Translate this page into:
Antioxidant and enzyme inhibitory activities of Zizypus jujuba, Adhatoda vasica and Berberis lycium from hilly areas
⁎Corresponding authors. drmubashar@upr.edu.pk (Syed Mubashar Sabir), shahbaz@kfueit.edu.pk (Shahbaz Ali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Berberis lycium, Adhatoda vasica, and Zizypus jujuba are common worldwide medicinal plants. These plants are used in different diseases by local people. However, the scientific rationale for the use of these plants is limited especially in diseases arising from oxidative stress. This study was therefore aimed to evaluate the antioxidant, antidiabetic, and anti-gout activity of these plants from Kashmir flora.
Methods
The antioxidant activities were studied by different in vitro assays which include lipid peroxidation assay, DPPH assay, ABTS assay, iron chelation assay, total phenolic and flavonoid contents. The antidiabetic activity of plants extracts was analyzed by the inhibition of alpha glucosidase, while anti-gout activities were observed by the inhibition of xanthine oxidase enzyme.
Results
All plants extract exhibited good antioxidant activities which are due to significant metal chelating ability and inhibition of lipid peroxidation. The maximum inhibitory effect against lipid peroxidation was observed in water extract of Berberis lycium root extracts. The maximum antidiabetic activity was shown by acetone extract of Berberis lyceum roots justifying its popular use in diabetes. Acetone extract of Zizypus jujuba showed excellent inhibition of xanthine oxidase justifying its popular use in gout. The high antioxidant and enzyme inhibitory activities of these plants might be due to high phenolic and flavonoid contents.
Conclusion
Based on these results, it is concluded that Zizpus jujuba, Adhatoda vasica and Berberis lycium are rich source of antioxidants and may be utilized as antidiabetic and antigout agents.
Keywords
Medicinal plants
Lipid peroxidation
Radical scavenging activity
Alpha glucosidase inhibition
Anti-gout activity
1 Introduction
It is well known that reactive oxygen species (ROS) such as O2 (superoxide anion), H2O2 (hydrogen peroxide), and OH (hydroxyl radical) results in different degenerative diseases such as Alzheimer's disease, cancer, inflammation, aging, rheumatoid arthritis, and atherosclerosis (Singh, 1998). Reactive oxygen species are produced during metabolism or by the effect of ionizing radiations and cause deleterious effects including cancer (Wiseman and Halliwell, 1996, Shinwari and Gilani, 2003). Natural antioxidants have capacity to neutralize reactive oxygen species and thus protect against human diseases (Shinwari, 2010). Medicinal plants are rich source of natural antioxidants (Farooq et al., 2021) and modern medicine is based on plant derived compounds as many diseases have no cure in allopathy (Verpoorte, 2000, Sarwat et al., 2012).
Oxidative stress is the main cause of damage in biomolecules that results in lipid peroxidation, cell injury, abnormal tissue, and gene function (Hussain et al. 2018). Free radicals are continuously produced in cells and causes different diseases which include aging, cardiovascular disorders, cancer, neurodegenerative diseases, and inflammation (Pham-Huy et al., 2008). The food is spoiled rapidly during processing and storage when lipid peroxidation is enhanced (Donnelly and Robinson, 1995). These days the studies of natural antioxidant are popular due to their high therapeutic and nutritive values.
Berberis lyceum Royle belongs to family Berberidiaceae. It is found in hilly areas of Azad Kashmir and Khyber Pakhtunkhwa province of Pakistan. The flowers are seen in the month of April- June on this plant. This plant has several medicinal properties and are listed in British and Indian pharmacopeias (Jan et al., 2011; Matthews, 1994; Srivatava et al., 2006). Roots, bark, and berries of the plant are used in medicines. This plant is popularly used to treat wound and Jaundice (Manan et al., 2007). Ziziphus Jujuba belongs to Rhamnaceae family and the Ziziphus genus. Zizpus jujuba fruit posses’ cytotoxic, anti-termite and insecticidal activities (Ahmad et al., 2006, 2011). Triterpenic acids, saponins and flavonoids were detected the leaves of Ziziphus species (Guo et al., 2011). Zizyphus Jujuba protects against the seizure and reduces the impairment in cognition (Hwang et al., 2011).
