Translate this page into:
Antioxidant and organic acid evaluation of Geaster saccatum mushroom by chemical and electrochemical assay at carbon nanotube paste electrode
⁎Corresponding author. ufaq07@gmail.com (Javed Ahmad Wagay)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The study was aimed to investigate the antioxidant and organic acid composition of Geaster saccatum mushroom by chemical and electrochemical assay. The antioxidant activity was done by using DPPH and ABTS reagents while the cyclovoltametry (CV) and differential pulse voltamery (DPV) were used to determine its electrochemical behavior at single walled carbon nano tube paste electrode in 0.02 M acetate buffer (pH 5.0), to explore the adsorptive character of its antioxidant compounds at working electrode as a function of pH, scan rate and potential. The organic acid analysis of this mushroom was carried out by High performance liquid chromatography. In DPPH, the scavenging rate was 56.4 ± 0.78%, at 20 µg/ml, reached to 93.4 ± 0.93% at 100 µg/ml, with 61.01 μg/ml as its IC50. Similarly, in ABTS, at same concentrations, scavenging rates were 38.14 ± 0.54% and 95.16 ± 0.43%, with 58.03 μg/ml as its IC50, respectively. HPLC quantified eight organic acids with gallic acid at highest concentration (193.34 mg/kg dry wt) followed by ketoglutaric acid (181.16 mg/kg dry wt.) and oxallic acid (69.54 mg/kg dry wt.). The CV produced a clear irreversible anodic peak at Epa = 0.69 ± 0.01 V, while, ascorbic acid produced a single anodic peak at Epa = 0.29 ± 0.01 V and gallic acid produced two anodic peaks at Epa = 0.41 ± 0.01 V and second at Epa = 0.69 ± 0.01 V. However, only one anodic peak of mushroom was found in DPV at 0.56 ± 0.01 V, which increased proportionally at each increasing concentration of mushroom extract. The results declared this mushroom as one of the best source of organic acids which characterized it as a better antioxidant food as well as medicine.
Keywords
Electrochemical
Antioxidant
High performance liquid chromatography
Cyclovoltametry
CV
DPV
1 Introduction
The ideal food is the lucent enigma behind the better health, act as medicine and an appropriate source of energy. The most mushrooms witnessed the best source of proteins (30–35% dry basis), with essential amino acids, vitamins including thiamin, riboflavin, ascorbic acid, vitamin D2, minerals like iron, zinc, selenium, and sodium (Mattila et al., 2000). Besides, an appropriate wellspring of bioactive compounds, mushrooms have pharmaceutical impacts like antibacterial, antioxidant, antiviral, anticancer, anti-inflammatory and, anti-obesity activities (Ozen et al., 2011). Antioxidants are thoroughly being used by the living cells to combat the oxidant load to counterbalance the toxicity of reactive oxygen species (ROS), reactive nitrogen species (RNS) and reactive sulphur species (RSS) (Carocho and Ferreira, 2013). However, defense against these oxidants, nature enriched the human body by enzymes like superoxide dismutase, catalase, glutathione peroxidases, glutathione reductase and, coenzymes like, glutathione and vitamin like α-tocopherol (Gutteridge and Halliwell 2000). But this primary defense network is comparatively deficient and inadequate to minimize the oxidant load of cells. Therefore, antioxidants with natural origin like mushrooms had been thoroughly focused as a supplementary antioxidant support to endogenous defense system for maximum possible protection from oxidative loss. Because of the fact, mushrooms have higher antioxidant capabilities as they contain polyunsaturated fatty acids, immunomodulatory proteins, phenols and organic acids (Chai et al., 2016). These significant ingredients of mushrooms are being used as pharmaceutics for treating diseases like, rheumatoid, immune disorders, acute radiation syndrome, hemophilia and used to protect DNA, proteins and lipids which are susceptible to oxidants (Valko et al., 2007). The type and composition of organic acids in mushrooms are crucial factors affecting their organoleptic characters, and are early relievers of cellular oxidative loads (Silva et al., 2004). The chemical structure and proportion of organic acids in mushrooms determine their antioxidant capability (Valentao et al., 2005). The present work was designed to evaluate the antioxidant activity of Geaster saccatum mushroom by chemical and electrochemical methods including cyclo voltametry (CV) and differential pulse voltametry (DPV) at multi walled carbon nano tube paste electrode (MWCNTPE) and to determine its organic acid profile by using HPLC. However, no literature review and reports are available about all such aspects of this mushroom, hence the work strictly carries an innovative mass of new openings and opportunities in mushroom research both as food and pharmaceutics.
2 Materials and methods
2.1 Chemicals and reagents
The standards used namely, oxalic acid, ascorbic acid, Succinic acid, malic acid, ketoglutaric, gallic acid, quinic acid, fumaric acid were purchased from Sigma (St. Louis, MO, USA). All the solvents (ethanol, methanol) used were of analytical grade and were purchased from either Anala R or Himedia Laboratories Pvt. Ltd. Mumbai.
