Translate this page into:
Antioxidant, anti-inflammatory and anti-apoptotic effects of amentoflavone on gentamicin-induced kidney damage in rats
⁎Corresponding author. umar.ijaz@uaf.edu.pk (Muhammad Umar Ijaz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Gentamicin (GEN) is an extensively used aminoglycoside. Contrary to its antibacterial potential, it can induce oxidative stress in several organs, including kidney. Amentoflavone (AMN) is a biflavonoid with conspicuous pharmacological activities. The current study was held to estimate the curative efficacy of AMN to antagonize the nephrotoxic effects instigated by GEN. 48 albino rats were separated into four groups: control group, GEN administrated group (80 mgkg−1 intraperitoneally), GEN + AMN treated group (80 mgkg−1 + 40 mgkg−1) and only AMN administrated group (40 mgkg−1). Following 30 days of administration, results showed that GEN disturbed antioxidant enzymes i.e., glutathione (GSH), glutathione reductase (GSR), glutathione peroxidase (GPx), glutathione S-transferase (GST), superoxide dismutase (SOD), catalase (CAT) activity, besides elevated malondialdehyde (MDA) along with reactive oxygen species (ROS) level. Besides this, level of inflammatory cytokines involving interleukin-6 (IL-6), nuclear factor-kappa B (NF-κB), interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α) & cyclo-oxygenase-2 (COX-2) were escalated. Furthermore, treatment with GEN enhanced the level of apoptotic proteins comprising of Bax, caspase-9 along with caspase-3 besides lessened the level of Bcl-2. In addition to this, GEN reduced the level of albumin, creatinine clearance & augmented the level of creatinine, urea, urobilinogen, urinary protein, kidney injury molecules-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL) as well as instigated various histopathological damages. However, co-treated group (AMN + GEN) revoked abovementioned renal dysregulations instigated by GEN. Taken together, AMN could significantly counteract GEN-instigated nephrotoxic effects due to its antioxidant capabilities.
Keywords
Gentamicin
Amentoflavone
Kidney damage
Oxidative stress
Inflammation
Apoptosis
1 Introduction
Aminoglycosides are potential semisynthetic or natural antibiotics obtained from actinomycetes. They placed among the first-line medicines used clinically to treat infectious disturbance (Krause et al., 2016). Gentamicin (GEN) is an amino-glycosidic medicine which are employed to treat multiple life-threatening bacterial ailments, especially from gram-negative bacteria (Virani et al., 2016). Nephrotoxicity can be induced by various sorts of agents through several mechanisms, causing cellular apoptosis and necrosis either by radiation or chemicals. About 20% of renal toxicity is provoked by drugs while this proportion is much higher in aged people following long-term exposure to these causative drugs. GEN-induced nephrotoxicity results due to the intensive production of ROS that eventually leads to renal dysfunction. About 90% of gentamicin is excreted unaltered from proximal convoluted tubules in the kidney, resulting structural variations in renal tissues. Chronic and higher doses of GEN causes in-vitro and in-vivo free radical generation triggering multiple life-threatening mechanisms (Bhatia et al., 2017).
Bioaccumulation of GEN in the proximal convoluted tubules (PCT) causes inflammation, damages in the mesangial cells, renal tubular damages, enhance iron mediated lipid peroxidation and ultimately renal disruption due to receptor-mediated endocytosis (Nagai and Takano, 2004). In the kidneys, aggregation of GEN results in structural alterations such as removal of the brush borders and tubular deterioration (Sardana et al., 2015). The underlying mechanism of GEN-instigated renal toxicity is poorly known. Previous literature linked the GEN-caused renal toxicity with oxidative stress and brought down the defense system of nephron as well as elevated the risk of kidney dysfunction (Balakumar et al., 2010).
Flavonoids are characterized by their polyphenolic structural composition and are known to possess various pharmacological and biological properties (Maleki et al., 2019). Among these flavonoids, amentoflavone (AMN) stands out as a widely distributed biflavonoid. It consists of two apigenin molecules that are connected in a dimeric form and it contains six hydroxyl (OH) groups. AMN is primarily obtained from species of Selaginella, a genus of plants. It is eminent for its pharmacological abilities involving anti-senescence, anti-diabetes, anti-tumor and anti-inflammation (Yu et al., 2017). The objective of this study was to assess the therapeutic potential of AMN in mitigating the renal damage caused by GEN.