Adhatoda vasica belongs to family Acanthaceae. The colors of leaves are dark green and pale yellow at the bottom. Flowers are white in color and arranged in spike. It is used in the treatment of cough, bronchitis, asthma and common cold (Sharav and Dhara, 2015).
As we are aware of the high medicinal and nutritional uses of Zizypus jujuba, Berberis lycium and Adhota vesica the pharmacological properties were studied in detail. Moreover, literature on the practical use of these plant species in the management of oxidative stress related diseases such as diabetes and gout are limited. The present study involves the use of iron and sodium nitroprusside as known prooxidants to induce the lipid peroxidation and the potential inhibitory effect of these plantsa gainst lipid peroxidation was investigated. The antidiabetic and antigout effect of these plant extracts was also determined by in vitro methods.
2 Material and methods
2.1 Preparation of plants extracts
The leaves, flowers and roots bark of plants were collected from various regions of Rawalakot, District Poonch, Azad Kashmir, Pakistan during March-August 2019 and were identified by taxonomist Dr. Ahmad Shafique at Botany Department, University of Poonch Rawlakot, Pakistan. Ziziphus jujuba fruits, Adhatoda vasica flowers and Berberis lycium roots were extracted (Khan et al., 2012). For aqueous extraction, twenty-five grams of leaves, flowers and roots was ground then mixed with 100 mL of hot water for fifteen minutes, permitted to cool and then filtered by using Whatman filter papers. For solvent extraction, ten grams of leaves, flowers and roots extract mixed with 500 mL of acetone and ethanol and kept for three days at room temperature and then filtered by using Whatman filter paper (Hussain et al., 2021). The residue was further extracted, and the extract was concentrated in a rotary evaporator at low temperature and used for experiments after proper dilutions.
2.2 Test animals
All Animal procedures were in strict guidance of the NIH Guide for Care and Use of Laboratory animals. Ethnical committee approval was sought from department of Zoology, University of Poonch, Rawalakot (UPR 101). BALB/c male mice (22–27 g) were housed in separate cages acclimatized and were used for in vitro studies.
2.3 Lipid peroxidation in animal tissues
Lipid peroxidation was carried out by using modified procedure (Ohkawaet al., 1979). To anesthetize the animal’s diethyl ether was used. The liver of mice was homogenized in TRIS-HCl (PH 7.4). The homogenates (100 µl) were incubated with 50 µl of Fe (II) and sodium nitroprusside (SNP), different plant samples and with deionized water. The reaction mixture was incubated at 37 °C for one hour. Then finally 200 µl of 8.1% sodium dodecyl sulphate, 500 µl of acetic acid (pH 3.4) and 500 µl of 0.6% TBA was finally added and incubated at 97 °C. The absorbance was finally read at 532 nn in a spectrophotometer (D-20; Spectronic, West Yorkshire, UK).
2.4 DPPH radical scavenging activity
DPPH activity was measured by following the method of Hatano et al. (1988). 0.25 mM solution of DPPH radical (0.5 mL) was added into ethanol, acetone, and aqueous extract solution (1 mL) in concentration from (37.5–600 µg/mL). After shaking the mixture put it into dark for 30 min and then absorbance was checked in spectrophotometer at 517 nm.
2.5 Determination of ABTS radical cation scavenging activity of extract
ABTS+method will be followed by the method of Re et al. (1999) with modifications.
2.6 Metal chelating activity
The iron chelating capacity of extracts was checked by using the protocol of Puntel et al. (2005). To the reaction mixture which contained 150 μl of freshly prepared 2 mM FeSO4 solution, 168 μl of 0.1 M Tris-HCl solution and 218 μl of 0.9 % NaCl solution and different concentrations of extract were added. All the test tubes were incubated for five minutes and 13 μl of o-phenanthroline was added finally. After that absorbance was measured spectrophotometrically at 510 nm.