2.2 Sample preparation and extraction
Geaster saccatum fruiting bodies were collected from some parts of central India (Jabalpur, Sagar) during the rainy season in 2019 and dried in an oven at 40 °C for 4 h. The dried fruiting bodies were crushed to powder by using electronic blender. Twenty grams (20 g) of powder was taken in 200 ml of 95% methanol in soxhlet extraction unit for extraction at 30C0 for 12–18 h. The extraction was filtered through Whatman no.4 filter paper. The methanolic extract was then evaporated in vacuum rotary evaporater at 40 °C to dryness. The extract was re-dissolved in acid water (0.1% formic acid). The aqueous solution was then passed through an Isolute C18 column at a ratio of 3:7(methanol and acid water). The organic acids (polar compounds) eluted with aqueous solution while non polar compounds hold back, respectively. Under reduced pressure the aqueous mushroom extract was evaporated to dryness and re-dissolved in 1 ml of H2SO4 (0.02 N), out of which 30 µL was thoroughly analyzed by HPLC at UV detector. Meanwhile, a standard mixture was prepared in H2SO4 (0.02 N) out of which the same volume was analyzed. The organic acids were separated at 0.1 ml/min flow rate at 110 min with 0.02 N H2SO4 as mobile phase. The quantitative analysis of organic acids were done by comparing the absorbance chromatograms of standards by using their peak areas and retention time values.
2.3 DPPH radical scavenging assay
The DPPH assay was done as per authentic protocol with some modifications. Different concentrations of mushroom methanolic extract (0.1–1 ml) were thoroughly mixed with 1 ml of DPPH (0.2 mM) solution. The mixture was thoroughly whir pooled vigorously and then left stagnant for 50 min at 20 °C in dark. The absorbance of the resulting solution was measured at 517 nm against the blank using a UV/Vis spectrophotometer (Elico SL164 Double beam UV–VIS spectrophotometer). Similarly, the absorbance of standards were also measured for comparison. On reduction by antioxidant compound, the DPPH is decolorized, which was quantitatively recorded on spectrophotometer, as absorbance changes. The IC50 value (mg/ml) was recorded as interject by using linear regression analysis.
2.4 ABTS radical scavenging assay
The antioxidant assay of mushroom was determined by using ABTS•+ (2,2′ –azinobis, 3-ethylbenzothiazoline-6-sulphonic acid) with small modifications in pH (7.8). The radicals were produced by reacting ABTS with potassium per sulfate (K2S2O8). 3 mmol/L of ABTS was prepared by dissolving it in 60 ml phosphate buffered saline as a stock solution, from which 60 ml were taken and kept in dark at normal room temperature for overnight. The various concentrations of mushroom extract solutions (0.5 ml) were mixed with 1.5 ml ABTS•+ solution, and absorbance was measured at 734 nm against blank (PBS). The sample analysis were carried in triplicates and their average values were taken as a statistical tool. The absorbance of mushroom extract concentration where 50% radicals were scavenged was taken its IC50 value. The average radical-scavenging assay of samples were calculated as: where A control; absorbance of the control (ABTS•+ alone) and Atest is absorbance of the test sample (ABTS•+ with mushroom extract).
2.5 Electrochemistry
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were performed on a Ω Metrohm 757 VA Computrace (Ion analyzer, version 3.1) using a compact standard triple electrode cell. A single walled carbon nanotube paste electrode (SWCNPE) was used as the working electrode and a platinum (Pt) wire as the counter electrode. All potentials are refer to an Ag/AgCl 3 M KCl reference electrode (Methrom). Prior to use, the working electrode was polished in an aqueous suspension of 0.3 lm alumina (Beuhler) on a Master-Tex (Beuhler) polishing pad, then rinsed with water. Subsequently, in a chemical treatment, the electrode was soncated in 6 M HCl for 1 min, and then in methanol, as a cleaning procedure before any electrochemical measurements. The nitrogen gas was purged for 5 min. A systronics digital µpH meter model-361 was used for pH measurements.
