Translate this page into:
Antioxidant and antineoplastic activities of methanolic extract of Kaempferia galanga Linn. Rhizome against Ehrlich ascites carcinoma cells
⁎Corresponding author. Tel.: +88-01556312361. yeasmin_bio@yahoo.com (Tanzima Yeasmin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
As sources of natural products, medicinal plants bear a great interest for researcher in recent decades and this interest has increased considerably in finding naturally occurring antioxidant and antineoplastic compounds. Kaempferia galangal Linn., is an important member of medicinal flora available in Bangladesh and used traditionally for the prevention of numerous diseases. The present study was designed to investigate the antioxidant and antineoplastic activities of methanol extract of Kaempferia galanga rhizome (MEKGR). In vitro models and MTT assays were used to determine the antioxidant and in vitro antineoplastic properties of MEKGR. Antineoplastic effect of MEKGR against Ehrlich ascites carcinoma (EAC) were assessed in vivo by evaluating the viable tumor cell count, survival time, body weight gain due to tumor burden, observing morphological changes and nuclear damage of EAC cells by fluorescence microscope and estimating hematological profiles of experimental mice. Chemical composition was also analyzed by GC–MS. Treatment with MEKGR significantly (p < 0.05) reduced viable EAC cells and weight gain and increased life span. MEKGR restored all hematological parameters, such as RBC, WBC, hemoglobin (Hb%) of EAC-bearing mice towards normal level. Membrane blebbing, chromatin condensation, nuclear fragmentations were observed after treatment with MEKGR. MEKGR exhibited strong antioxidant activity. TPC (Total phenolic content) and TFC (Total flavonoid content) were found strongly correlated (P < 0.05) with antioxidant activities of MEKGR. 2-Propenoic acid, phthalic acid, palmitic acid, sandaracopimaradiene, oleic acid, octadecanoic acid, 2-[2-(4-nonylphenoxy) ethoxy] ethanol and glycidyl stearate were identified as the major constituents of MEKGR by GC–MS analysis. The overall findings of this study suggest that MEKGR may provide a natural source of antioxidant and antineoplastic activities.
Keywords
Kaempferia galanga L. rhizome
Antioxidant activity
Anticancer activity
Apoptosis
GC–MS analysis
- MEKGR
-
methanol extract of Kaempferia galanga rhizome
- GC–MS
-
gas chromatography–mass spectrometry
- EAC
-
Ehrlich ascites carcinoma cells
- IC50
-
inhibitory concentration at which 50% inhibition is observed
- MTT
-
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide
- CA
-
catechin
Abbreviations
1 Introduction
The body’s normal uses of oxygen such as respiration and some cell-mediated immune functions have continuously produced reactive oxygen species (ROS). Excessive production of ROS was found to play important roles in tissue damage and loss of function in a number of tissues and organs. ROS may also be very harmful as they leads to damage of proteins, DNA and lipid thereby commencing various chronic diseases like cancer, atherosclerosis, diabetes, cardiovascular disease, ageing and inflammatory diseases (Islam et al., 2013). There is a close relation between excessive production of ROS and induction of cancer (Islam et al., 2014a,b). So butylated hydroxyl toluene and butylated hydroxyl anisole are largely included in human diets as synthetic antioxidants but they are suspected to possess harmful effects on health (Li et al., 2014). In this aspect, plants and their products are considered as rich sources of phytochemicals with less side effects and these phytochemicals have been found to possess a variety of biological activities including antioxidant and antineoplastic activities (Devasagayam et al., 2004; Chen et al., 2011; Ramful et al., 2011; Harsha et al., 2013; Habib Et al., 2010; Shynu et al., 2011; Rafik et al., 2014) Kaempferia galangal Linn, also known as chandramulika, belongs to the Zingiberaceae family and has a wide spread occurrence in Bangladesh, India, China and Malaysia (Ridtitid et al., 2008). Many ayurvedic drugs are prepared from this herbal spice and are traditionally used to cure various ailments such as asthma, malaria, skin disease, bronchitis, wounds and spleenic disorders (Kirtikar and Basu, 1997). Previously it was reported that this spice possess expectorant, carminative, diuretic, anti-inflammatory and antimicrobial activities (Hanumantharaju et al., 2010; Shirin et al., 2000; Patnibul et al., 2008). Therefore, in the present study, the effects of methanol extract of Kaempferia galanga rhizome against Ehrlich ascites carcinoma (EAC) were analyzed. The study also focused on the chemical composition as well as antioxidant potency of methanol extract of Kaempferia galanga rhizome.