2.7 Determination of alpha glucosidase inhibitory activity of extract
Glucosidase inhibitory activity of sample extract was carried out by method of Sancheti et al. (2011) with slight modifications. This reactive mixture has 250 μl of 100 mM (potassium phosphate) buffer pH 7.0, 150 μl of 0.5 mM of 4-nitrophenyl-α D glucopyranoside, 50 μl of extract and 150 μl of α-glucosidase from (Saccharomyces Cerevisiae 0.1unit/mL in 10 mM (potassium phosphate) buffer with pH 7. The it was incubated for 30 min 37 °C. This reaction reaction was stopped by adding 600 μl of 200 mM sodium carbonate. The absorbance was checked at 400 nm.
2.8 Xanthine oxidase inhibitory activity assay
The inhibitory effect on xanthine oxidase was estimated spectrophotometrically by Unno et al. (2004). Reaction mixture comprised of 300 μl of 50 mM (sodium phosphate) buffer pH 7.5, 100 μl of test solution mixed in appropriate solvent, 100 μl of fresh prepared solution of enzyme 0.2 units/ml of xanthine oxidase in phosphate buffer and 100 μl of distilled water. Then this mixture was incubated for 30 min at 37 °C. At that point, 200 μl of substrate mixture (0.15 mM of xanthine) was included into mixture. The whole mixture was incubated for 30 min at 37 °C. Next reaction was halted with addition of 200 μl of 0.5 M HCl. The absorbance was estimated utilizing UV/VIS spectrophotometer at 280 nm.
2.9 Phenolic content estimation
Total phenolic content was estimated by using the method of Singleton et al. (1999) using Folin-Ciocalteu,s reagent.
2.10 Total flavonoid content estimation
Total flavonoid content as quercetin equivalent/g sample were estimated by the method of Kosalec et al. (2004).
2.11 Statistical analysis
The results were reported as means ± SD. The data was subjected to One Way ANOVA and different group means were compared by Duncan Multiple Range test (DMRT) where necessary. The software package STATISCA 7.1 was used.
3 Results
The DPPH activity of plant extracts is shown in Fig. 1. The activity was the highest at concentration of 600 μg/mL, the order of DPPH radical scavenging is Zizypus jujuba > Adhota vesica > Berberis lycium. The results were compared with vitamin C as reference antioxidant.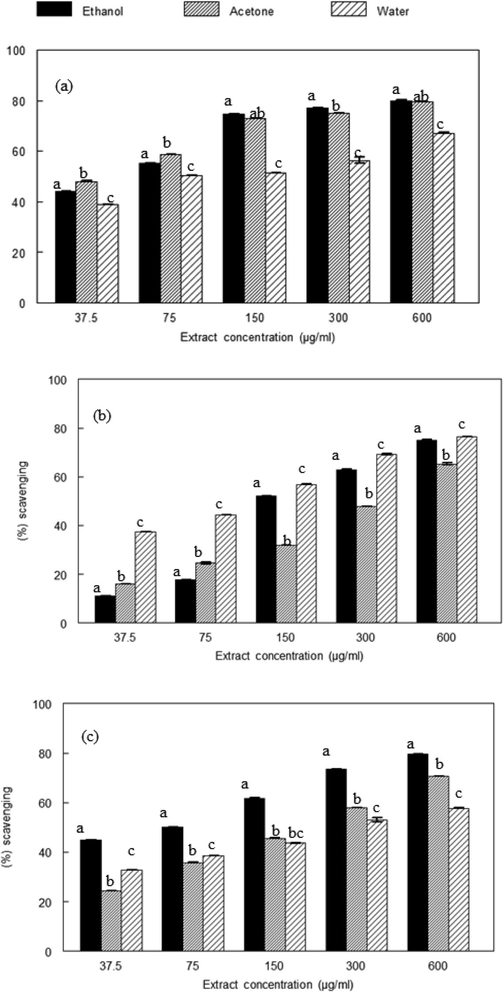
DPPH radical scavenging activity of plant extracts. (a). Ziziphus jujuba fruits extracts (b). Adhatodavasica flower extracts (c). Berberis lycium root extract. Values represent the means of three separate experiments in duplicate ± SD. Values in figures which share different letters are significantly (p < 0.05) different from each other by DMRT.