2 Material and methods
2.1 Chemicals
GEN was procured from Alexandria Pharmaceuticals, Egypt. AMN was bought from Chengdu Must Biotechnology, China.
2.2 Animals
Experiment was held on 60–70 days old 48 albino rats weighing 190–220 g. Rats were housed at the University of Agriculture Faisalabad (UAF). Constant temperature (25 ± 2 °C) and twelve hours of dark and light period were maintained. The selection of 12-hour dark/light photoperiod in experimental trial was predicated on its ability to maintain the circadian rhythm of organisms as it offered a balanced alteration between light and dark periods. It is a standard procedure that ensures comparability across studies while minimizing stress and disruption and closely emulates natural day length. Its implementation in experimental setups is also highly practical. Moreover, accessibility to food and water was ensured. Experiment was conducted in accordance with European Union of Animal Care and Experimentation (CEE Council 86/609) protocol.
2.3 Layout of experiment
4 groups of animals were made (6 rats in each). Control group received an administration of normal saline. The GEN-treated group received (80 mgkg−1) of GEN intraperitoneally. Co-treated rats were exposed with AMN (40 mgkg−1) orally & GEN (80 mgkg−1) intraperitoneally. Only AMN supplemented animals were administered with (40 mgkg−1) on daily basis during the entire period. The dosage of GEN, 80 mgkg−1 was administered in accordance with the previous research of Kopple et al. (2002), while the usage of AMN at a dosage of 40 mgkg−1 was employed, it was based on the experiment conducted by Alherz et al. (2022). On the 30th day of examination, rats were anesthetized with the help of ketamine (60 mgkg−1) as well as xylazine (6 mgkg−1), then decapitated & blood sample were taken for further analysis. After dissection, kidneys were separated, one of them was preserved in 10% formalin for histology and the other was collected in plastic bag and kept at −80 °C for the analysis of biochemical profile.
2.4 Biological analysis of antioxidants
The activity of CAT was analyzed using Aebi (1984) protocol while SOD’s activity was analyzed with the techniques of Sun et al. (1988). The assessment of GPx activity was conducted by using the methodology developed by Lawrence and Burk. Ellman’s, 1959 method was used for the assessment of activity of the GSH. GST activity was analyzed by Habig et al. (1974). The Carlberg and Mannervik (1975) approach was employed to assess GSR activity.
2.5 Analysis of ROS, MDA and H2O2
The concentration of ROS in the kidneys was evaluated using methodology by the Hayashi et al. (2007). Analysis of MDA level was carried out in-line with the process given by Ohkawa et al. (1979). The Pick & Keisari (1981) method was used to detect the concentration of H2O2.
2.6 Analysis of urine, serum profile
Employing standard diagnostic kits (MediScreen kit France), urea, urinary proteins, albumin, urobilinogen, creatinine and creatinine clearance levels were evaluated.
2.7 Analysis of KIM-1 & NGAL
NGAL & KIM-1 concentration was detected via KIM-1 Quantikine ELISA kits and NGAL Quantikin ELISA kits, respectively in compliance with the manufacturer’s advice (R & D System China Co. Ltd., Changning, China).
2.8 Analysis of inflammatory markers
ELISA kits from Shanghai-YL-Biotech. Co. Ltd., China was applied for estimating the concentration of IL-1β (CSB-E08055r), NF-κB (CSB-E13148r) IL-6 (CSB-E04640r), TNF-α (CSB-E07379r) as well as COX-2 (CSB-E04640r).
2.9 Analysis of apoptotic markers
Utilizing ELISA kits from Cusabio Technology Llc, Houston, TX, USA. Caspse-3 (CSB-E08857r), Bax (CSB-EL002573RA), Bcl-2 (CSB-E08854r) and caspase-9 (CSB-E08863r) concentrations were analyzed.