2 Materials and methods
2.1 Chemicals and reagents
Hoechst 33342, RPMI 1640-medium and MTT were obtained from Sigma–Aldrich, USA. Penicillin-streptomycin and fetal calf serum were purchased from Invitrogen (USA). Methanol was obtained from Labscan, Thailand. Trypan blue and all other chemicals/reagents were of analytical grade obtained commercially.
2.2 Collection of plant materials and authentication
Rhizome of Kaempferia galanga L. was collected from the hilly areas of Chittagong, Bangladesh. Authentication of the plant material was identified by a taxonomist at the Department of Botany, University of Rajshahi. The voucher sample (No. 06) of this collection was deposited for further reference.
2.3 Preparation of extract
The dried and powdered rhizome of Kaempferia galanga L. was used for the extract preparation with methanol (200 g powder in 500 ml methanol) at room temperature and after filtration, filtrates were evaporated under reduced pressure at 40 °C using a rotary evaporator to have methanol extract (about 2% yield) and it was designated as MEKGR (Methanol extract of Kaempferia galanga rhizome).
2.4 Estimation of total phenolic and flavonoid content
Previously described methods were used to estimate the total phenolic (TPC) and flavonoid (TFC) content of MEKGR (Singleton and Rossi, 1965; Dewanto et al., 2002). TPC of MEKGR was calculated as gallic acid (used as reference standard) equivalents (y = 0.117x + 0.051, R2 = 0.998) per gram of dry weight whereas TFC was calculated from the standard curve of catechin (y = 0.005x + 0.047, R2 = 0.998) as catechin equivalents per gram of dry weight.
2.5 Determination of total antioxidant and ferrous reducing capacity
The total antioxidant and ferrous reducing potential of the extract/catechin as standard was assessed by the method of Prieto et al. (1999) and Oyaizu (1986).
2.6 DPPH, ABTS and nitric oxide radical scavenging assay
The DPPH free radical scavenging capacity of MEKGR was measured by the method Choi et al. (2000) with some modification whereas the ABTS method was used according to Cai et al. (2004). Nitric oxide scavenging activity of MEKGR extract was carried out as reported by Marcocci et al. (1994) with some modification. The absorbance was taken at 517 nm for DPPH assay, 734 nm for ABTS assay and 546 nm for nitric oxide assay. Catechin was used as reference in all cases. Percentage of DPPH/ABTS/nitric oxide radical scavenging was calculated using the following formula:
where Asample = Absorbance of sample and Acont = Absorbance of control.
IC50 values (µg/ml) were determined from the plotted graphs of scavenging activity against the concentration of the extract.
2.7 Animals and ethical clearance
Swiss-albino mice of both sexes, average weighing 28–32 g were collected from the Animal Research Branch of the International Centre for Diarrhoeal Diseases Research, Bangladesh (ICDDR,B). The mice were grouped and housed in polypropylene cages (maximum six mice per cage). Sterile paddy husk was used as bedding material and the mice were fed with standard mouse food-pellets (collected from ICDDR’B) and water ad libitum. The room was maintained at a temperature of 25 ± 2 °C, humidity 55 ± 5% with 12:12 h light–dark cycle. Protocol used in this study for the use of mice as a animal model for cancer research was approved by the Institutional Animal, Medical Ethics, Biosafety and Biosecurity Committee (IAMEBBC) for Experimentations on Animal, Human, Microbes and Living Natural Sources, (225/320-IAMEBBC/IBSc), Institute of Biological Sciences, University of Rajshahi, Bangladesh.