The ABTS radical scavenging activity of plant extract is shown in Fig. 2. The activity was highest at 300 μg/mL, the order of ABTS radical scavenging activity was Adhota vesica > Berberis lycium > Zizypus jujuba. The iron chelating ability of plants is shown in Fig. 3. The activity was highest at 300 μg/mL, the order of Fe(II) chelating ability was Zizypus jujuba > Adhota vesica > Berberis lycium.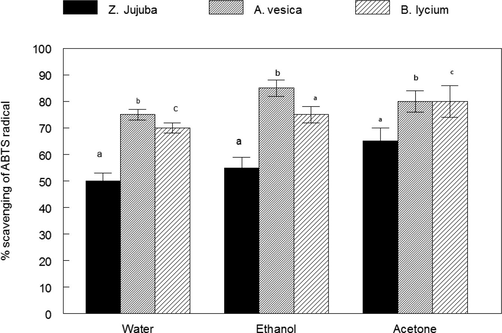
ABTS radical scavenging of the plant extracts of Ziziphus jujuba fruits extracts, Adhatoda vasica flower extracts, and Berberis lycium root extract. Values in the figures which share the different letters are significantly (p < 0.05) different from one another by DMRT.
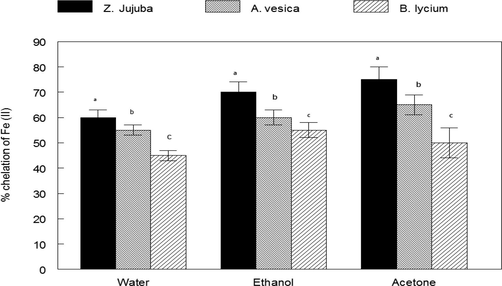
The iron chelating ability of the plant extracts of Ziziphus jujuba fruits extracts, Adhatoda vasica flower extracts and Berberis lycium root extract.
The inhibitory effect of plants extracts against lipid peroxidation is shown in Fig. 4. All the extracts showed higher percentage scavenging of lipid peroxidation. The order of anti-lipid peroxidation activity was Berberis lycium > Zizypus jujuba > Adhota vesica.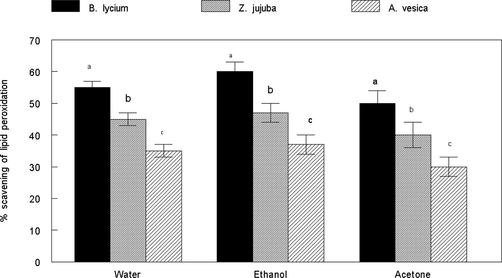
Inhibition of lipid peroxidation induced by iron by plant extracts. There is significant (p < 0.05) difference among different plant species by DMRT.
The antidiabetic activity of plant extracts was analyzed by inhibition of alpha glucosidase enzyme and is shown in Fig. 5. All the plant extracts were capable of more than 50% inhibition of alpha glucosidase enzyme at maximum tested concentration. However, the order of inhibitory effect was Berberis lycium > Zizypus jujuba > Adhota vesica.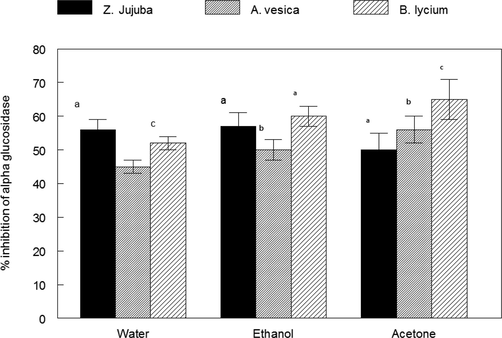
Inhibition of alpha glucosidase activity by plant extracts at 100 µg/ml. (a). Ziziphus jujuba fruits extracts b). Adhatoda vasica flower extracts c). Berberis lycium root extract. There is significant (p < 0.05) difference among different plant species by DMRT.