3 Results and discussion
3.1 Antioxidant activity
3.1.1 Radical scavenging at DPPH
The reducing ability of our mushroom extract, was determined from the bleaching of methanol solution of DPPH, a stable organic free radical having deep violet color, with λmax at 510–530 nm (Sultana et al., 2007). This characterization presets to reduce the DPPH in solution by an antioxidant substance as it gives an electron or hydrogen, causing the DPPH lighter in color, measured in terms of its absorbance, as higher the antioxidant concentration, lesser will be the absorbance of reagent (Gulcin et al., 2003). Here the absorbance was measured at two time intervals (10–70 min), at various concentrations (20–100 µg/ml) of mushroom extract. At an initial concentration of 20 µg/ml of extract, scavenging was measured 56.4 ± 0.78%, whereas, at the similar concentration the scavenging percent of ascorbic acid (standard) was 70.2 ± 0.52%, a difference of 13.8%. At 100 µg/ml of mushroom, scavenging was increased to 93.4 ± 0.93%, whereas, it was 103.3 ± 0.87% of standard, a difference of 09.9%, respectively. The IC50 value of mushroom extract was 61.01%, whereas, it was 27.11% of standard, a difference of 33.9%, respectively (Table 1). The radical scavenging activity of our studied mushroom shown an excellent efficiency at lesser concentrations when compared to other mushrooms such as, G. tsugae and Pleurotus ostreatus (Tseng et al., 2008; Jayakumar et al., 2009). The lower IC50 of this mushroom was found more significant in comparison to other reports (Jagadish et al., 2009). However, the antioxidant capability of this mushroom was credited to its known organic acid compounds as one of the best reducing agents at specific pH and temperature. Values: Mean ± Standard Deviation (n = 3), Superscript alphabetic letters in columns indicate significant differences (p < 0.05), PI = percent inhibition, AA = Ascorbic acid.
Group
Conc. (µg/ml)
PI (DPPH)
PI (ABTS)
Geaster saccatum
20
56.4 ± 0.78 a
38.14 ± 0.54
40
62.13 ± 0.52
62.05 ± 0.33
60
71.11 ± 0.43
73.21 ± 0.12 a
80
82.32 ± 0.54
84.11 ± 0.32
100
93.4 ± 0.93
95.16 ± 0.43
IC50 (μg/ml)
61.01
58.03
Standard (AA)
20
70.2 ± 0.52
49.06 ± 0.66
40
78.12 ± 0.11 a
74.32 ± 0.25
60
84.11 ± 0.32
86.15 ± 0.54
80
92.51 ± 0.13
97.41 ± 0.44
100
103.3 ± 0.87b
112.31 ± 13
IC50 (μg/ml)
27.11
33.09
3.1.2 Scavenging activity on ABTS radical
ABTS is considered the best free radical reagent to be used to measure electron/hydrogen donating potential of natural substances and is also an appropriate substrate for enzymes like peroxidases (Choi et al., 2007). The percent inhibition of ABTS radicals of our studied mushroom extract measured significant results at different concentrations. At 20 µg/ml of mushroom extract, the radical scavenging was 38.14 ± 0.54%, whereas, it was 49.06 ± 0.66% by ascorbic acid (standard) at the same concentration, hence a difference of 10.92%,respectively. The rate of percent inhibition of radicals were continuously increased at each increasing concentration of mushroom extract. At 40 µg/ml extract concentration, the radical scavenging was 62.05 ± 0.33 and 74.32 ± 0.25% that of standard, hence a difference of 12.27%, at 100 µg/ml extract concentration, the radical scavenging increased to 95.16 ± 0.43%,respectively (Table 1). The pattern of higher scavenging in dose dependant manner of this mushroom was characterizing of its higher electron donating property. The simultaneous, absorbance decreasing property of this mushroom was found comparable with other antioxidant mushrooms (Lee et al., 2008).
The IC50 of mushroom was 58.03 µg/ml and 33.09 µg/ml to that of standard, again a difference of 24.94 µg/ml, means standard scavenge half of the radicals of ABTS only by 25% more than mushroom. Scavenging of ABTS radicals at such a small IC50 by this mushroom was being highly appreciated by earlier reports (Yang et al.,2002).