2.8 In vitro cell viability test by MTT colorimetric assay
MTT assay was performed to assess the viability of EAC cells as reported by Kabir et al. (2016). In shortly, the dose-dependent effects of extract on the viability of EAC cells were investigated by MTT assay. The cells (2 × 106) were plated in 200 µl RPMI 1640 media in the presence of various concentrations (3.125–100 µg/ml) of extracts and allowed to stand for 24 h at 37 °C in CO2 incubator. Following incubation, aliquot were removed and 180 µl of PBS and 20 µl of MTT were added to each well and again incubated at 37 °C for 8 h. After clearing away the supernatant, 200 µl of acidic isopropanol was put into each well of the culture plate and finally incubation was carried out at 37 °C for 1 h. Titer plate reader was used to measure the absorbance at 570 nm.
2.9 Determination of cell growth inhibition (in vivo)
To explore the in vivo cell growth inhibition properties of the extract, three groups of Swiss albino mice (n = 6) weighing 26 ± 3 gm were used (Islam et al., 2016). For this purpose, 1.6 × 106 EAC mice cells were inoculated into each group of mice on the first day. After 24 h of tumor injection treatments were commenced and continued for five days. In brief, groups 1 and 2 were received MEKGR at the doses of 5 and 10 mg/kg/per day, respectively, via intraperitoneal injection. Group 3 was used as untreated control mice. On day 6, mice were sacrificed and viable tumor cells of MEKGR-treated groups were compared with those of control group.
2.10 Survival time and tumor weight
Studies on survival time and tumor weight of extract were carried out using the method of Khanam et al. (2010). In summary, Swiss albino mice were divided into three groups (six in each group) and each group of mice received 1.6 × 106 EAC cells. Group 1 and group 2 mice were administered with MEKGR at 5 and 10 mg/kg/mouse/day, respectively for 10 days whereas group 3 was used as untreated control. Then tumor weight gain and mean survival time of each group were recorded.
2.11 Observation of EAC cells morphological change
The nuclear morphological changes of EAC cells in MEKGR-treated (10 mg/kg/mouse/day) and untreated control mice were investigated using a fluorescence microscope (Olympus iX71, Korea). Briefly, the EAC cells from both groups of mice were collected and stained with Hoechst 33342 at 37 °C for 10 min in the dark. The cells were then washed with PBS. Finally, the morphological changes were studied using a fluorescence microscope.
2.12 Studies on hematological parameters
Studies on hematological profiles of extract were carried out using the method of Islam et al. (2014a). Experimental mice were divided into four groups (n = 6) and each mice were injected with EAC cells (1.6 × 106 cells/mouse) intraperitoneally except the normal group (Group 1). Group 2 was used as control. Group 3 and group 4 mice were administered with MEKGR at 5 and 10 mg/kg/mouse/day, respectively for 10 days. On the 12th day, after tumor transplantation, tail vein blood was collected which was used to measure hematological profile.
2.13 Brine shrimp lethality bioassay
Brine shrimp lethality bioassay was carried out according to published method for the determination of cytotoxic property of the sample extracts (Kabir et al., 2011). Briefly, 3 mg of the extract was prepared in 0.6 ml of distilled water to get a concentration of 5 μg/μl and solution of different concentrations such as 10, 20, 30, 40 and 50 μg/ml were obtained. After 24 h. of incubation the percent of lethality of the brine shrimp nauplii was calculated for each concentration.
2.14 GC–MS analysis of MEKGR
Separation and identification of the components of ethanol extract were performed by GC–MS agilent 6890 N gas chromatography hooked to agilent 5973 N mass selective detector. They equipped with a flame ionization detector and capillary column with HP-5MS (30 m × 0.25 mm × 0.25 μm). In GC settings: the initial oven temperature was set at 60 °C for 1 min and ramped at 10 °C min−1to 180 °C for 1 min and then ramped at 20 °C min−1 to 280 °C for 15 min. The temperature of the injector was controlled at 270 °C. The samples (1 μl) were injected neat, with a split ratio of 1:10. Helium was used as the carrier gas at a flow rate of 1.0 ml min−1. Spectra were scanned from 20 to 550 m/z at 2 scans s−1. Identification of most constituents by gas chromatography was done by comparing their retention indices with those reported in the literature or with those of authentic components available in database.
