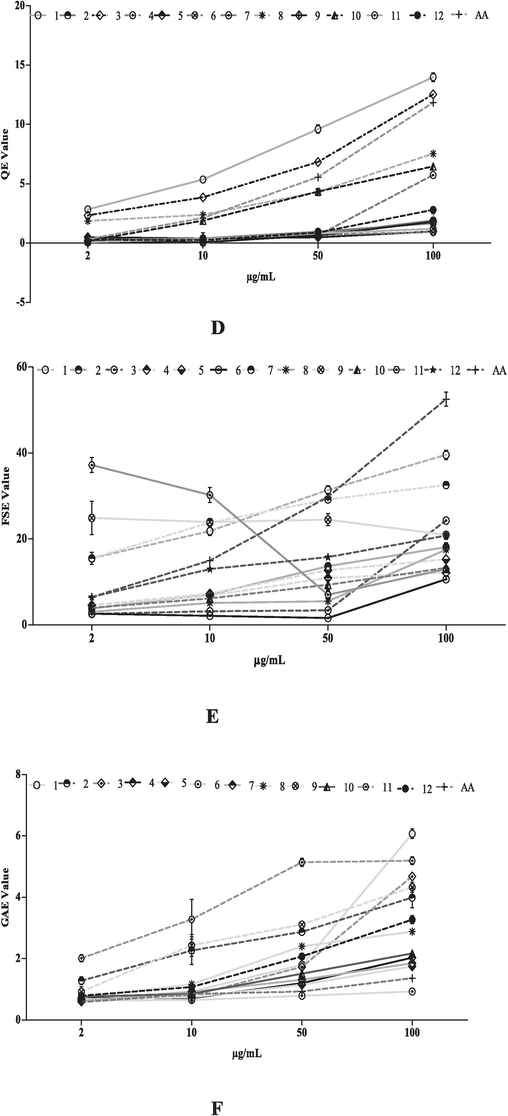
Antioxidant activity of extract and fractions of T. procumbens crude methanol leaves extract (1); hexane (2); chloroform (3);butanol (4) fractions of leaves, crude methanol stems extract (5); hexane (6); chloroform (7); butanol (8) fraction of stem and crude methanol flowers extract (9); hexane (10); chloroform (11) and butanol (12) flower fractions in concentration dependent manner: (A) DPPH free radical inhibition; (B) Reducing power assay; (C) Nitric oxide inhibition; (D) Total Phenolic activity; (E) Total Flavonoid content; (F) Total Antioxidant capacity and (G) Ferric reducing antioxidant power. Values are presented in mean ± SD.
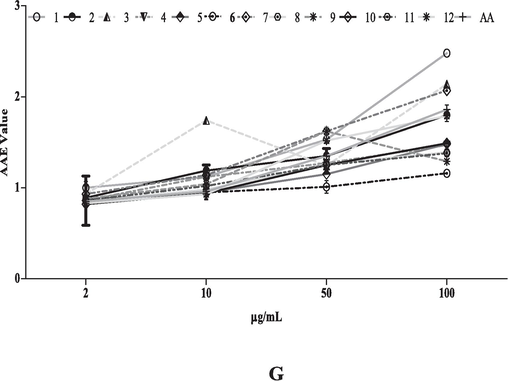
Antioxidant activity of extract and fractions of T. procumbens crude methanol leaves extract (1); hexane (2); chloroform (3);butanol (4) fractions of leaves, crude methanol stems extract (5); hexane (6); chloroform (7); butanol (8) fraction of stem and crude methanol flowers extract (9); hexane (10); chloroform (11) and butanol (12) flower fractions in concentration dependent manner: (A) DPPH free radical inhibition; (B) Reducing power assay; (C) Nitric oxide inhibition; (D) Total Phenolic activity; (E) Total Flavonoid content; (F) Total Antioxidant capacity and (G) Ferric reducing antioxidant power. Values are presented in mean ± SD.
The total phenolic content was examined by Folin-Ciocalteu reagent of extract of T. procumbens. The result revealed that the phenolic contents in 12 tested extracts were ranged from 6.07 ± 0.86 to 0.93 ± 0.03 GAE (Fig. 3D). In addition, the total flavonoid content of T. procumbens extracts was measured by aluminum chloride reagent. The flavonoid content of the extract (12) was ranging from 13.97 ± 0.37 to 0.93 ± 0.06 QCE (Fig. 3E).
