Translate this page into:
Antioxidant potential of biotransformed green tea catechin metabolites and their impact on peripheral blood mononuclear cells
⁎Corresponding authors. rkrishnamoorthy@ksu.edu.sa (Rajapandiyan Krishnamoorthy), alshatwi@ksu.edu.sa (Ali A. Alshatwi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Catechin absorption, bioavailability, and distribution in human tissue and excretion were low. Colonic bacteria degrade catechin and few studies suggest that biotransformed metabolites may increase or decrease biological activities. However, the antioxidant potential of biotransformed metabolites is still unexplored. Hence, it is necessary to investigate the antioxidant potential of colonic bacterial transformed catechin metabolites.
Methods
The In vitro antioxidant potential of biotransformed catechin metabolites was assessed by DPPH, Hydrogen peroxide, and ABTS assay. Cytotoxic effect of biotransformed catechin metabolites on human mesenchymal stem cells and their response to antioxidant genes GPX1 and POR expression in human peripheral blood mononuclear cells (PBMC) were evaluated.
Results
Free radical scavenging activity of partially purified bacterial transformed catechin metabolites was low compared to pure catechin. The results revealed that variations in the antioxidant potential of pure catechin and colonic bacterial altered catechin metabolites. The IC50 value of partially purified biotransformed catechin metabolites exhibits free radical scavenging activity at high concentrations, while pure catechins act as a potent inhibitor at lower concentrations. The expression of GPX1 and POR gene increased four folds in a concentration dependent manner in pure catechin-treated cells. In contrast, partially purified biotransformed catechin metabolites treated cells expressed the downregulation of GPX1 and upregulation of POR gene.
Conclusion
Therefore, it can be concluded that the functional group alteration in biotransformed catechin metabolites may reduce the hydrogen donating ability, thus In vitro antioxidant potential of biotransformed catechin metabolites was low compared to pure catechin. Hence, GPX1 and POR exhibit different expression patterns in treated peripheral blood mononuclear cells. These findings suggest that pure catechin and colonic bacterial transformed catechin metabolites differed functionally.
Keywords
Catechin
Biotransformation
Antioxidants
Peripheral blood mononuclear cells
Human mesenchymal stem cells
1 Introduction
Imbalance of free radicals or reactive oxygen species (ROS), such as superoxide anion radical (O2–) or hydroxyl radical (HO), hydrogen peroxide (H2O2) or singlet oxygen (1O2) which are produced during the cellular metabolic process, counteracts with their detrimental effects via neutralization that cause oxidative stress (OS). However, both metabolic and environmental sources can increase ROS production. Metabolic sources have occurred in cells where the oxygen consumption rate is high, like mitochondria, inclusion bodies, and endoplasmic reticulum. Environmental sources such as pollution, smoking, heavy metals deposities, pesticides, temperature variations and drugs (Phaniendra et al., 2015). These ROS are molecular species that possess more than one unpaired valence electrons, hence highly reactive and may cause OS that leads to chemical alteration of biological macromolecules causing structural and functional modifications (Lobo et al., 2010). The OS induced damage that may lead to pathological conditions in inflammations, cancer, and diabetic conditions. In general, the normal cell metabolic process in humans produces ROS/free radicals, which cause irreversible oxidation in functional carbohydrates, glycolipids, proteins, and nuclear materials (Sisein, 2014). The irreversible oxidation in protein may lead to the breakdown of peptide chains due to oxidized amino acid, and oxidation of glycolipids inhibits semipermeable membrane synthesis and cause membrane damage. If OS occurs in the protein sulfhydryl group switches, significant conformational changes in native structures lead to functional alterations, deterioration, and unfolding (Neha et al., 2019). OS in glycolipids provoke unsaturated aldehydes and isoprostanes and disable the protein functions (Carocho et al., 2013).
Antioxidants are capable of mitigating the free radicals and detoxify the ROS to sustain the normal cellular process. Naturally, wide varieties of antioxidants were found in the phytochemicals that are rich in polyphenols. Polyphenols are naturally occurring plant secondary metabolites and have been reported with numerous biological effects eg, antioxidant, antimicrobial, anti-cancer, and anti-obesity potential (Ganesan and Xu, 2017). Polyphenols chemical structure consists of several hydroxyl groups on aromatic rings. Thus structure and substitution relate to its reduction potential and its ability to stabilize and delocalize the unpaired electron. Hence, polyphenols act via inhibition of free radical formation, neutralizing the highly reactive radicals involved in the chain of reactions leads to OS-mediated cellular damage (Mut-Salud et al., 2016). Catechin is the dietary polyphenol that abundantly exists in green tea, vegetables, fruits, cereals, and beverages. Wide literatures has suggested that intake of catechin-rich food prevents many chronic diseases by inhibiting excessive oxidative stress through activation of superoxide dismutase, glutathione peroxidase and catalase (Fan et al., 2017). Catechin molecular structure favors functionally potent quenchers to neutralize highly reactive singlet oxygen and other ROS via Fenton reaction (Singh et al., 2008). However, in humans, absorption, distribution, metabolisms, and excretion (ADME) of catechin in human tissues were low. Current, research have focused that dietary catechins can be modified by the human gut bacteria (Takagaki and Nanjo, 2015). Nevertheless, research in human gut microbes, the role of gut bacteria conversion of food compounds is a glooming field of research. Few studies suggest that biotransformed compounds may increase or decrease biological activities. Extensive research was conducted on bioactive potential of catechin. However, the biological potential of gut bacterial transformed catechin is yet to be explored. Hence, we aimed to investigate the In-vitro antioxidant efficacy of partially purified biotransformed catechin metabolites. More so, it evaluated their impact on the expression of antioxidant genes GPX1 and POR of human peripheral blood mononuclear cells.