3.1.3 Organic acid analysis
The organic acid evaluation of Geaster saccatum mushroom by HPLC-UV chromatogram revealed the presence of eight organic acids (Fig. 1), reported first time in the studied mushroom. However, some of these compounds were also been reported from some other mushrooms as well (Valentao et al., 2005). The quantified data of organic acids in the investigated mushroom ranges from 536 mg/kg to 664 mg/kg. Among all the identified organic acids, gallic acid was found in the highest concentration (193.34%), followed by Ketoglutaric acid (181.16%) and oxalic acid (64.54%). The other organic acid were found in absolutely good proportion namely, ascorbic acid (57.22%), succinic acid (43.74%) and mallic acid (40.66%),respectively. Quinic acid (4.04%) and fumaric acid (8.86%) were found in lower concentrations among all the quantified organic compounds (Table 2). Values are: Mean ± SD (standard deviation) of five concentrations, RT = Retention time, S = Sample, c1,c2,c3 = three different concentrations of each sample, GM = Grand Mean, NI = Not identified, nq = Not quantified.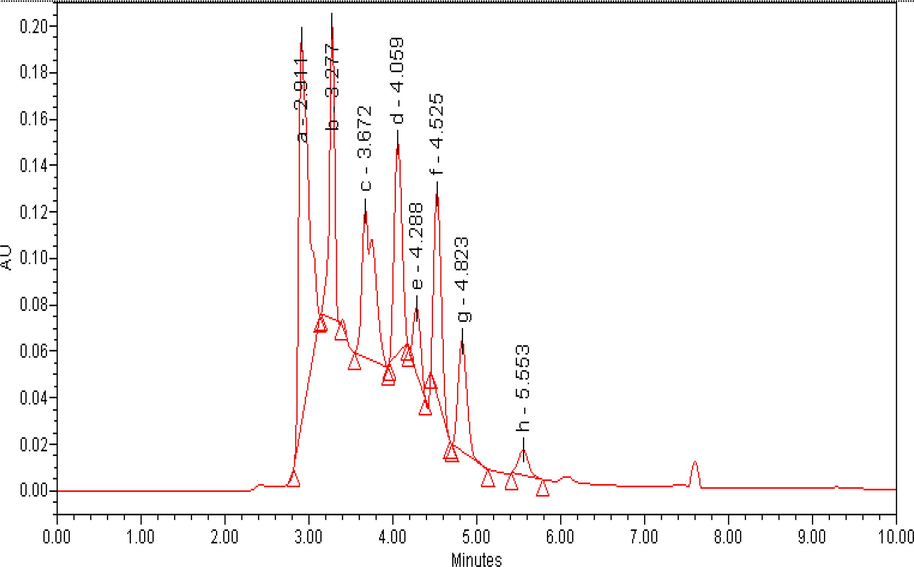
Chromatogram (HPLC-UV) of organic acids of Geaster saccatum, Detection at 214 nm: (a) oxalic acid, (b) ascorbic acid, (c) succinic acid, (d) malic acid, (e) ketoglutaric acid,(f) gallic acid, (g)Not identified, (h) Quinic acid, (i) fumaric acid.
Organic acids
RT
S1 (c1,c2,c3)
S2 (c1,c2,c3)
S3 (c1,c2,c3)
S4 (c1,c2,c3)
S5 (c1,c2,c3)
G.M
Oxallic acid
2.37
33.4 ± 04
64.7 ± 0.2
69.0 ± 1.1
76.2 ± 0.2
79.4 ± 1.2
64.54
Ascorbic acid
2.99
45.4 ± 1.4
49.3 ± 1.2
57.7 ± 0.6
64.5 ± 1.6
69.2 ± 1.3
57.22
Succinic acid
3.12
37.9 ± 1.3
40.4 ± 0.4
43.9 ± 0.3
45.8 ± 1.4
50.7 ± 0.2
43.74
Malic acid
3.46
31.6 ± 1.3
37.4 ± 0.4
40.2 ± 0.3
45.3 ± 1.6
48.8 ± 0.2
40.66
Ketoglutaric acid
3.75
168.2 ± 1.2
174.6 ± 0.2
179.4 ± 1.3
187.2 ± 0.3
196.4 ± 1.5
181.16
Gallic acid
3.90
185.2 ± 1.4
187.3 ± 0.8
193.6 ± 1.5
197.4 ± 0.5
203.2 ± 0.6
193.34
NI
4.67
nq
nq
nq
nq
nq
Quinic acid
6.1
2.5 ± 0.7
3.2 ± 1.3
4.0 ± 0.4
4.8 ± 1.2
5.7 ± 0.6
4.04
Fumaric acid
7.1
7.1 ± 0.5
8.2 ± 0.4
8.9 ± 1.1
9.7 ± 0.7
10.4 ± 1.3
8.86
Summation (Σ)
536.3 ± 8.1
565.1 ± 4.9
596.7 ± 6.6
630.9 ± 7.5
663.8 ± 6.9
The organic acid profile of our studied mushroom was found in higher concentration than most of the earlier reports (Valentao et al., 2005). The HPLC-UV confirms only a few organic acids in L. giganteus mushroom with highest concentration of citric acid as main organic acid (Ribeiro et al., 2007), which was absent among the eight organic acids of our studied mushroom. Barros et al. (2009) used UFLC-PDA of some mushroom species and found five organic acids namely, oxalic acid, quinic acid, malic acid, citric acid and fumaric acid, with mallic acid at higher concentration (6 g/100 g), respectively, but comparatively at lower concentration than our studied mushroom. The presence of oxalic acid in our studied mushroom was also been found in other mushrooms as well including Amanita spissa, F. hepatica and B. nigrescens and most of the plants including tea as main antioxidant compounds (Ribeiro et al., 2008) but was in slightly lower proportion comparatively. Patriacia et al. (2005), reported nine organic acids in S. collinitus and X. chrysenteron mushrooms, supported our results with only the absence of shikimic acid in our mushroom. The organic acid analysis of this investigated mushroom revealed a virgin report in its nature so far, indicated its chemo taxonomic importance, a key to food composition and identification.