The reduction potential of testing extracts ranges from 2.21 ± 0.01 to 1.16 ± 0.01 at higher tested concentration (100 µg/mL). Among all the extracts the extract 1(methanolic leaves extract) showed better reducing potential (2.21 ± 0.01) other tested extracts Fig. 3B. Conversely, nitric oxide scavenging activity of all tested extracts of T. procumbens was ranged from58.23 ± 0.12 to 14.02 ± 2.90 % in a concentration dependent manner. However, extracts 7and 9 showed the maximum inhibition of radicals with anIC50 value 29.90 µg/mL and 34.26 µg/mL respectively (Table 2, Fig. 3C).
Antioxidant capacity (uttered ascorbic acid equivalent) for crude methanolic extracts as well as fractions of selected plant was shown in Fig. 3F. Our tests showed the ferric reducing antioxidant potential of all the 12 tested extracts of T. procumbens which ranges from 10.62 ± 0.10to 39.57 ± 1.05FSE (Fig. 3G).
3.4 HPLC quantification
Analytical HPLC was used to quantitatively estimate the compounds present in the methanolic extract of T. procumbens. The leaves stem, and flowers of T. procumbens were measured for the presence of kaempferol and ferulic acid. HPLC chromatogram of methanolic extract of leaves, stem and flower of T. procumbens showing kaempferol and ferulic acid is presented in Fig. S1 while HPLC chromatogram of pure kaempferol and ferulic acid obtained from methanolic extract of T. procumbens presented in Fig. S2.
Within the plant, there were significant variations in the amounts of kaempferol and ferulic acid. Kaempferol levels varied from 1.1 mg to 4.95 mg/g dry wt., with leaves having the highest concentration and flowers having the lowest (Table 4). Ferulic acid content varied from 0.55 mg/g to 2.65 mg/g dry weight, with stem content being the lowest (0.55 mg/g) and flower content being the highest (2.65 mg/g). Ferulic acid was discovered to be abundant in T. procumbens. There are traces of some other derivative compounds.
Compounds
Concentration (mg/g dry wt. sample)
Leaves
Stem
Flower
Kaempferol
4.95
2.19
1.11
Ferulic acid
1.29
0.55
2.65
4 Discussion
Cancer is one of the most prominent ailments in both developing and urban countries of the world and is still not curable in most cases. Generally, the herbal medicines are exercised to cure a number of ailments, including cancer (Benzie et al., 1996). Seeing that, naturally occurring bioactive compounds such as secondary metabolites and antioxidants (Andriana et al., 2019) are the potential sources of plants from traditional herbs and biologically active chemical constituents isolated from medicinal plants are able to scavenge superoxide free radicals and helpful in minimizing the risk of cancer. According to earlier studies the plant has demonstrated positive effects in treating or managing specific health conditions like infections, inflammation, or other ailments therefore Tridax procumbens could be used as an alternative or complementary treatment to conventional medicine. Recent studies have highlighted the anticancer potential of Tridax procumbens, focusing on its bioactive compounds, particularly luteolin (Muruganathan et al., 2022; Ali, 2001). Research shows that luteolin from Tridax procumbens demonstrates significant anticancer activity through various mechanisms, including apoptosis induction and inhibition of cancer cell proliferation. Computational studies and in-vitro experiments have supported these findings, indicating that luteolin might be effective against different types of cancer, such as lung, breast, and prostate cancers. The ongoing research emphasizes the potential of this plant in cancer therapy (Almatroodi et al., 2024; Nandakumar et al., 2022). Thus, the antiproliferative activity of T. procumbens has been investigated in organ specific human cancer cell lines via MTT assay. The Fig. 1 confirmed that tested extracts of T. procumbens inhibited the growth of human cancer cell lines (PC-3, A549, A431, MDA-MB-231, MDA-MB-468, K-562and COLO-205). But the best efficacy was observed by extract 5 which inhibited the growth of MDA-MB-231 cell line above 50 % with IC50 values 23.41 µg/mL (Fig. 2D). Interestingly, Fig. 2C confirmed that fraction7 has potent activity against A431 with IC50 values 29.45 µg/mL.