The anti-gout activity of acetone extracts of plants was tested by inhibition of xanthine oxidase enzyme and is shown in Fig. 6. All the plant extracts showed a significant inhibition (P < 0.05) of xanthine oxidase. However, the order of reactivity was Zizypus jujuba > adhota vesica > Berberis lycium.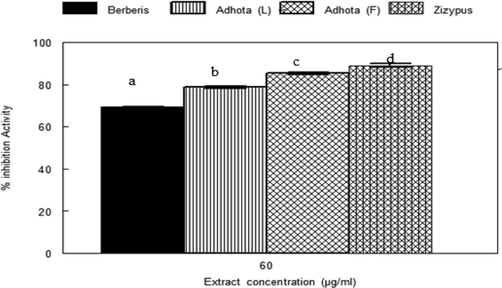
Xanthine oxidase inhibitory effect of plant extracts at 60 µg/ml. Values represent the means of three separate experiments in duplicate ± SD. Values in figures which share different letters are significantly (p < 0.05) different from each other by DMRT.
The total phenolic and flavonoid contents are shown in Tables 1. The ethanolic and acetone extracts of all plant species were effective in extracting the highest content of phenolics and flavonoids. From the results of ANOVA table (Table 2) it is clear that the antioxidant activities and enzyme inhibitory activities are significantly different (p < 0.05) among different plant species. SS = Sum of squares, MS = Mean squares, DF = Degrees of freedom, * indicates significant differences at 95% probability.
Sample
Phenoilc (mg/g)
Flavonoid (mg/g)
Ethanol extract of Zizypus jujuba
780 ± 3.1
1070 ± 7.1
Acetone extract of Zizypus jujuba
1430 ± 7.2
1310 ± 2.1
Water extract of Zizypus jujuba
970 ± 1.1
540 ± 4.1
Ethanol extract of Adhota vesica
940 ± 1.3
1030 ± 9.6
Acetone extract of adhota vesica
1130 ± 3.1
1610 ± 6.1
Water extract of adhota vesica
1410 ± 7.1
91 ± 0.5
Acetone extract of Berberis lycium
720 ± 9.4
930 ± 5.1
Ethanol extract of Berberis lycium
1200 ± 6.3
1440 ± 7.3
Water extract of Berberis lycium
340 ± 5.5
350 ± 2.1
Sample
SS
DF
MS
F
P
DPPH radical scavenging activities of Adhota vesica
5892.93
4
1473.23
14.8
0.0003*
DPPH activities of Berberis lycium
4144.27
4
1036.07
16
0.0002*
ABTS radical scavenging activities of plants
1400
2
700
111
0.0000*
Metal chelation activities of plants
889.56
2
444.78
22.1
0.0017*
Lipid peroxidation activities of plants
986.89
2
493.444
23.1
0.0015*
Alpha glucosidase inhibition of plants
886.19
2
393.444
19.1
0.0013*
Xanthine oxidase inhibition of plants
536.5
2
178.33
142
0.0002*
4 Discussion
The present study is based on the in vitro antioxidant activities and enzyme inhibitory effect of three commonly used plants. Various in vitro assays were carried out to obtain the results. Berberis lycium is widely used in diabetes, whereas Adhatoda vasica is used as antitussive and antihypertensive agents. The fruit of Zizypus jujuba is nutritious and provide additional health benefits. Free radicals stimulate the lipid peroxidation which is involved in the clinical pathogenesis of cancer, diabetes, gout, and cardiovascular diseases (Halliwell et al., 1992). Antioxidants act at different stages and involve different mechanisms such as donating hydrogen atoms, by scavenging of reactive oxygen and by deactivating metal ions. The ABTS•+ and DPPH assays are mostly used to determine the antioxidant capacities of plant extracts. All of the extracts showed higher percentage of scavenging against DPPH and ABTS radicals and thus can be effectively utilized in diseases arising from radical attack. Our results are in agreement to previous studies (Sabir et al., 2013; Koley et al., 2016).