4 Electrochemical assay
4.1 Cyclic voltammetry (CV)
The CV of Geaster saccatum was used to assess the constant chemical reactions following electrochemical oxidation reaction. Both standards shown normal irreversible oxidation phenomenon, a diagnostic feature of antioxidant compounds (Cosio et al., 2006). The CV of gallic acid produced a single anodic peak at Epa = 0.29 ± 0.01 V (Fig. 2a), while CV of ascorbic acid produced double anodic peaks, at Epa = 0.41 ± 0.01 V and at Epa = 0.69 ± 0.01 V (Fig. 2b), respectively. The mushroom extract also produced, a diagnostic irreversible anodic peak at 0.69 ± 0.01 V, The E1/2 (half-wave potential) value of mushroom in CV is 0.78 V (Fig. 2c), confirmed that mushroom extract undergone oxidation at relatively higher positive potential in comparison to standards used. The voltammogram showed a linear relation from 2 mg/ml to15mg/ml of mushroom concentration to positive potential in calibration plot (Fig. 2d), with R2 = 0.969, confirmed the phenomenon of diffusion restrain. The positive potential as a peak current shown by the mushroom ensured its concentration dependence, electron transfer kinetics and diffusion coefficient of its electroactive compounds which was agreeable with earlier studies (Arulpriya et al., 2010). This CV character of our studied test mushroom accepted the fact that mushrooms having lower oxidation potential do carry higher oxidative current densities possessing excellent antioxidant property and in turn higher antibiotic capacity, and was found comparable with prior studies (Cosio et al., 2010). The results also indicated that both gallic and ascorbic acids went oxidation reaction slightly at low positive potentials than mushroom extract, forming an electrochemical responses, clearly shown the absence of used standards in test mushroom. The electron flow as a current assessment of the mushroom extract ahead and after pulse potential developed unprecedented character because of adsorption on working electrode and were proportionally same at main potential intervals, this electrochemical feature of the mushroom extract was found similar to other reports (Dandamudi and Nageswara, 2011). The mushroom showed extremely low oxidation potential, produced higher oxidative current density, hence possessed excellent antioxidant activity, a character found in other reports as well (Barros et al., 2008).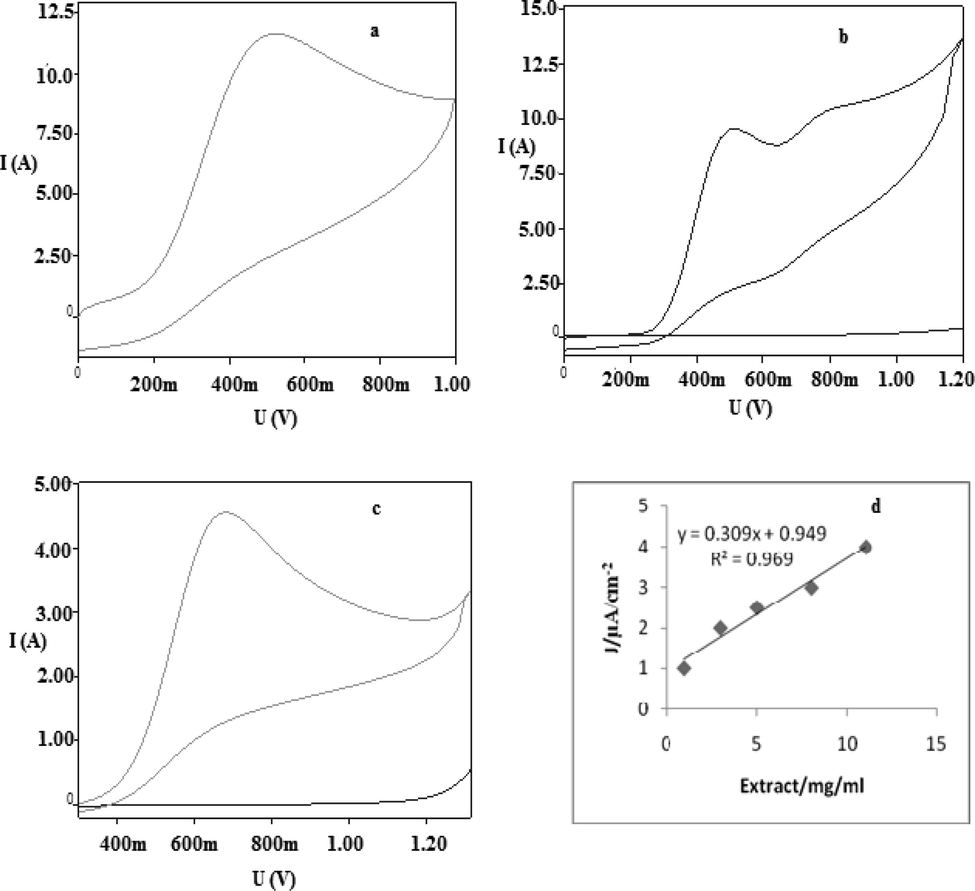
CV, in 0.1 M methanol/acetate buffer at scan rate of 100 mV/s (pH 4) solutions of: (a) 0.5 mM ascorbic acid, (b) 0.5 mM gallic acid, (c) 5 mg/ml of mushroom extract, (d) 2–15 mg/ml of mushroom extract.