However, the result of Fig. 1 signified that all the 12 tested extracts and fractions were nontoxic in and inhibited the cell proliferation 50 % and less at the higher tested concentration (100 µg/mL, 37.50 ± 1.87 %). Interestingly, the anticancer activity of T. procumbens were not explored very much and no report has been found so far against PC-3, A549, A431, MDA-MB-231, MDA-MB-468, K-562 and COLO-205 cell lines. Moreover, the Fig. 2A demonstrated that the fraction 11 has 84.37 ± 1.24 % cytotoxicity against PC-3 cells (37.44 µg/mL) which is in support of Priya et al. (2011b), reported that the acetone extract of flower inhibits the cell growth. But the phytochemical constituents and their property may differ depending on geographical as well as extraction techniques (Baharvand-Ahmadi et al., 2016).
Previous literature suggested that antioxidants inhibit cancer cell growth by targeting the overproduction of intracellular oxidizing free radicals (Rai et al., 2013), Fig. 3A-3H demonstrated that extracts of T. procumbens hold significant antioxidant activity. The extracts confirmed IC50 values ranging from 14.70 µg/mL to 93.40 µg/mL. Precisely, Fig. 3A specified that fraction 7 has strong free radical scavenging activity with an IC50 of 14.70 µg/mL. Furthermore, Fig. 3C exhibits that fractions 7 and 9 effectively inhibit the production of NO, with IC50 values of 29.90 µg/mL and 34.26 µg/mL, respectively. At the same time, Fig. 3F and 3G further confirmed the antioxidant activity of the crude extract and its fractions of tested plant, measured in terms of AAE and FSE.
The antiproliferative and antioxidant activity of T. procumbens may be due to the presence of different phytochemicals. Plant extracts with high antioxidant may hold numerous types of phytochemicals, in which phenolic compounds are ubiquitous. Phenolics typically have strong antioxidant properties (Vander et al., 1999) and are crucial for human health (Rice-Evans et al., 1997). Fig. 3D confirmed that all the extracts of T. procumbens have phenolic contents.Previous reports suggested that flavonoid also significant role to protect cells from oxidative damage and control diseases by shielding proteins, lipids, and DNA (Moskaug et al., 2005; Chen et al., 2013). Therefore, Fig. 3E indicated that tested extracts have good flavonoid content (13.97 ± 0.37 to 0.93 ± 0.06).
According to the HPLC results, kaempferol and ferulic acid present in plant exhibit antioxidant potential and have the potential to lessen free radicals such as reactive oxygen species (ROS). The reduction of ROS can change the phenotype of malignant cancer cells (Le Marchand, 2002; López-Lázaro, 2010; Salehi et al., 2018a; Sharifi-Rad et al., 2018). An earlier report claimed that, at micro molar concentrations, kaempferol inhibits the growth of lung adenocarcinoma A549 cells as well as breast cancer cells (Salehi et al., 2018b; Azevedo et al., 2015; Zhu and Xue, 2018). The aforementioned research on the antiproliferative and high antioxidant activity of various solvent extracts of the Native American species of T. procumbens showed that it has the necessary biological activity in various human cancer cell lines that are specific to various organs (Han et al., 2018).
5 Conclusions
The present study highlights the significant antioxidant and antiproliferative activities exhibited by various solvent fractions of Tridax procumbens. The promising antiproliferative properties observed, particularly in the methanolic crude extract and the chloroform fraction of the stem with IC50 23.41 µg/mL and 29.45 µg/mL against breast cancer (MDAMB-231) and skin cancer (A-431) cells, suggest that the bioactivity may be attributed to the secondary metabolites present. The study also focused on the usefulness of the plant for medicinal use by simultaneously detecting two major bioactive phenols using reverse-phase high-performance liquid chromatography and photodiode arrays. These findings underscore the potential of T. procumbens as a source of bioactive compounds with therapeutic relevance. However, further research is essential to isolate and characterize the specific compounds responsible for these and techniques such as HPLC-MS and NMR can be useful. Also, study the mechanisms of action of the active compounds, such as their effects on apoptosis, cell cycle regulation, and signaling pathways involved in cancer progression. Animal studies should be done to evaluate the efficacy, safety, and potential side effects of T. procumbens extracts or their isolated compounds. The potential synergistic effects of T. procumbens extracts when combined with other anticancer agents needed to explore.