2.10 Histological study of renal tissues
The right kidneys from each rat were divided longitudinally and placed in a solution (10% formalin) for about 24 h. Kidneys were encased in paraffin wax and sliced into small pieces by using microtome machine. These slices were stained with eosin following hematoxylin & analyzed using light microscope, Olympus BX51 (Imagetec Pvt. Ltd., Chennai, India), for histo-pathological examination.
2.11 Statistical evaluation
Results were demonstrated as MEAN ± SEM. One-way ANOVA following Tukey’s test was employed for comparison between treated animals. P < 0.05 was set as a level of significance. Minitab (software) Version 21.1.0 was utilized for the accomplishment of analysis of data.
3 Results
3.1 Effect of AMN on the activity of antioxidant enzymes
GEN-exposed rats manifested a notable (p < 0.05) decline in anti-oxidant enzyme (GPx, GST, SOD, GSH, GSR) activity contrary to control condition animals. In the co-administered rats, GEN + AMN, resulted in a notable (p < 0.05) improvement in the aforementioned enzyme’s activity when matched with GEN-administered rats. Whereas, AMN (alone) administered animals displayed the normal level of enzymes (Table 1). Values exhibiting dissimilar letters varies notably from other groups.
Groups
CAT (U/mg protein
SOD (nanomole)
GSR (Nm NADPH oxidized/min/mg tissue)
GPX (U/L)
GSH (Nm/min/mg protein)
GST (mg/dI)
Control
8.73 ± 0.49a
8.11 ± 0.15a
6.25 ± 0.12a
27.25 ± 1.55a
15.67 ± 0.93a
21.97 ± 1.69a
GEN
4.50 ± 0.18c
3.25 ± 0.20c
2.75 ± 0.19c
7.02 ± 0.23c
4.51 ± 0.72c
9.20 ± 0.55c
GEN + AMN
7.29 ± 0.22b
6.48 ± 0.27b
4.83 ± 0.09b
16.69 ± 2.10b
12.18 ± 0.59b
15.42 ± 2.18b
AMN
8.79 ± 0.48a
8.15 ± 0.18a
6.27 ± 0.13a
27.43 ± 1.74a
15.69 ± 0.94a
22.03 ± 1.67a
3.2 Effect of AMN on the concentration of MDA, H2O2 & ROS