2 Materials and method
2.1 Chemicals
Catechin (Tea polyphenol), ABTS: 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), DPPH; 1,1-diphenyl-2-picrylhydrazyl. Ascorbic acid, potassium persulphate, and Trolox; (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid were purchased from Sigma Aldrich (St. Louis, Mo., USA). Histopaque-1077 (Sigma Aldrich) and RPMI-1640 medium (gibco, life technologies, USA), Obtained Ultra purity water was from Milli-Q system (Millipore Laboratory Bedford, MA). All other chemicals and reagents used were of analytical grade.
2.2 Biotransformed catechin metabolites
In our previous research, we performed In vitro catechin biotransformation. The biotransformed crude (Cr) catechin metabolites were collected and further partially purified by preparative thin-layer chromatography and analyzed by Gas chromatography-mass spectrometry and High-performance liquid chromatography-mass spectrometry (Krishnamoorthy et al., 2019). We obtained two sets of partially purified metabolites (PPM); PPMA consists of dehydroquinic acid, 4-ethylphenol, 4-methoxyphenyl propan-2-ol, 3-phenyl propionic acid, 2-phenoxyethanol, benzene tricarboxylic acid,1,2-dimethyl ester, catechol-1,4-benzenediol, benzene-1,3,5-tris1-methylpropyl, 3,5,7-trihydroxy-2H-chromen-2-one, and 4-hydroxyphenylpropionic acid. PPMB consist dimethoxycinnamic acid.
2.3 DPPH radical scavenging assay
The catechin biotransformed metabolites' effect on DPPH radicals was evaluated as previously reported with some modifications (Kumari et al., 2016). Briefly, the reaction mixture was prepared by adding DPPH (0.1 mM) in 3.9 mL methanol and 0.1 mL of different concentration (5, 10, 20, 40 and 80 μg/mL) of biotransformed metabolites. The mixture was vortexed and then incubated in the darkroom at room temperature for 30 mins, and after incubation, absorbance was recorded at 517 nm. All the assays were performed in triplicates, for standard Ascorbic acid and Trolox were used. The percentage of DPPH scavenging activity of tested samples and standard was calculated using the bellow equation.
A0 - absorbance of the control, As - absorbance of the test samples.
The IC50 (required concentration to scavenge 50% free radicals) for of the test samples and the standard was estimated using CalcuSyn1 software.
2.4 ABTS+ radical cation decolorization assay
The ABTS (2,2′- azino-bis (3-ethylbenzothiazoline-6-sulfonicacid) diammoniumsalt) radical cation scavenging ability was analysed as previously described with some modifications (Re et al., 1999). Briefly, the radical solution was prepared by mixing an equal quantity of 7 mM ABTS aqueous solutions and 2.45 mM potassium persulfate solution. This mixture was incubated 16 h in dark conditions at room temperature. The assay was carried out with freshly prepared working solutions by mixing 3 mL of ABTS+ solution with 0.2 mL of different concentrations of pure and partially purified catechin biotransformed metabolites. After 6 min, the absorbance was measured at 734 nm. All the assays were performed in triplicates. Inhibition (%) = ODc-ODs/ODc × 100
ODc – absorbance of the control, ODs- absorbance of the samples.
2.5 Hydrogen peroxide (H2O2) radical scavenging activity
The method, as previously described, determined the hydrogen peroxide radical scavenging activity of the catechin and partially purified biotransformed metabolites (Sarma et al., 2016). Briefly, the reaction mixture was prepared by mixing different concentrations of pure and partially purified catechin biotransformed metabolites and 0.6 mL 40 mM hydrogen peroxide prepared with phosphate buffer (pH 7.4) and incubated this reaction mixture at 37°Cin for 10 min. After incubation, the absorbance was measured at 230 nm against the blank. All the assays were performed in triplicates. Inhibition (%) = (OD1-OD2)/OD1 × 100
OD1 - absorbance of hydrogen peroxide, OD2 - absorbance of reaction mixture along with test samples.