The processing of food often causes the contamination with metal ions (Morgan, 1999). Bivalent metal ions such as iron speed up the oxidation process due to the production of hydroxyl radicals which is involved in Fenton reaction and decomposes hydroperoxide (Wang and Fordham, 2007). These processes can be delayed by iron chelation which deactivates metals. A number of plant extracts were found effective in chelating iron due to the presence of phenolics and flavonoids (Sabir et al., 2021, Fatima et al., 2021). Iron reacts with phenanthroline to form a red color complex. However, in the presence of extract the iron is chelated from the solution which disrupts the complex formation. All the plant extracts showed the higher percentage in chelating the iron.
This study also analyzed the percentage inhibition of lipid peroxidation by aqueous, acetone and ethanol extracts in mice liver. Iron is a prooxidant which stimulates the lipid peroxidation by increasing the production of reactive oxygen species. Iron overload results in different degenerative diseases which includes cancer, liver, heart, brain disorder and neurodegenerative disorders (Miller and Megson, 2007). Lipid peroxidation results in malondialdehyde (MDA) which is the main product of the reaction. The MDA is an index of lipid peroxidation and reacts with thiobarbituric acid at high temperature and low pH (Jadhav et al., 1996). The acetone, ethanolic and aqueous extracts of plant showed excellent inhibition of lipid peroxides which is partly due to their iron chelating abilities. Berberis lycium was found to be the potential candidate of lipid peroxidation inhibition.
Diabetes is one the leading disease effecting 171 million people and most of the patients suffer from type II diabetes (Gershell, 2005). Type 2 diabetes mellitus is a widespread disease and accounts for 9 % of deaths, there is urgent need to find out new potential therapeutic agents. The treatment of diabetes mellitus has improved; however, drug resistance is still needed to be addressed. There is need to maintain the blood glucose level and reduce its production to small intestine. When we eat carbohydrate rich diet it is promptly absorbed by the human intestine as α-glucosidase enzyme acts on it and convert disaccharides into absorbable monosaccharides. The extracts which inhibit α-glucosidase stop the digestion of disaccharides and glucose absorption is made smooth (Casirola and Ferraris, 2006). Natural products have diverse chemical nature and have the ability to inhibit different enzymes. The search of new and safe biologically active photochemical has initiated this study. All the tested extracts showed higher percentage in inhibiting alpha glucosidase enzyme. However, maximum antidiabetic activity was shown by Berberis lycium. Berberis lyceum root extracts has maximum antidiabetic activity due to the presence of berberine which is raw material for pharmaceutical industries (Gulfraz et al., 2007). Berberis lyceum Royle is rich in alkaloids (Khare, 2004) but its major alkaloid is berberine (Khosla et al., 1992) and is found in root and bark. Berberine and palmatin are present in Berberis lyceum which show anticancer effects (Khan et al., 2010) and reduce serum cholesterol in chickens (Chand et al., 2007).
The evaluation of XO inhibitory activity of different parts of Zizypus jujuba, Adhota vesica and Berberis lyceum at 60 µg/ml is higher than 50% of the crude extracts. This justified the fact that medicinal plants from Pakistan have well diverse chemical structures from their secondary metabolite and chemical diversity (Ahmad et al., 2005) which makes them promising remedies for gouty ailments in humans. Maximum inhibition of xanthine oxidase was demonstrated by Zizypus jujuba (81.2%). This is the first report on the xanthine oxidase inhibitory effect of Zizypus jujuba, Berberis lycium and Adhatoda vasica.
Phytochemical analysis revealed that Berberis lycium, Adhatoda vasica and Zizypus jujube relatively contained high amount of phenolic and flavonoid contents. Presence of these photochemical contributes antioxidant activities and is responsible for observed activities against alpha glucosidase and xanthine oxidase.