GEN exposure exhibited a remarkable (p < 0.05) escalation in levels of H2O2, MDA, ROS when matched with control rats. The animals with combine treatment of (GEN + AMN) expressed a notable (p < 0.05) decrease in MDA, H2O2, & ROS concentration in contrast to rats intoxicated with GEN. AMN administration (alone) showed similar level of abovementioned markers as in the untreated animals (Table 2). Values exhibiting dissimilar letters varies notably from other groups.
Groups
MDA (micro mole/Liter)
ROS (µmol/g)
H2O2 (µM/min/mg protein)
Control
0.80 ± 0.08c
0.74 ± 0.10c
1.19 ± 0.09c
GEN
4.92 ± 0.18a
8.19 ± 0.35a
4.9 ± 0.19a
GEN + AMN
1.74 ± 0.10b
3.40 ± 0.18b
1.86 ± 0.11b
AMN
0.79 ± 0.06c
0.72 ± 0.11c
1.17 ± 0.08c
3.3 Effect of AMN on urine and serum profile
Treatment with GEN instigate a substantial (p < 0.05) elevation in concentration of urinary protein, urobilinogen, urea, serum creatinine as well a notable (p < 0.05) reduction was noticed in creatinine clearance & albumin when matched with the control. However, co-administration of GEN + AMN represented a remarkable (p < 0.05) decrease in the concentration of urobilinogen, urinary proteins, creatinine along with urea but a notable increase in the albumin and creatinine clearance was found in contrast to GEN administration. The treatment of AMN alone doesn’t express any considerable difference when matched with the control animals (Fig. 1).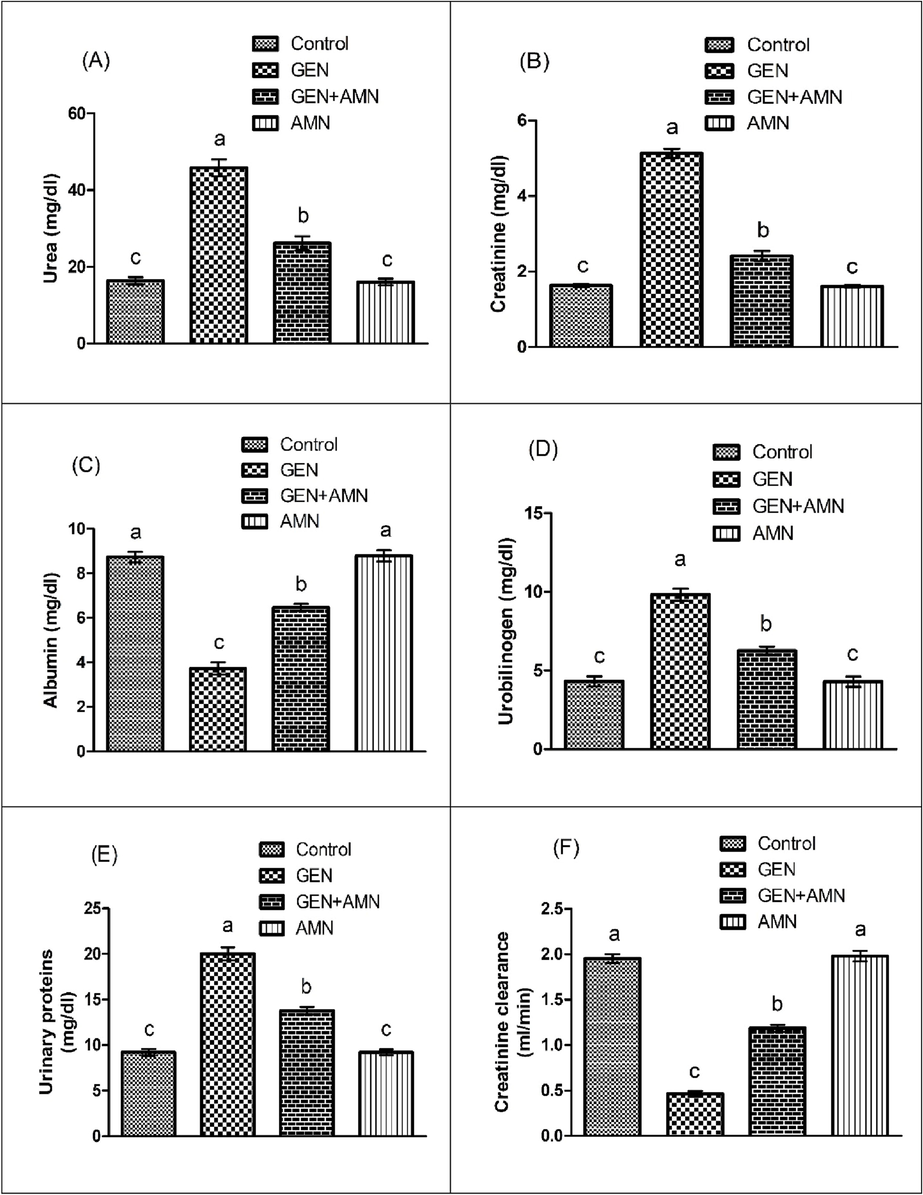
Effect of GEN and AMN on (A) Urea, (B) Creatinine, (C) Albumin, (D) Urobilinogen, (E) Urinary Protein, (F) Creatinine Clearance Levels. Bars Represent Mean ± SEM Values. Diffrent superscripts on bars indicate significant differences at p < 0.05.
3.4 Effect of AMN on the concentration of KIM-1 and NGAL
Treatment with GEN prompted a profound (p < 0.05) rise in the KIM-1 & NGAL’s level as compared to control condition animals. GEN + AMN significantly regulated the aforesaid dysregulation contrary to GEN group. AMN administration alone showed similar as in normal rats. (Table 3). Values exhibiting dissimilar letters varies notably from other groups.