2.6 Cytotoxicity effects on human mesenchymal stem cells
The cytotoxicity of the test samples was analyzed using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. At the density of 10,000 cells/mL human mesenchymal stem cells (hMSCs) were seeded in the respective wells in 96 well plates. The pure catechin and partially purified biotransformed metabolites were treated at different doses and incubated for 24 and 48 h at 37 °C in a humidified environment maintained with 5% CO2. After incubation of MTT 20 µL/well was added and incubated overnight. After incubation, the supernatant was removed from the wells by centrifugation. The formazan crystal was dissolved by adding dimethyl sulfoxide (100%). Using a microplate reader (Promega, Madison, WI, USA) optical density of the samples was measured at 570 nm and a reference filter (630 nm). The cell viability was calculated as percentage from the obtained values by comparing survival of control cells. All the analyses were performed in triplicates/dose, and data are presented as the percentage mean ± SD.
2.7 Peripheral blood mononuclear cells (PBMC)
The experiment was performed in conformity with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of King Saud University College of Medicine (E-19–3703). The volunteers were educated with the purpose and the implications of the study, and informed consent was obtained. The venous blood 100 mL was collected from a healthy volunteer, who was accepted and not consumed antioxidant-rich foods for 24 h before blood collection. Using density gradient method, the collected fresh blood was immediately treated with Histopaque-1077 (Sigma, St Louis, MO), and PBMC were separated by centrifugation for 15 min at 2500 g (Carrie et al., 2005). The PMBC concentration was diluted to 1 × 106 cells/mL in RPMI 1630 culture medium and maintained at 37 °C for 24 h in a humidified environment supported with 5% CO2.
2.8 Quantitative real-time reverse transcription-polymerase chain reaction (qPCR)
RT-qPCR experiment was performed using Applied Biosystems 7500 Fast real-time PCR system. Cell to cDNA synthesis kit (FastLane Cell cDNA Kit (Qiagen, Germany) was used to synthesis cDNA. The cDNA quantification and purity were carried out using the NanoDrop 2000 UV-spectrophotometer (Thermo Fisher, USA). qPCR reaction was carried out with the SYBR Green PCR Master Mix (Qiagen, Chatsworth, CA, USA) and Quantitect primer assays in an ABI 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA) as per the manufacturer’s instruction (Table 1). Reaction mixtures and thermal-cycling conditions were followed as per the manufacturer’s instructions. Relative changes in the GPX1 and POR gene expression were calculated using ΔΔCt method, and reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used for normalization. Data were presented as mean fold change over control with standard deviations.
S. No
Primers
Cat No
Accession No
1
GPX1
QT00203392
NM_000581
2
POR
QT00045339
NM_000941
3
GAPDH
QT 00,079,247
NM_002046
2.9 Statistical analyses
All the experiments were carried out in triplicates, and the results were presented as mean ± SD. The data were analyzed using the SPSS software, version 22.0 (Chicago, IL, USA) and Microsoft excel. One-way analysis of variance (ANOVA) was carried performed. The statistically significant differences were expressed as *p ≤ 0.05.
3 Results
3.1 DPPH radical scavenging activity
Pure catechin and partially purified biotransformed catechin metabolites reduction potential on DPPH radicals were assessed by the decreased absorbance at 517 nm and expressed as a percentage of inhibition. The free radical scavenging potential is based on the hydrogen donating ability of the compound to convert unpaired electrons to paired electrons (Ozsoy et al., 2008). Fig. 1 represents the scavenging activity of ascorbic acid, Trolox, catechin, and catechin biotransformed metabolites PPMB, crude and PPMA inhibition at a concentration 80 µg/mL were 79.66 ± 0.50, 70.45 ± 0.70, 63.24 ± 0.32, 57.31 ± 0.73, 50.40 ± 0.50, and 43.59 ± 0.71% respectively. The obtained results were statistically significant (p < 0.05). The partially purified biotransformed catechin metabolites exhibited low inhibition potential compared to pure catechin. The IC50 of positive control ascorbic acid and Trolox, catechin and their biotransformed metabolites PPMB, crude, and PPMA were 15.60, 23.58, 40.60, 86.32, 111.25, and 137.64 µg/mL, respectively. The current study demonstrated that the IC50 value of partially purified catechin biotransformed metabolites was twofold higher than pure catechin.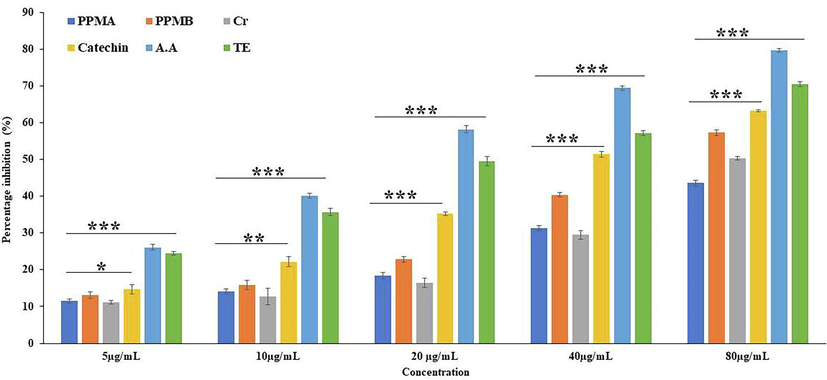
2–2- Diphenyl-1-picryl-hydrazyl (DPPH) free radical scavenging activity of pure catechin and partially purified colonic bacterial transformed catechin metabolites at different concentrations. Data represented as mean ± SD of triplicates along with the error bar, ***, **, * statistically significant differences at P < 0.001, P < 0.01, and P < 0.05. Partially purified biotransformed catechin metabolites A - PPMA, Partially purified biotransformed catechin metabolites B - PPMB, Crude metabolites - Cr, Catechin, Ascorbic acid -AA, Trolox - TE.