5 Conclusion
In conclusion the crude extracts of studied plants showed broad range of biological activities which include DPPH, ABTS radical scavenging activities, metal chelation activities and anti-lipid peroxidative properties. These plants have significant enzyme inhibitory properties against alpha glucosidase and xanthine oxidase enzymes which justifies their popular use in diabetes and gouty arthritis. This study highlights the use of these plants extracts in different food and pharmaceutical industries. However further research on vivo studies are required to demonstrate the antioxidant and enzyme inhibitory properties of plants.
Acknowledgements
We are highly thankful to Higher Education Commission, Islamabad (NRPU, 2438) for providing research project to carry out this work. The authors extend their appreciation to the Researchers Supporting Project number (RSP2022R483), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ecotype diversity in autumn olive (Elaeagnus umbellata) a plant with multiple micronutrient genes. Chem. Ecol.. 2006;6:509-521.
- [Google Scholar]
- The antifungal, cytotoxic, antitermite and insecticidal activities of Zizyphus jujuba. Pak. J. Pharm. Sci.. 2011;24:489-493.
- [Google Scholar]
- Role of Berberis lycium in reducing serum cholesterol in Broilers. Asian Aust. J. Anim. Sci.. 2007;20(4):563-568.
- [Google Scholar]
- Alpha glucosidase inhibitors prevent diet- induced increases in intestinal sugar transport in diabetic mice. Metabolism. 2006;55:832-884.
- [Google Scholar]
- Induction of secondary metabolites on nanoparticles stress in callus culture of Artemisia annua L. Braz. J. Biol.. 2021;81:474-483.
- [Google Scholar]
- Characteristics and methods to release seed dormancy of two ground cherry (Physalis) species. J. Appl. Res. Med. Aromatic Plants. 2021;25:100337.
- [Google Scholar]
- Foliar application of seed water extract of Nigella sativa improved maize growth in cadmium-contaminated soil. Plos one. 2021;16(7):e0254602.
- [Google Scholar]
- Drought stress in sunflower: physiological effects and its management through breeding and agronomic alternatives. Agri. Water Manage.. 2018;201:152-166.
- [Google Scholar]
- Allelopathic plant water extracts tank mixed with reduced doses of atrazine efficiently control Trianthema portulacastrum L. Zea mays. J. Animal Plant Sci.. 2012;22(2):339-346.
- [Google Scholar]
- The New Plantsman. UK: Royal Horticultural Society, London; 1994. p. :68.
- Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci.. 2008;4:89-96.
- [Google Scholar]
- Antihyperglycemic effects of Berberis lycium royle in Alloxan induced diabetic rats. DiabetologiaCroatica. 2007;36:49-54.
- [Google Scholar]
- Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC-PDA-MS/ELSD. J. Pharm. Biomed. Anal.. 2011;56:264-270.
- [Google Scholar]
- Zizyphus enhances cell proliferation and neuroblast differentiation in the subgranular zone of the dentate gyrus in middle-aged mice. J. Med. Food. 2011
- [CrossRef] [Google Scholar]
- Free radicals, antioxidants and human disease: Where are we now? J. Lab. Clin. Med.. 1992;119:598-620.
- [Google Scholar]
- Two new flavonoids and other constituents in licorice root;their relative astringency and radical scavenging effects. Chem. Pharm. Bull.. 1988;36:2090-2097.
- [Google Scholar]
- Influence Of herbal dye extracted from dry wood of indigenous Berberis petiolaris wall. In plant histological staining. Pak. J. Bot.. 2011;43(5):2597-2600.
- [Google Scholar]
- Lipid oxidation in biological and food systems. In: Madhavi D.L., Deshpande S.S., Salunkhe D.K., eds. Food Antioxidants: Technological, Toxicological, and Health Perspectives. New York: Marcel Dekker Inc.; 1996. p. :5-63.