Groups
KIM-1 (ng/day)
NGAL (mg/ml)
Control
0.27 ± 0.03c
0.60 ± 0.05c
GEN
0.86 ± 0.05a
1.82 ± 0.14a
GEN + AMN
0.52 ± 0.05b
1.13 ± 0.10b
AMN
0.25 ± 0.04c
0.59 ± 0.06c
3.5 Effect of AMN on the concentration of inflammatory markers
GEN supplemented rats represented remarkable (p < 0.05) elevation in concentrations of pro-inflammatory markers (IL-6, TNF-α, COX-2, IL-1β, NF-κB) compared to the control, demonstrating GEN’s role in potentially triggering inflammatory damage. The co-treated animals expressed a notable (p < 0.05) reduction in pro-inflammatory markers when matched to the GEN administered rats. AMN when given (alone) displayed same results as in untreated group (Table 4). Values exhibiting dissimilar letters varies notably from other groups.
Groups
NF-κB (ng/g tissue)
TNF-α (ng/g tissue)
IL-1β (ng/g tissue)
IL-6 (ng/g tissue)
COX-2 (ng/g tissue)
Control
13.65 ± 1.71c
7.26 ± 1.12c
23.21 ± 1.42c
8.44 ± 1.23c
15.94 ± 1.79c
GEN
85.26 ± 2.39a
26.12 ± 2.42a
88.06 ± 3.19a
51.18 ± 3.52a
84.78 ± 2.69a
GEN + AMN
35.44 ± 2.21b
14.09 ± 1.25b
34.63 ± 1.71b
17.57 ± 2.21b
29.63 ± 2.53b
AMN
13.50 ± 1.73c
7.19 ± 1.13c
23.14 ± 1.43c
7.06 ± 0.67c
15.57 ± 1.51c
3.6 Effect of AMN on the level of apoptotic markers
Concentrations of caspase-9, caspase-3 & Bax’s level were escalated in the GEN treated group while concentration of Bcl-2 was reduced in comparison to the control, confirming that GEN has the ability to cause apoptotic injuries. The co-treated (GEN + AMN) group manifested a remarkable reduction in the pro-apoptotic marker’s level relative to GEN exposed group. Whereas AMN (alone) supplementation showed similar activity of aforesaid markers with control rats (Table 5). Values with different superscripts varies notably from other groups.
Groups
Bax (pg/mL)
Bcl-2 (ng/mL)
caspase-3 (pg/mL)
caspase-9 (pg/mL)
Control
1.97 ± 0.14c
16.98 ± 1.36a
3.45 ± 0.27c
2.69 ± 0.32c
GEN
7.44 ± 0.22a
3.59 ± 0.53c
15.94 ± 0.34a
13.91 ± 1.32a
GEN + AMN
2.92 ± 0.17b
13.53 ± 1.19b
7.58 ± 0.29b
4.67 ± 0.41b
AMN
1.94 ± 0.11c
17.03 ± 1.38a
3.43 ± 0.26c
2.66 ± 0.32c
3.7 Effect of AMN on tissue histology
The histopathological observation of the control and AMN group demonstrated the presence of a normal and intact structure of glomerulus, Bowmen’s capsule and convoluted tubules (Fig. 2A and D). In contrast, the glomeruli of rats treated with GEN exhibited significant congestion, accompanied by pronounced damage to Bowman’s capsule & alterations in the tubular system of the kidney (Fig. 1B). However, the histopathological analysis of the co-treated group (GEN + AMN) revealed an improved morphology of the glomerular structure and restoration of the normal lining of tubular renal epithelium (Fig. 2C).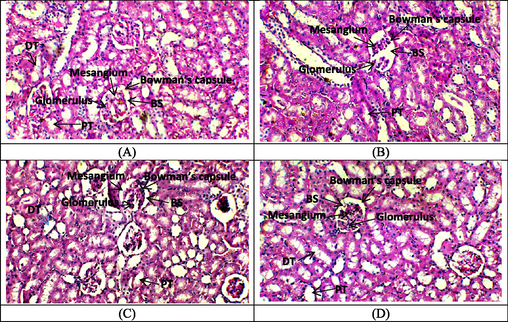
Effect of GEN and amentoflavone on renal cortex of the rats. (A) Control group presenting normal distal convoluted tubules, renal glomerulus and Bowman’s space, (B) GEN treated group displaying epithelial flattening in tubules with vacuolar degeneration, atrophied glomeruli and widening of Bowman’s spaces, (C) Co-treated group of GEN + AMN representing recovering distal convoluted tubules, renal glomerulus and Bowman’s space almost near to normal architecture, (D) Only AMN treated group with normal glomerulus.