6 Institutional Review Board Statement
Not applicable.
CRediT authorship contribution statement
Rida Sagheer: Software, Resources, Methodology, Investigation, Formal analysis. Annie Gupta: Writing – review & editing, Methodology, Investigation, Formal analysis, Conceptualization. Suaib Luqman: Software, Resources. Harshpreet Kaur: . Kajal Srivastava: Writing – review & editing. S.M. Kawish: Resources, Methodology, Data curation. Sunil Kumar Panda: . Muzaffar Iqbal: Writing – review & editing, Resources, Funding acquisition, Data curation.
Acknowledgments
The authors express their sincere thanks to Department of Chemistry, Integral University, Lucknow; Department of Biochemistry King George’s Medical University Lucknow and CSIR-CIMAP, Lucknow to encourage them through research atmosphere. The authors extend their appreciation to the Researchers Supporting Project number (RSPD2024R734), King Saud University, Riyadh, Saudi Arabia for their support.
Authors contributions
All authors scientifically contributed in the preparation and execution of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- New constituents from the roots of Oenothera biennis and their free radical scavenging and ferric reducing activity. Ind. Crop. Prod.. 2014;58:125-132.
- [CrossRef] [Google Scholar]
- A new flavonoid from the aerial parts of Tridax procumbens. Fitoterapia. 2001;72(3):313-315.
- [CrossRef] [Google Scholar]
- Effects and Mechanisms of Luteolin, a Plant-Based Flavonoid, in the Prevention of Cancers via Modulation of Inflammation and Cell Signaling Molecules. Molecules. 2024;29(5):1093.
- [CrossRef] [Google Scholar]
- Antihyperuricemia, antioxidant, and antibacterial activities of Tridax procumbens L. Foods. 2019;8(1):21.
- [CrossRef] [Google Scholar]
- The chemo preventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer. 2015;67(3):504-513.
- [CrossRef] [Google Scholar]
- An ethno-medicinal study of medicinal plants used for the treatment of diabetes. Journal of Nephropathology. 2016;5(1):44.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analyiochem. 1996;15:70-76.
- [CrossRef] [Google Scholar]
- A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem.. 2013;138(4):2099-2107.
- [CrossRef] [Google Scholar]
- Han, X., Liu, C.F., Gao, N., Zhao, J. and Xu, J., 2018. RETRACTED: Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. DOI: 10.1016/j.biopha.2018.09.087.
- Cytotoxic and Apoptogenic Effects of a Bioactive Fraction Isolated from The Leaves of a Traditional Medicinal Plant Tridax Procumbens. Int. J. Pharm. Sci. Res.. 2019;10(11):5075-5086.
- [Google Scholar]
- Cancer preventive effects of flavonoids—a review. Biomed. Pharmacother.. 2002;56(6):296-301.
- [CrossRef] [Google Scholar]
- A new view of carcinogenesis and an alternative approach to cancer therapy. Mol. Med.. 2010;16:144-153.
- [Google Scholar]
- Luqman, S., Kumar, R., Kaushik, S., Srivastava, S., Darokar, M.P. and Khanuja, S.P., 2009. Antioxidant potential of the root of Vetiveria zizanioides (L.) Nash.
- Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid. Based Complement. Alternat. Med.. 2012;2012(1):519084
- [CrossRef] [Google Scholar]
- The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun.. 1994;201(2):748-755.
- [CrossRef] [Google Scholar]
- Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem.. 2005;91(3):571-577.
- [CrossRef] [Google Scholar]
- Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr.. 2005;81(1):277S-278S.
- [CrossRef] [Google Scholar]
- Pharmacology of Tridax procumbens a weed. Int J Pharm Tech Res. 2010;2(2):1391-1394.
- [Google Scholar]
- Recent updates on source, biosynthesis, and therapeutic potential of natural flavonoid luteolin: A review. Metabolites. 2022;12(11):1145.
- [Google Scholar]
- Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharm. Biol.. 2011;49(6):669-673.