2.15 Statistical analysis
All results were represented as mean ± standard deviation (SD). SPSS software (version 16) was used to perform one way analysis of variance (ANOVA) followed by Dunnett’s ‘t’ test and IC50 was calculated using GraphPad Prism 6. P < 0.05 were considered to be statistically significant.
3 Results
3.1 Total phenolic and flavonoid contents
The TPC and TFC of MEKGR which were 15.40 ± 0.35 mg of gallic acid equivalent/g of extract and 37.72 ± 0.50 mg of catechin equivalent/g of extract, respectively (Table 1). Data are expressed as mean ± SD.
Sample
TPC (mg of gallic acid equivalent/g of extract)
TFC (mg of catechin equivalent/g of extract
DPPH radical scavenging activity (IC50 values in µg/ml)
ABTS radical scavenging activity (IC50 values in µg/ml)
Nitric oxide scavenging activity (IC50 values in µg/ml)
MEKGR
15.40 ± 0.35
37.72 ± 0.50
16.58
8.24
38.16
Catechin
–
–
2.67
4.53
3.18
Total antioxidant and reducing power capacity of MEKGR and catechin
MEKGR
Total antioxidant capacity
Ferrous reducing capacity
Concentration (µg/ml)
Absorbance
Concentration (µg/ml)
Absorbance
3.125
0.086
3.125
0.113
6.25
0.211
6.25
0.265
12.5
0.341
12.5
0.343
25
0.452
25
0.479
50
0.685
50
0.565
100
0.771
100
0.865
Catechin
Total antioxidant capacity
Ferrous reducing capacity
3.125
0.123
3.125
0.217
6.25
0.246
6.25
0.434
12.5
0.418
12.5
0.733
25
0.673
25
1.176
50
1.327
50
2.040
100
1.855
100
2.509
3.2 Total antioxidant and ferrous reducing capacity
As shown in Table 1, The MEKGR was found to increase the total antioxidant and ferrous reducing capacities with the increasing concentration of the extract.
3.3 DPPH, ABTS and nitric oxide radical scavenging activity
Table 1 shows DPPH, ABTS and nitric oxide radical scavenging activities of MEKGR and catechin (CA). In each radical scavenging assay, the activity of MEKGR was found to be increased with increasing its concentration. In case of DPPH, ABTS and nitric oxide scavenging assay, the IC50 values of MEKGR were 16.58, 8.24 and 38.16 µg/ml, respectively whereas for catechin these were 2.67, 4.53 and 3.18 µg/ml, respectively.