4 Discussion
In the present experiment, GEN administration resulted in reduction in the antioxidant enzyme’s activity involving GSH, SOD, GPx, CAT, GST and GSR (Bansal et al., 2005). CAT performs a pivotal function in the substantial conversion of H2O2 into H2O and molecular oxygen, consequently, it offers cellular defense against oxidative injury caused by H2O2 and hydroxide ions (Aitken and Roman, 2008). SOD functions as a crucial catalyst in the transformation of superoxide anion into H2O2 & oxygen (Liochev and Fridovich, 2010). GSR mediated the oxidation of glutathione disulfide, thereby altering it into its reduced form (GSH) (Kaneko et al., 2002). GSH exhibits its protective effects against oxidative stress in mammalian cells via reducing the levels of not only hydrogen peroxide but also other peroxides (Deponte, 2013). GST, being a versatile enzyme, participate in a wide range of detoxification processes via catalytically conjugating electrophilic toxins with the abundant glutathione molecules it contains (Tirmenstein and Reed, 1989). GEN administration induced a remarkable escalation in lipid peroxidation (MDA), H2O2 & ROS production. Lipid peroxidation along with oxidative stress (OS) reduces the ability of proximal convoluted tubules to function properly which eventually results in renal failure (Ishtiaq et al., 2022; Al-Kuraishy and Al-Gareeb, 2016). The aforementioned antioxidant enzymes play a remarkable task in decreasing oxidative stress prompted deformities (Zia-Ul-Haq, 2021). CAT predominantly alters H2O2 into H2O (Aitken and Roman, 2008). Toxic superoxide is primarily converted by SOD into H2O2 and 1O2 (El-Boshy et al., 2019). GPX acts as a quencher of H2O2 (Ballatori et al., 2009). In the present research, co-treatment GEN + AMN enhanced the activity of the above-mentioned antioxidants and diminish H2O2, ROS & MDA concentrations, indicating the curative action of AMN against GEN. Thus, the ability of AMN in mitigating the GEN instigated oxidative disturbance in kidneys is attributed to its radicle scavenging ability as determined by invitro analysis from Bajpai et al. (2019).
Administration of GEN enhanced the concentration of renal pro-inflammatory proteins viz. IL-1β, TNF-α, IL-6 along with NF-κB which are involved in acute inflammatory responses and other severe infections correlated with escalated level of ROS (Widowati et al., 2022). Intensive upregulation of these cytokines specifically NF-κB alters renal cellular configuration, causing damage to glomerulus, proximal convoluted and distal convoluted tubules, including collecting duct, affecting rate of filtration and reabsorption which disturbs normal activity of nephron (Irkin and Öztürk, 2022; Akbaribazm et al., 2021). Our findings demonstrated that the co-treatment with AMN and GEN significantly reduced the concentration of pro-inflammatory markers. Whereas, AMN exerts therapeutic action against elevated level of above-mentioned pro-inflammatory cytokines due to its anti-inflammatory action (Kim et al., 1998).
Administration of GEN elevated the concentration of apoptotic proteins such as caspase-3, Bax & caspase-9 although down-regulated the concentration of anti-apoptotic protein Bcl-2 in rats which is related with acute apoptotic responses (Kandeil et al., 2018). This imbalance in Bcl-2 & Bax stimulate the secretion of cytochrome complex from the inner membrane of mitochondria & enhances production of caspase-3 which is a major protein that induces process of apoptosis in kidneys (Berens and Tyler, 2011). Furthermore, our findings showed that co-treatment with AMN + GEN maintained the level of pro-apoptotic markers while upregulating the concentration of Bcl-2. AMN attenuated the GEN instigated apoptotic impairments that may be attributed to its anti-apoptotic nature (Zhang et al., 2015).