- [CrossRef] [Google Scholar]
- Antibacterial, antifungal and cytotoxic activities of eight Asteraceae and two Rubiaceae plants from Colombian biodiversity. Braz. J. Microbiol.. 2006;37:566-570.
- [CrossRef] [Google Scholar]
- Bivalent furostene carbamates as antiproliferative and antiinflammatory agents. The Journal of Steroid Biochemistry and Molecular Biology. 2019;194:105457
- [CrossRef] [Google Scholar]
- Pharmacognostical and Preliminary Phytochemical Studies of Leaves of Tridax procumbens L. Ethnobotanical Leaflets. 2008;2008(1):172.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269(2):337-341.
- [CrossRef] [Google Scholar]
- Preliminary phytochemical screening of members of Lamiaceae family: Leucas linifolia, Coleus aromaticus and Pogestemon patchouli. International Journal of Pharmaceutical Science Review and Research. 2013;21(1):131-137.
- [Google Scholar]
- Antioxidant properties of phenolic compounds. Trends Plant Sci.. 1997;2(4):152-159.
- [CrossRef] [Google Scholar]
- Exploration of hepatoprotective potential of methanolic extract of Tridax procumbens against isoniazid-rifampicin induced toxicity in albino rats. Journal of Pharmacognosy and Phytochemistry. 2018;7(3):384-390.
- [Google Scholar]
- Effect of aqueous leaf extract of Tridax procumbens on blood pressure and heart rate in rats. Afr. J. Biomed. Res.. 2004;7(1)
- [Google Scholar]
- Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol.. 2018;80:104-122.
- [CrossRef] [Google Scholar]
- Saraf, S., Pathak, A.K. and Dixit, V.K., 1991. Hair growth promoting activity of Tridax procumbens.
- In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica Poir. Cell. Mol. Biol.. 2018;64(8):57-64.
- [CrossRef] [Google Scholar]
- Lamuela-Raventos: Analysis of total phenoles and other oxidation substartes and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol.. 1999;299:152.
- [Google Scholar]
- Vegetable peel waste for the production of ZnO nanoparticles and its toxicological efficiency, antifungal, hemolytic, and antibacterial activities. Nanoscale Res. Lett.. 2016;11:1-10.
- [Google Scholar]
- RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. Journal of Photochemistry and Photobiology b: Biology. 2016;162:550-557.
- [CrossRef] [Google Scholar]
- In-vitro antibacterial, antioxidant potentials and cytotoxic activity of the leaves of Tridax procumbens. Saudi Journal of Biological Sciences. 2020;27(2):757-761.
- [CrossRef] [Google Scholar]
- Bioactivity studies of extracts from Tridax procumbens. Phytomedicine. 2000;7(3):235-238.
- [CrossRef] [Google Scholar]
- Immunomodulatory effects of aqueous extract of Tridax procumbens in experimental animals. J. Ethnopharmacol.. 2004;92(1):113-119.
- [CrossRef] [Google Scholar]
- Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell. 1999;3(2):159-167.
- [CrossRef] [Google Scholar]
- Vilwanathan Ravikumar, V.R., Shivashangari, K.S. and Thiruvengadam Devaki, T.D., 2005. Hepatoprotective activity of Tridax procumbens against D-galactosamine/lipopolysaccharide-induced hepatitis in rats.
- In vitro anticancer activity of aqueous and acetone extracts of Tridax procumbens leaf on pc 3 cell lines. Int J Pharm Pharm Sci. 2011;3(4):356-358.
- [Google Scholar]
- Evaluation of anti-cancer activity of Tridax procumbens flower extracts on PC 3 Cell lines. Pharmanest. 2011;2(1):28-30.
- [Google Scholar]
- Evaluation of anticancer activity of Tridax procumbens leaf extracts on A549 and Hep G2 cell lines. Asian J Pharm Clin Res. 2015;8(3):129-132.
- [Google Scholar]
- Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem.. 1995;43(1):27-32.
- [CrossRef] [Google Scholar]
- Zhu, L. and Xue, L., 2019. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res., 27(6), p.629. DOI: 10.3727%2F096504018X15228018559434.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103474.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