3.2 ABTS+ radical cation decolorization assay
The ABTS+ radical scavenging assay was employed as an index that revealed the antioxidant activity of chain-breaking antioxidants (Leong and Shui, 2002). Compounds with higher reduction potential to neutralize the active pronated radicals via accepting or donating electrons capable of terminating radical chain reactions (Khan et al., 2013). The cationic radical inhibition potential was high in ascorbic acid followed by Trolox, catechin, crude, PPMB, and PPMA; 75.40 ± 0.64, 69.72 ± 1.02, 68.00 ± 0.56, 66.61 ± 1.11, 56.70 ± 0.90 and 53.81 ± 0.82% respectively (Fig. 2). The IC50 value of ascorbic acid, Trolox, catechin, crude, PPMB, and PPMA was 15.61, 19.39, 30.74, 55.27, and 69.09 µg/mL, respectively. The IC50 values indicate that partially purified catechin biotransformed metabolites were demonstrated significant scavenging capacity at high concentrations compared to pure green tea catechin.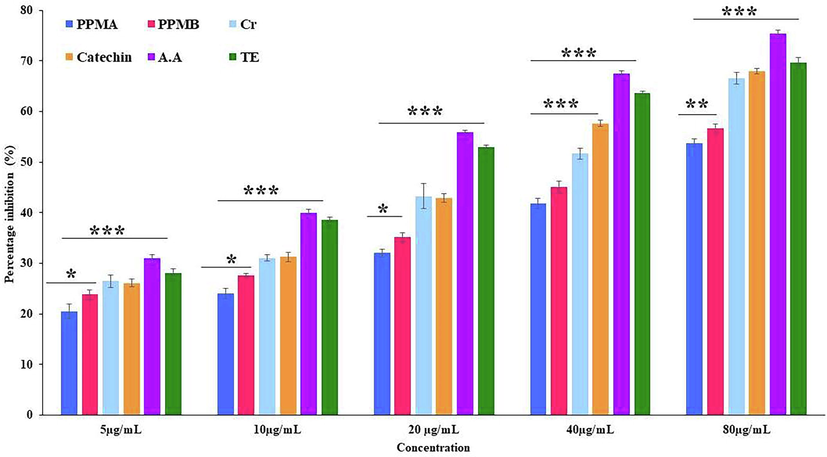
ABTS+ radical scavenging activity catechin and partially purified colonic bacterial transformed catechin metabolites at different concentrations. Data represented as mean ± SD of triplicates along with the error bar, ***, **, * statistically significant differences at P < 0.001, P < 0.01, and P < 0.05.