In the present experiment GEN treatment instigated a considerable escalation in the creatinine, urea, urinary proteins and urobilinogen whereas we found a remarkable decrease in creatinine clearance and albumin. Besides this, concentration of KIM-1 & NGAL were also escalated substantially which are the major indicators of renal failure (Lei et al., 2015). Variation in glomerular architecture, dysregulation of filtration rate and subsequent disruption in the functions of nephron are all associated with elevated the level of nitrogenous end products (urea & creatinine) (Alvi et al., 2022; Laurent et al., 1990). Aforementioned dysregulations in the concentrations of NGAL & KIM-1 resulted in lethal conditions such as vascular damage along with proximal tubular dysfunctions (Dobrek et al., 2016). It was found that co-administration of AMN and GEN effectively downregulated the urobilinogen, urinary proteins, creatinine NGAL & KIM-1 concentrations. Besides this, the concentration of creatinine clearance & albumin was escalated. This mitigative effect may be ascribed to Reno-protective role of AMN.
In the present investigation, GEN instigated histopathological alterations including apoptotis, degradation of the epithelium in the renal tubules and contraction of glomerular mesangial cells (Lopez-Novoa et al., 2011). According to Quiros et al. (2011), GEN aggregates over a prolonged period of time in the collecting tubule’s epithelial cells, resulting in intracellular alterations that eventually cause necrosis of the brush border and tubular cells. GEN instigated nephrotoxicity causes reduction in renal blood flow through vasoconstriction, blockage of vasodilators and promotion of leukocyte marginalization (Samiee-Zafarghandy and van den Anker, 2013). Our result manifested that when AMN was given to rats in combination with GEN, overt therapeutic response was observed in the aforesaid renal damages generated by GEN. Therefore, histoprotective potential could be ascribed to its anti-apoptotic, anti-oxidant & anti-inflammatory nature of AMN.
5 Conclusion
The evidences from the present investigation illustrate that AMN possesses the ability to mitigate the detrimental nephrotoxic effects induced by GEN via modulating the levels of antioxidant enzymes. AMN supplementation was observed to significantly reduce renal reactive oxygen species, lipid peroxidation, serum urea and creatinine concentration. Furthermore, it demonstrated the capacity of AMN to restore the elevated levels of pro-inflammatory & pro-apoptotic markers as well as alleviate the disruption in renal tissues. These results provide evidence that AMN exhibits defensive properties that can be assigned to its anti-apoptotic, anti-oxidant, anti-inflammatory & nephroprotective capabilities.
Acknowledgement
This work was funded by Researchers Supporting Project number (RSP2023R26), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell Longev.. 2008;1:15-24.
- [Google Scholar]
- Anti-inflammatory, anti-oxidative and anti-Apoptotic effects of Heracleum persicum L. extract on rats with gentamicin-induced nephrotoxicity. Asian Pac. J. Trop. Biomed.. 2021;11:47-58.
- [Google Scholar]
- Potential cardioprotective effects of Amentoflavone in doxorubicin-induced cardiotoxicity in mice. Biomed. Pharmacother.. 2022;154:113643
- [Google Scholar]
- Potential effects of pomegranate on lipid peroxidation and pro inflammatory changes in daunorubicin-induced cardiotoxicity in rats. Int. J. Prev. Med.. 2016;7:85.
- [Google Scholar]
- Nephroprotective Effects of Delphinidin against Bisphenol A Induced Kidney Damage in Rats. Pak. Vet.. 2022;43:189-193.
- [Google Scholar]
- Antioxidant and antimicrobial efficacy of a biflavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chem.. 2019;271:239-247.
- [Google Scholar]
- Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmacol. Res.. 2010;62:179-186.
- [Google Scholar]
- Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem.. 2009;390:191-214.
- [Google Scholar]
- Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact.. 2005;156:101-111.
- [Google Scholar]
- The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis. J. Virol.. 2011;85:3858-3871.
- [Google Scholar]
- Ameliorative role of esculetin-mediated renoprotection against gentamicin-induced nephrotoxicity and possible involvement of N-methyl-D-aspartate receptors. Asian J. Pharm. Clin. Res.. 2017;10:322-328.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [Google Scholar]
- Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. Gen. Subj.. 2013;1830:3217-3266.