4.2 Differential pulse voltammetry (DPV)
The DPV of mushroom was featured by higher resolution, absolute for peak separation, promotes the characterization of electro-oxidation process to measure the differential current (di). The DPV of gallic acid at 0.2 mg/ml produced two anodic peaks at 0.41 V and 0.71 V (Fig. 3a), while ascorbic acid produced one anodic peaks at 0.29 V (Fig. 3b). DPV of mushroom was done at 2–5 mg/ml, produced a well defined peak current at 0.56 ± 0.01 V (Fig. 3c). At 2 mg/ml, peak current was low, while increasing to 5 mg/ml, the peak current also increased, simultaneously. The DPV of gallic acid produced peak currents at different concentrations such as, 0.0125 mg/ml, 0.025 mg/ml, 0.05 mg/ml, 0.1 mg/ml, 0.15 mg/ml and 0.2 mg/ml each and concentration of ascorbic acid were 0.5 mg/ml, 1 mg/ml, 2 mg/ml, 3 mg/ml, 4 mg/ml and 5 mg/ml, respectively (Fig. 4). At each increasing concentration of standards, a linear relation existed between the concentration and its peak current. Similar character was seen in mushroom extract also where the peak current increased along the different concentrations namely, 2 mg/ml, 4 mg/ml, 6 mg/ml, 8 mg/ml, 10 mg/ml and 12 mg/ml (Fig. 5). The slope of peak current density (j) of mushroom was 0.798 (Table 3), while the slope of peak current density of ascorbic and gallic acid were 203.5 and 237.6, respectively. The slope values of mushroom and standards were different because it depends upon concentration, kinetics of electron transfer and diffusion coefficient of electro-active species (Brett and Brett, 1993). The relation between current density (j) and mushroom concentration was different from standards, because neither of standards were present in mushroom extract. However, on slope comparison of two standards (Table 3), which depicted their different diffusion coefficients, but while comparing with mushroom extract, the difference was due to electro-active mass amount, and was found comparable with other reports (Cosio et al., 2006). Therefore, DPV of mushroom extract confirmed the presence of antioxidant compounds which undergone either oxidation or reduction and produced differential current (DI) which increased at each increasing concentration of mushroom. The electrochemical method used in the present study was an accurate and authentic drive to assess the antioxidant activity of the studied mushroom due to its precision and cheap disbursement (Blasco et al., 2005). The main compounds present in this mushroom were organic acids, acted as reducing agents, undergone oxidation in electrolytic set up cell at inert electrodes. In view of this fact, enormous cited authors developed a relationship between electrochemical behavior of the organic acids and their resultant antioxidant activity, where low oxidation potential corresponds to high antioxidant rate (Cosio et al., 2006). Values: E1/2/V – Anodic peak, AP – Antioxidant power, AA – Ascorbic acid, GA – Gallic acid.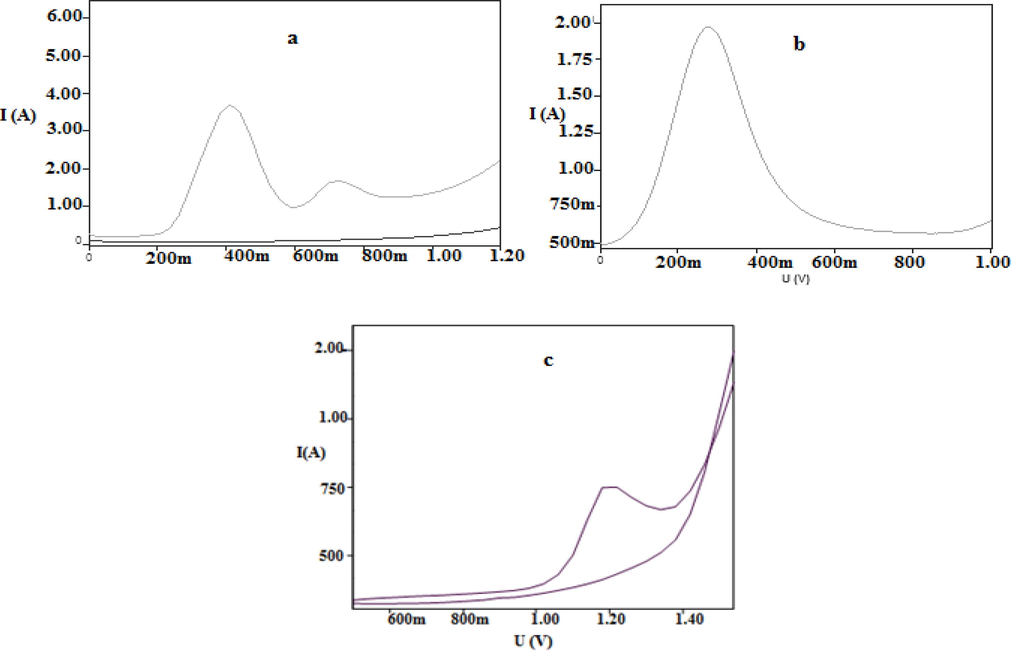
Differential pulse voltagram of 0.2 mg/ml of (a) gallic acid (b) ascorbic acid (c) 2–5 mg/ml of mushroom extract in methanol/acetate buffer 0.1 M (pH = 4) solution at 100mv/s scan rate in methanol/ acetate buffer 0.1 M (pH = 4) solution at 100mv/s scan rate.