3.4 Inhibition of cancer cell growth with MEKGR (in vitro)
In MTT assay, MEKGR induced EAC cell death in a dose dependent manner (Fig. 1A). A reduced cell growth was observed with MEKGR at a concentration as low as 3.125 µg/ml which markedly increased with increasing concentration of MEKGR as compared to control. A strong inhibition (76.56%) of EAC cell growth was observed at concentration 50 μg/ml which is further increased (89.37%) at concentration 100 μg/ml of MEKGR. The IC50 value of the MEKGR was determined as 17.10 µg/ml against EAC cell (Fig. 1B).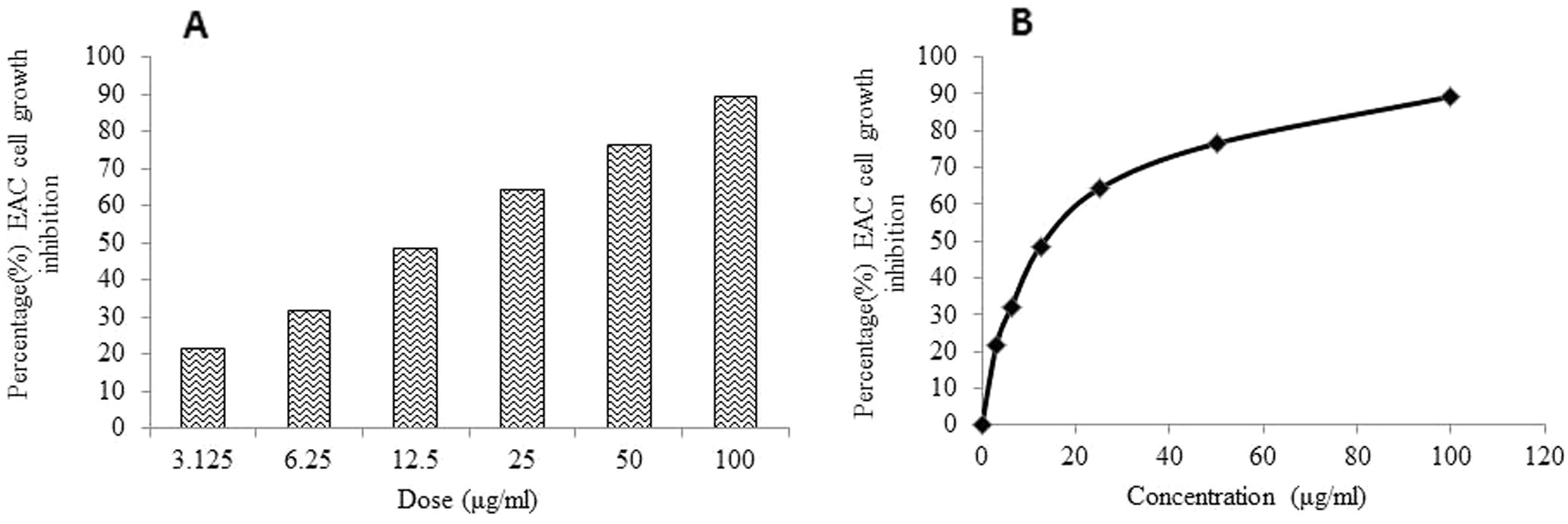
(A) Growth inhibition of EAC cells by MEKGR when EAC cells were treated with various doses of MEKGR for 5 days. The inhibition ratios were measured by the MTT assay (n = 3, Mean ± SD). (B) IC50 value of MEKGR was calculated from the dose–response curve.
3.5 Cell growth inhibition (in vivo)
Table 2 showed the cell growth inhibition of MEKGR. Maximum cell growth inhibition was observed with the treatment of MEKGR at the dose of 10 mg/kg (i.p.) (70.58% inhibition). Data are expressed as mean ± SD for six animals in each group. P < 0.05: against EAC control group.
Group
Treatment
Viable EAC cells on day 6 after inoculation (×107 cells/ml)