- [Google Scholar]
- Urinary kidney injury molecule-1 (KIM-1) excretion in rats with experimental cystitis induced by oxazaphosphorines. Prz Lek. 2016:73.
- [Google Scholar]
- The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Res.. 2019;26:22736-22746.
- [Google Scholar]
- Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. - Genet. Toxicol.. 2007;631:55-61.
- [Google Scholar]
- Ameliorative Effects of Ulva rigida (C. Agardh, 1823) on Cadmium-induced Nephrotoxicity in Wistar Albino Rats. Pak. Vet.. 2022;42:419-423.
- [Google Scholar]
- Therapeutic Effect of Oroxylin A Against Bisphenol A-induced Kidney Damage in Rats: a Histological and Biochemical Study. Pak. Vet.. 2022;42:511-516.
- [Google Scholar]
- Wheat germ and vitamin E decrease BAX/BCL-2 ratio in rat kidney treated with gentamicin. Beni-Suef Univ. J. Basic Appl. Sci.. 2018;7:257-262.
- [Google Scholar]
- The expression of glutathione reductase in the male reproductive system of rats supports the enzymatic basis of glutathione function in spermatogenesis. Eur. J. Chem.. 2002;269:1570-1578.
- [Google Scholar]
- Amentoflavone, a plant biflavone: a new potential anti-inflammatory agent. Arch. Pharm. Res.. 1998;21:406-410.
- [Google Scholar]
- l-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol. Dial. Transplant.. 2002;17:2122-2131.
- [Google Scholar]
- Aminoglycoside-induced renal phospholipidosis and nephrotoxicity. Biochem. Pharmacol.. 1990;40:2383-2392.
- [Google Scholar]
- Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun.. 1976;71:952-958.
- [Google Scholar]
- Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci. Rep.. 2015;8:1-9.
- [Google Scholar]
- Mechanism of the peroxidase activity of Cu, Zn superoxide dismutase. Free Radic. Biol. Med.. 2010;48:1565-1569.
- [Google Scholar]
- New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int.. 2011;79:33-45.
- [Google Scholar]
- Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab. Pharmacokinet.. 2004;19:159-170.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages-induction by multiple nonphagocytic stimuli. Cell Immunol.. 1981;59:301-318.
- [Google Scholar]
- An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol. sci.. 2011;119:245-256.
- [Google Scholar]
- Nephrotoxic effects of aminoglycosides on the developing kidney. J. Pediatric Neonatal Individ. Med.. 2013;2 020227-020227
- [Google Scholar]
- Nephroprotective effect of catechin on gentamicin-induced experimental nephrotoxicity. Clin. Exp. Nephrol.. 2015;19:178-184.
- [Google Scholar]
- A simple method for clinical assay of superoxide dismutase. Clin. Chem.. 1988;34:497-500.
- [Google Scholar]
- Role of a partially purified glutathione S-transferase from rat liver nuclei in the inhibition of nuclear lipid peroxidation. Biochim. Biophys. Acta Proteins Proteom.. 1989;995:174-180.
- [Google Scholar]
- Aloe vera attenuates gentamicin-induced nephrotoxicity in wistar albino rats: histopathological and biochemical changes. Asian J. Pharm. Clin. Res.. 2016;9:113-117.
- [Google Scholar]
- Protective Effect of Ethanolic Extract of Jati Belanda (Guazuma ulmifolia L.) by Inhibiting Oxidative Stress and Inflammatory Processes in Cisplatin-induced Nephrotoxicity in Rats. Pak. Vet.. 2022;42:376-382.
- [Google Scholar]
- A review on the phytochemistry, pharmacology, and pharmacokinetics of amentoflavone, a naturally-occurring biflavonoid. Molec.. 2017;22:299.
- [Google Scholar]
- Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen. Res.. 2015;10:1125.
- [Google Scholar]
- Zia-Ul-Haq, M., 2021. Historical and introductory aspects of carotenoids. In: Carotenoids: Structure and Function in the Human Body. pp. 1–42.