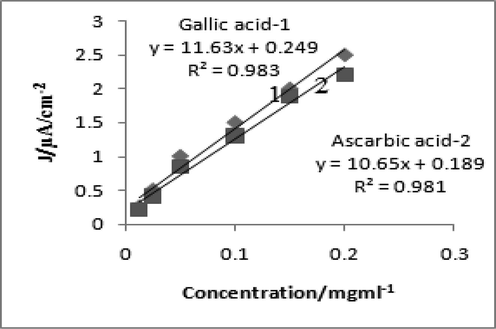
Calibration plot of concentration of gallic acid and ascorbic acid versus their oxidation currents in DPV (1-gallic acid,2-ascarbic acid) at 0.0125 mg/ml, 0.025 mg/ml, 0.05 mg/ml, 0.1 mg/ml, 0.15 mg/ml and 0.2 mg/ml concentration each.
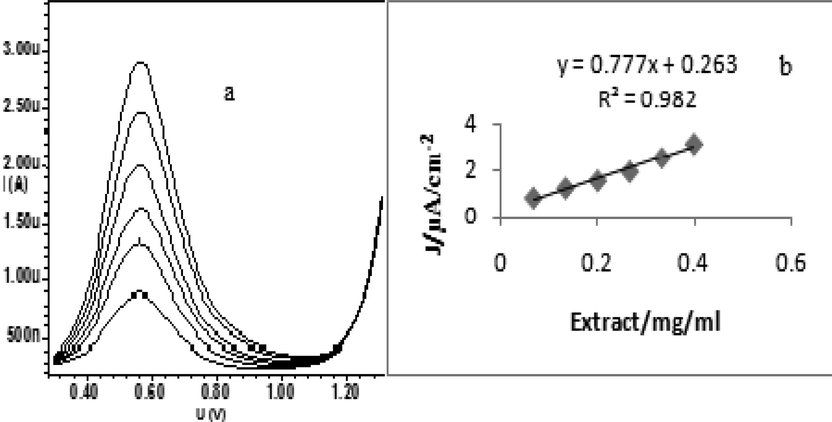
(a) DPV of 2 mg/ml, 4mg/ml, 6mg/ml, 8mg/ml, 10 mg/ml and 12 mg/ml mushroom extract in methanol/acetate buffer 0.1 M (pH = 4). (b) Variation of the peak current in DPV of mushroom extract at different concentrations.
Mushroom/Standard
E1/2/V
Slope/μAmg/ml
A.P. (AA)mg/g
A.P. (GA)mg/g
Geaster saccatum
0.56 ± 0.01
0.798
4.6 ± 0.35
4.3 ± 0.25
Ascorbic acid
0.29 ± 0.01
203.5
1000
602
Gallic acid
0.71 ± 0.01/s0.39 ± 0.01
237.6
1120
1000
4.3 Total anti-oxidant power (TAP)
The TAP of Geaster saccatum mushroom is a measure of its quenching free radical creation in terms of chemical measurement, but in terms of electrochemical measurement, it is the capacity of any substance to undergo redox in an electrochemical reaction and its transfer ability of ions to move from the electrolytic solution to the surface of working electrode. The TAP of this mushroom was found 4.6 ± 0.35 mg g−1 in terms of ascorbic acid and 4.9 ± 0.25 mg g−1 in terms of gallic acid, respectively (Table 3). While, TAP of ascorbic acid was measured 1005 mg g−1 in terms of ascorbic acid, whereas, 710 mg g−1 in terms of gallic acid, respectively. The antioxidant power of gallic acid was measured 1120 mg g−1 in terms of ascorbic acid, whereas, 1005 mg g−1 in terms of gallic acid, respectively. The slope variation of ascorbic and gallic acids were because of difference in their diffusion coefficients. Whereas, On comparison, slopes of mushroom with slopes of standards, the difference was due to electro active mass of mushroom rather than the diffusion coefficients, which in turn resulted in single peak potential characterization of CV. The higher TAP of this mushroom produced more current density (j), and was found highly agreeable with other reports as well (Blasco et al., 2005).