3.3 Hydrogen peroxide (H2O2) radical scavenging activity
Free hydroxyl radicals are highly reactive and formed during the cellular metabolic process in the biological system. However, in humans metabolic enzymes cannot act to neutralize them (Liu et al., 2005). The scavenging ability of hydroxyl radical was shown in Fig. 3. At high concentration inhibition percentage of ascorbic acid, Trolox, catechin, biotransformed metabolites crude, PPMA and PPMB were exhibited 79.23 ± 0.73, 77.64 ± 0.70, 74.16 ± 0.34, 76.77 ± 1.06, 73.37 ± 0.83, and 63.60 ± 0.89% respectively, and the obtained values are statistically significant. The IC50 value of ascorbic acid, Trolox, catechin, crude, PPMA, and PPMB biotransformed metabolites was 13.23, 16.68, 22.18, 23.94, 41.22, and 29.03 µg/mL, respectively. The present study found that pure catechin, partially purified catechin biotransformed metabolites B, and positive control exhibits a similar hydroxyl radical scavenging activity at high concentration, with varying IC50 values.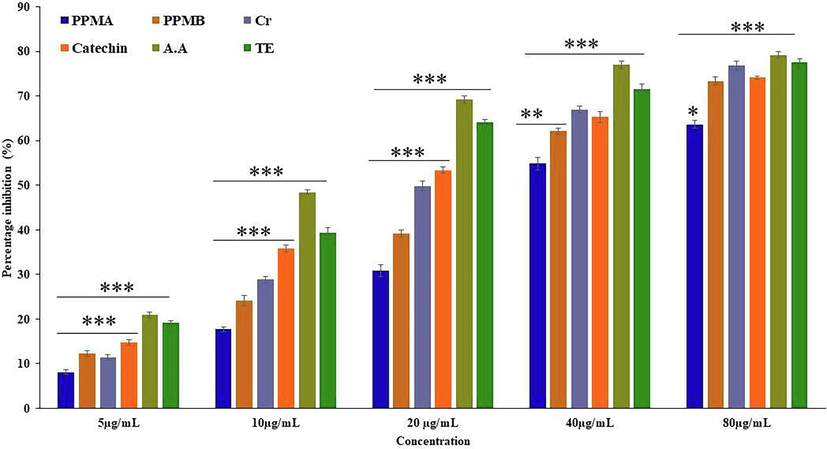
Hydrogen peroxide (H2O2) radical scavenging activity catechin and partially purified colonic bacterial transformed catechin metabolites at different concentrations. Data represented as mean ± SD of triplicates along with the error bar, ***, **, * statistically significant differences at P < 0.001, P < 0.01, and P < 0.05.
3.4 Cytotoxic effect on human mesenchymal stem cells
The pure catechin, catechin biotransformed metabolites crude, PPMA and PPMB were treated at different concentrations ranging from 10 to 80 µg/mL on hMSCs, and showed viability percentage after 24 and 48 h in Figs. 4 and 5. The pure catechin showed a statistical difference (p < 0.05) cytotoxic effect in a dose-dependent manner. In contrast, catechin biotransformed metabolites PPMA showed mild proliferative activity at the lower concentrations at 24 and 48 h treatment. However, crude, PPMA, and PPMB metabolites exhibit non-cytotoxic impact on hMMSc even at higher concentrations (80 µg/mL) and longer treatment times.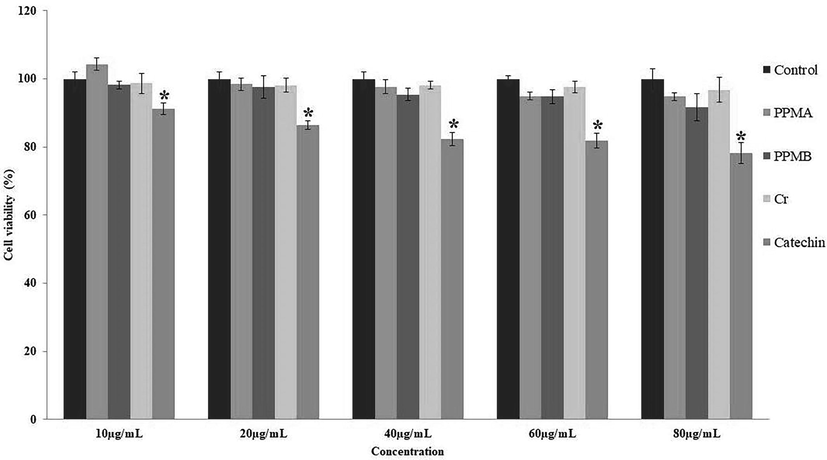
Cytotoxic effect of pure catechin and partially purified colonic bacterial transformed catechin metabolites at the different concentrations on human mesenchymal stem cells at 24 h. Data represented as mean ± SD of triplicates along with the error bar. * statistically significant differences at P < 0.05.
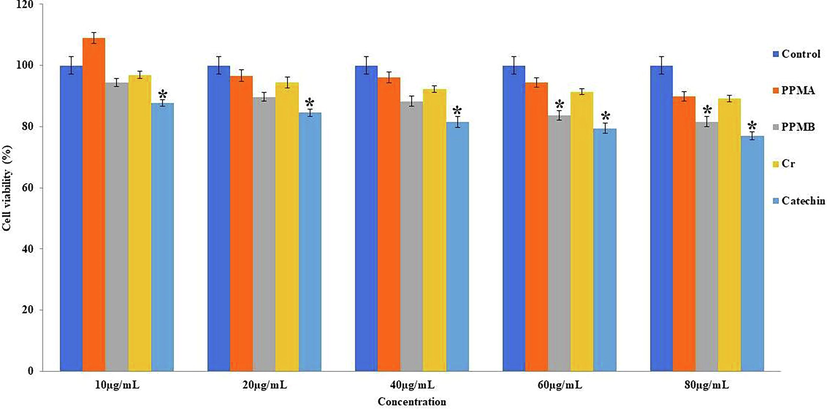
Cytotoxic effect of pure catechin and partially purified colonic bacterial transformed catechin metabolites at the different concentrations on human mesenchymal stem cells at 48 h. Data represented as mean ± SD of triplicates along with the error bar. * statistically significant differences at P < 0.05.