- [Google Scholar]
- Indian Herbal Remedies. New York: Springer; 2004. p. :98-100.
- Antioxidant activity and phenolic content in genotypes of Indian Jujube (ZizypusmauritianaLamk.) Arabian J. Chem.. 2016;9:S1044-S1052.
- [Google Scholar]
- Diversity of Medicinal plants in Wari subdivision District upper Dir. Pakistan. Pak. J. Pl. Sci.. 2007;13(1):21-28.
- [Google Scholar]
- Morgan, J.N. 1999. Effects of processing on heavy metal content of foods. In L. S. Jackson, M. G. Knize, J. N. Morgan (Eds.), Impact of Processing on Food Safety. Boston, MA: Springer US. pp. 195–211
- Recent developments in nitric oxide donor drugs. Br. J. Pharmacol.. 2007;151:305-321.
- [Google Scholar]
- Assay for lipid peroxides in animal-tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem. Res.. 2005;30:25-235.
- [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol.. 1999;26(9–10):1231-1237.
- [Google Scholar]
- Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu’s reagent. Methods Enzymol.. 1999;299:152-178.
- [Google Scholar]
- Estimation of heavy metals in different Berberis spp., and its mark samples. Environ. Mont. Assess.. 2006;116:315-320.
- [Google Scholar]
- Screening of Korean medicinal plant extracts for α-glucosidase inhibitory activities. Iranian J. Pharm. Res.. 2011;10:261-264.
- [Google Scholar]
- Phytochemical screening and antibacterial activity of Adhota vesica. Inventi Rapid Planta Activa. 2015;1:1-3.
- [Google Scholar]
- Singh, A. 1998. Physicochemical and physiological aspects. In: CRC Handbook of Free Radicals and Squadrito, G. L.; Pryor, W.A. Oxidative Chemistry of Nitric Oxide. Free. Radic. Biol. Med. 25, 392-403. 123-126.
- Screening of potential medicinal plants from district Swat specific for controlling women diseases. Pak. J. Bot.. 2012;44(4):1193-1198.
- [Google Scholar]
- Sustainable harvest of medicinal plants at Bulashbar Nullah, Astore (Northern Pakistan) J. Ethnophormacol.. 2003;84:289-298.
- [Google Scholar]
- Phytochemical and antioxidant studies of Berberis lycium. Pak. J. Pharm. Sci.. 2013;26:1165-1172.
- [Google Scholar]
- Phytochemical analysis and biological activities of ethanolic extract of Curcuma longa rhizome. Braz. J. Biol.. 2021;81:737-740.
- [Google Scholar]
- Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm.. 2004;54:65-72.
- [Google Scholar]
- Berberine, a potential drug for trachoma Rev. Int. Trach. Pathol. Occur. Trop. Subtrop. Sante. Publique.. 1992;69:147-165.
- [Google Scholar]
- Khan, M., B. Giessrigi, C. Vonach, S. Madlener, S. Prinz, I. Herbaceck, C. Holzi, S. Bauer, K. Viola, W. Mikulits, R.A. Qureshi, S. Knasmuller, M. Grusch, B. Kopp and G. Krupitizi. 2010. Berberine and a Berberis lycium extract inactivated Cdc25A and induce alpha-tubulin acetylation that corelate with micro liter-60 cell cycle inhibition and appoptosis. Mutat. Res. 5, 683(1-2): 123-30.
- Xanthine oxidase inhibitors from the leaves of Lagerstroemia speciosa (L.) Pers. J. Ethnopharmacol.. 2004;93:391-395.
- [Google Scholar]
- Difference in chemical composition and antioxidant capacity among different genotypes of666 autumn olive (elaeagnus umbellate thunb.) Food Tech. Biotech.. 2007;45(4):402-409.
- [Google Scholar]
- Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. J. Biochem.. 1996;313:17-29.
- [Google Scholar]
- Pharmacognosy in the New Millenium: lead finding and Biotechnology. J. Phar. Pharmacol.. 2000;52:253-262.
- [Google Scholar]