5 Conclusion
The antioxidant character of Geaster saccatum mushroom was an authentic feature to evaluate its importance as a food based medicine against different oxidative stress disorders. The results declared its organic acid composition as main reducing agents which reduced the highly reactive free radicals of DPPH and ABTS reagents, stabilizes the reactive free radicals at human physiology and cure the oxidant related diseases. Besides chemical method, the electrochemical method (CV and DPV) was being approved as an authentic tool to determine the antioxidant activity of mushrooms in electrochemical set up due to its consistent current response, drip potential at anode, short detection limits, electrode surface resurgence and stability, as the CV and DPV of the mushroom were based on proton electron dynamics through diffusion regulated phenomenon. Because of antioxidant character, the Geaster saccatum mushroom is endorsed and consigned as a competent natural antioxidant mushroom at food and pharmaceutics levels for the welfare of human health.
Acknowledgement
The authors are thankful to the Researchers supporting project number (RSP-2020/261) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cyclic voltammetric assessment of the antioxidant activity of petroleum ether extract of Samanea saman (Jacq.)Merr. Adv. Appl. Sci. Res.. 2010;1(3):24-35.
- [Google Scholar]
- Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem.. 2008;111(1):61-66.
- [CrossRef] [Google Scholar]
- Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushroom species. Food Chem. Toxicol.. 2009;47:1076-1079.
- [Google Scholar]
- “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: a proposal. Anal. Chim. Acta. 2005;539(1-2):237-244.
- [CrossRef] [Google Scholar]
- Electrochemistry, principles methods and applications. Oxford: Oxford University Press; 1993. p. :219.
- Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem.. 2007;103(1):130-138.
- [CrossRef] [Google Scholar]
- Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem.. 2006;97(4):725-731.
- [CrossRef] [Google Scholar]
- A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol.. 2013;51:15-25.
- [CrossRef] [Google Scholar]
- proteomic analysis of mushroom polysaccharide-treated Hep G2 cells. Sci. Rep.. 2016;6:23565.
- [Google Scholar]
- Antioxidant properties and electrochemical behavior of cultivated commercial Indian edible mushrooms. J. Food Sci. Technol.. 2011;13:338-346.
- [Google Scholar]
- Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem.. 2003;83(3):371-382.
- [CrossRef] [Google Scholar]
- Free radicals and antioxidants in the year. A historical look to the future. Ann. N. Y. Acad. Sci.. 2000;899:136-147.
- [Google Scholar]
- Comparative study on the antioxidant, anticancer and antimicrobial property of Agaricus bisporus before and after boiling. Afr. J. Biotechnol.. 2009;8(4):654-661.
- [Google Scholar]
- In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov. Food Sci. Emerg. Technol.. 2009;10(2):228-234.
- [CrossRef] [Google Scholar]
- Antioxidant properties of three extracts from Pleurotus citrinopileatus. LWT – Food Sci. Technol.. 2007;40(5):823-833.
- [CrossRef] [Google Scholar]
- Functional properties of edible mushrooms. Nutrition. 2000;16(7-8):694-696.
- [CrossRef] [Google Scholar]
- Screening of antioxidant, antimicrobial activities and chemical contents of edible mushrooms wildly grown in the Black Sea region of Turkey. Comb. Chem. High. T. Scr.. 2011;14:72-84.
- [Google Scholar]
- Quantitation of nine organic acids in wild mushrooms. J. Agric. Food Chem.. 2005;53:3626-3630.
- [Google Scholar]
- Phenolic compounds, organic acids profiles and antioxidative properties of beefsteak fungus (Fistulina hepatica) Food Chem. Toxicol.. 2007;45(10):1805-1813.
- [CrossRef] [Google Scholar]
- Leucopaxillus giganteus mycelium: effect of nitrogen source on organic acids and alkaloids. J. Agric. Food Chem.. 2008;56:4769-4774.
- [Google Scholar]
- Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem.. 2007;104(3):1106-1114.
- [CrossRef] [Google Scholar]
- Influence of jam processing upon the contents of phenolics, organic acids and free amino acids in quince fruit (Cydonia oblonga Miller) Eur. Food Res. Technol.. 2004;218:385-389.
- [Google Scholar]
- Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem.. 2008;107(2):732-738.
- [CrossRef] [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39(1):44-84.
- [CrossRef] [Google Scholar]
- Quantification of nine organic acids in wild mushrooms. J. Agric. Food Chem.. 2005;53:3626-3630.
- [Google Scholar]
- Antioxidant properties of several commercial mushrooms. Food Chem.. 2002;77(2):229-235.
- [CrossRef] [Google Scholar]