3.5 Gene expression
The role of pure catechin and partially purified biotransformed metabolites on antioxidant gene expression on PBMC was analyzed. After the treatment with crude PPMA, PPMB, and pure catechin, the GPX1 and POR gene expression results were depicted in Figs. 6 and 7. The GPX1 and POR gene expression was significantly increased four folds in a dose-dependent manner in pure catechin-treated cells. In contrast, crude, PPMA, and PPMB downregulated the GPX1 expression and upregulated the POR gene expression in a dose-dependent manner.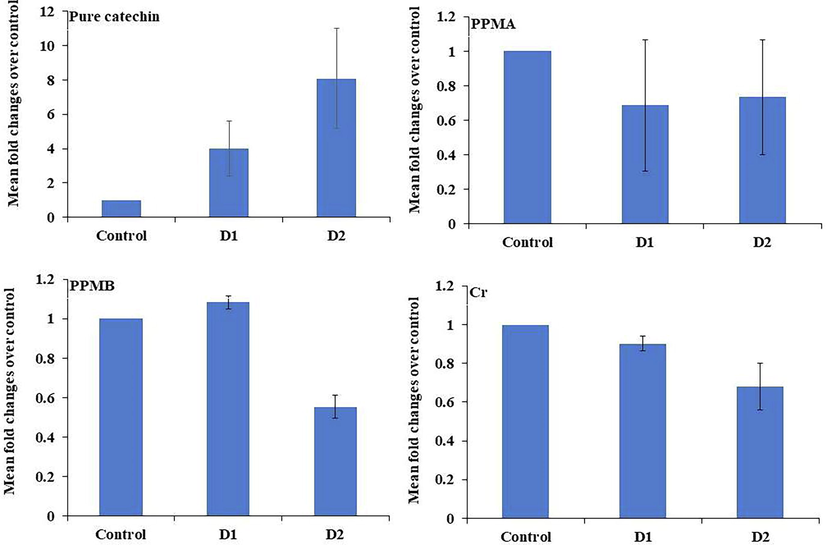
Antioxidant gene GPX1 expression in treated peripheral blood mononuclear cells at different concentrations of pure catechin and partially purified colonic bacterial transformed catechin metabolites.
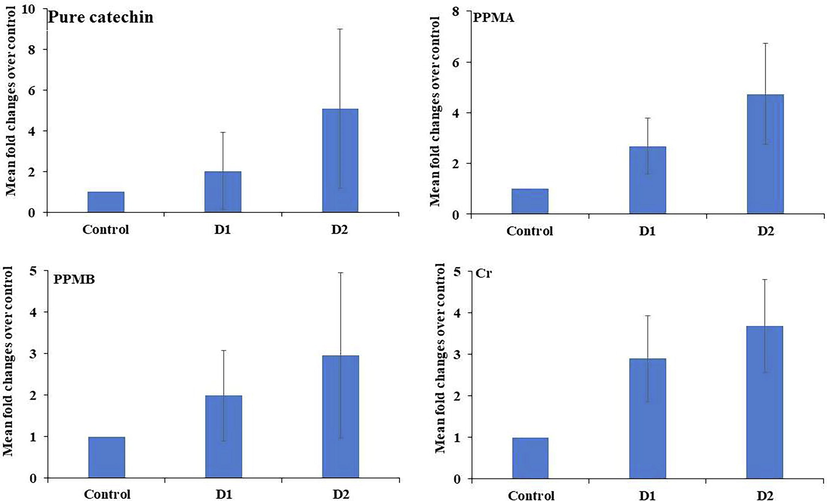
Antioxidant gene POR expression in treated peripheral blood mononuclear cells at different concentrations of pure catechin and partially purified colonic bacterial transformed catechin metabolites.
4 Discussion
The catechin molecular structure is extensively breakdown by colonic bacterial enzymes and transformed into many metabolites with a low molecular weight that is transportable and crosses the semipermeable membrane of the lumen, which may be responsible for health effects rather than the pure catechin in food (Cardona et al., 2013). Hence, in the current study, we attempt In vitro free radicals scavenging potential of partially purified colonic bacterial transformed catechin metabolites and their impact on antioxidant gene expression in PBMC. The experimental results showed that catechin transformed metabolites of crude, PPMA, and PPMB exhibit lower DPPH scavenging activity. However, the pure catechin compound exhibit strong DPPH radical scavenging potential, and the obtained results of this study were similar in comparison with previous reports (Grzesik et al., 2018). Thus, it is necessary to highlight that the functional groups of the catechin aromatic ring react with free radicals to stabilize via donating or accepting the unpaired electrons and significantly increase the radical scavenging activity (Cömert and Gökmen, 2017). However, the results revealed that colonic bacterial transformed catechin metabolites consist of the altered galoil group of flavanol skeleton, thus donating less electron that formed stable substances to terminate the DPPH free radicals scavenging activity (Deori et al., 2014), resulting the IC50 value of biotransformed catechin metabolites were higher than the pure catechin. The crude biotransformed metabolites showed synergistic effects at high concentrations on cationic ABTS radicals scavenging activity. It may be due to different biotransformed metabolites that consist of hydroxyl groups synergistically involved in the termination of chain reactions (Gülçin, 2012). The H2O2 scavenging activity of partially purified catechin biotransformed metabolites was relatively high compared to other antioxidant assays; however, the antioxidant pattern was similar. The H2O2 is a poor oxidizing agent that can cross the cell membrane and react with Fe2+ and Cu2+ ions to form a concentration gradient of radicals and stimulate lipid peroxidation via Fenton reaction resulting in toxic effects (Moniruzzaman et al., 2018). According to obtained results, catechin biotransformed metabolites exhibit less antioxidant activity compared to pure catechin and standards. This may be due to the purity of the tested compounds. However, the antioxidant potential of partially purified catechin metabolites PPMB exhibits higher activity than the PPMA in all experimental assays. The separated biotransformed catechin metabolites PPMB consists of cinnamic acid group metabolites (Gulcin, 2020). Hydroxycinnamic acid has been found to have significant antioxidant activity than the other biotransformed metabolites. This situation is due to the backbone structure of the hydroxycinnamic acid enhancing the conjugation and stabilization of free radicals. Direct exposure of biotransformed compounds to human cells in the intestine and their cytotoxicity is yet to explore; thus it is reasonable to perform cytotoxicity in hMSCs against these compounds. However, the current results revealed that the biotransformed catechin metabolites exhibit a non-toxic effect than the pure catechin in live cells. This fact brings the light that biotransformed catechin metabolites transport via porins of permeable membrane and actively participate in neutralizing ROS as typical cellular functional components for homeostasis even at higher concentrations (Li et al., 2016; Cruz-Chamorro et al., 2020). The neutralizing the free radicles in normal cellular functions in human cells involves a set of enzymes such as glutathione peroxidase and cytochrome P450 oxidoreductase etc. The active thiol site of these enzymes involves the neutralization of free radicals (Kasai and Kawai, 2006), demonstrating the importance of the regulation of these enzymes.
Also, the antioxidant system in human PBMC participates in maintaining homeostasis, hence investigating the PBMC expression pattern of GPX1 and POR genes. The present results revealed that the GPX1 gene was upregulated by pure catechin. In contrast, biotransformed catechin metabolites significantly down-regulate the GPX1 expression. The GPX1 is an effective suppressor of 5-Lipoxygenase (5-LO) that catalyzes the biosynthesis of leukotrienes in granulocytes, monocytes/macrophages, mast cells, and dendritic cells, and B –lymphocytes (Straif et al., 2000). Leukotrienes are mediators of inflammatory responses that PBMCs produce. Hence the present study speculates that biotransformed catechin metabolites enhance the leukotrienes biosynthesis via downregulating 5-Lo suppressor GPX1. However, all catechin biotransformed metabolites upregulated the POR expression in a dose-dependent manner. Previous research has shown that upregulation of the POR gene triggers the activation of a network of various CYP enzymes (electron donors), oxidative conversion of multiple steroid hormones, and detoxification and elimination of xenobiotics that are transported via peripheral blood (Gocek et al., 2014; Furukawa et al., 2004).
5 Conclusions
Therefore, it can be concluded that the bacterial action on the molecular structure of pure catechin suppresses the hydrogen donating ability to convert unpaired electron to paired electron, hence exhibiting low antioxidant potential under In vitro conditions. Hence, GPX1 and POR exhibit different expression patterns in treated peripheral blood mononuclear cells. These findings suggest that the functional variations between pure catechin and colonic bacterial transformed catechin metabolites. However, further In vitro and In vivo study is needed to assess the biological impact of colonic bacterial transformed catechin metabolites.
Acknowledgements
The authors acknowledge the support from the Researchers supporting project number (RSP-2020/178), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem.. 2013;24:1415-1422. PMID: 23849454
- [CrossRef] [Google Scholar]
- A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem.Toxicol.. 2013;51:15e25.
- [Google Scholar]
- Antioxidants bound to an insoluble food matrix: Their analysis, regeneration behavior, and physiological importance. Compr. Rev. Food Sci.. 2017;16(3):382-399.
- [Google Scholar]
- Immunomodulatory and Antioxidant Properties of Wheat Gluten Protein Hydrolysates in Human Peripheral Blood Mononuclear Cells. Nutrients.. 2020;4(12):1673.
- [CrossRef] [Google Scholar]
- Antioxidantand antigenotoxic effectsofpupaeofthemugasilkworm Antheraeaassamensis. Food Biosci.. 2014;5:108-114.
- [CrossRef] [Google Scholar]
- Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules. 2017;19:22-484. PMID: 28335502; PMCID: PMC6155401
- [CrossRef] [Google Scholar]
- Cytochrome p450 gene expression levels in peripheral blood mononuclear cells in comparison with the liver. Cancer Sci.. 2004;95:520-529.
- [CrossRef] [Google Scholar]
- A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients.. 2017;4:9-455. PMID: 28471393; PMCID: PMC5452185
- [CrossRef] [Google Scholar]
- NADPH-cytochrome P450 reductase is regulated by all-trans retinoic acid and by 1,25-dihydroxyvitamin D3 in human acute myeloid leukemia cells. PLoS One. 2014;9(3):e91752.
- [Google Scholar]
- Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem.. 2018;15(241):480-492. Epub 2017 Sep 1 PMID: 28958556
- [CrossRef] [Google Scholar]
- Antioxidant activity of food constituents: an overview. Arch. Toxicol.. 2012;86(3):345-391.
- [Google Scholar]
- Antioxidants and antioxidant methods: an updated overview. Arch. Toxicol.. 2020;94:651-715.
- [CrossRef] [Google Scholar]
- Oxidative DNA damage: mechanisms and significance in health and disease. Antioxid. Redox Signal.. 2006;8:981-983.
- [CrossRef] [Google Scholar]
- A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae) BMC Res. Notes. 2013;6:24.
- [Google Scholar]
- Colonic Bacteria-Transformed Catechin Metabolite Response to Cytokine Production by Human Peripheral Blood Mononuclear Cells. Biomolecules.. 2019;5:9-830. PMID: 31817548; PMCID: PMC6995598
- [CrossRef] [Google Scholar]
- In vitro and In vivo Antioxidant, Anti-hyperlipidemic Properties and Chemical Characterization of Centella asiatica (L.) Extract. Front Pharmacol. 2016:287-400. PMID: 27840607; PMCID: PMC5083837
- [CrossRef] [Google Scholar]
- An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem.. 2002;76(1):69-75.
- [Google Scholar]
- Protective Effect of Sinapine against Hydroxyl Radical-Induced Damage to Mesenchymal Stem Cells and Possible Mechanisms. Chem. Pharm. Bull. (Tokyo). 2016;64:319-325. Epub 2016 Feb 3 PMID: 26842908
- [CrossRef] [Google Scholar]
- On changes of activity of antioxidases in hippocampus rat with multi-infract dementia and the intervention effects of acupuncture. China J. Trad. Chin. Med. Pharm.. 2005;20:724-726.
- [Google Scholar]
- Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev.. 2010;4:118-126.
- [CrossRef] [Google Scholar]
- Melatonin ameliorates H2O2-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol. Res.. 2018;11:51-117. PMID: 29891016; PMCID: PMC5996524
- [CrossRef] [Google Scholar]
- Antioxidant intake and antitumor therapy: toward nutritional recommendations for optimal results. Oxid. Med. Cell. Longevity. 2016;2016:1-19.
- [Google Scholar]
- Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem.. 2019;15(178):687-704.
- [Google Scholar]
- Antioxidant activity of smilax excels L leaf extracts. Food Chem.. 2008;110:571-583.
- [CrossRef] [Google Scholar]
- Free radicals: properties, sources, targets, and implications in various diseases. Indian J. Clin. Biochem.. 2015;30:11-26.
- [Google Scholar]
- AntioxidantactivityapplyinganimprovedABTSradicalcation decolorizationassay. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [CrossRef] [Google Scholar]
- Polyphenol rich extract of Garcinia pedunculata fruit attenuates the Hyperlipidemia induced by High Fat Diet. Front. Pharmacol.. 2016;7:294.
- [CrossRef] [Google Scholar]
- Challenges for research on polyphenols from foods in Alzheimer's disease: bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem.. 2008;56(13):4855-4873.
- [Google Scholar]
- Biochemistry of free radicals and antioxidants, Scholars Acad. J. Biosci.. 2014;2 110e118
- [Google Scholar]
- Glutathione peroxidase-1 but not -4 is involved in the regulation of cellular 5-lipoxygenase activity in monocytic cells. Biochem. J.. 2000;349(2):455.
- [CrossRef] [Google Scholar]
- Biotransformation of (-)-Epigallocatechin and (-)-Gallocatechin by Intestinal Bacteria Involved in Isoflavone Metabolism. Biol. Pharm. Bull.. 2015;38(2):325-330.
- [Google Scholar]