Percentage (%) cell growth inhibition
1
EAC + Control
3.81 ± 0.62
–
2
EAC + MEKGR (5 mg/kg)
1.80 ± 0.21*
52.44 ± 4.52
3
EAC + MEKGR (10 mg/kg)
1.11 ± 0.08*
70.58 ± 2.71
3.6 Average tumor weight and survival time
The effect of MEKGR on survival time at different doses had been summarized in Fig. 2A. Administration of methanol extract at doses 5 and 10 mg/kg in EAC induced mice resulted in increase of life span markedly by 12.95% and 38.39%, respectively, compared with the control (p < 0.05) (Fig. 2B). MEKGR also reduced the tumor weight, compared with control group (p < 0.05) (Fig. 2C).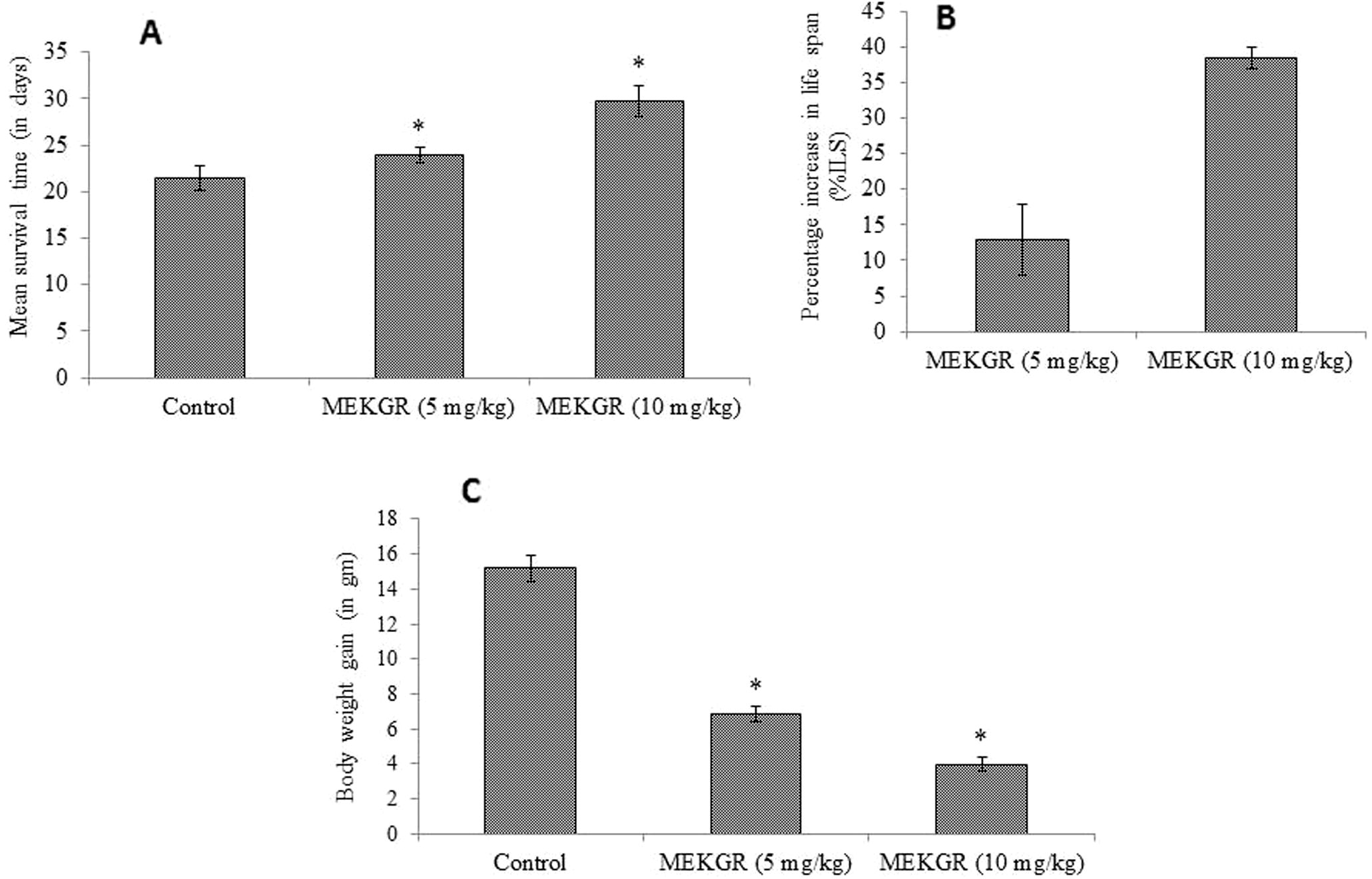
(A) Mean survival time of experimental mice. (B) Percentage of life span increase with the treatment and (C) reduction of body weight gain due to tumor burden at different doses. Data are expressed as mean ± standard deviation (n = 6). Level of significance *p < 0.05 when compared with that of control group.
3.7 Effect of MEKGR on cell morphological change
Hoechst 33342 staining was performed to confirm the methanol extract induced apoptosis of EAC cells and the results were presented in Fig. 3. In this morphological examination, the control cells were round, regular, and homogeneously stained with Hoechst 33342 (Fig. 3A) while treated EAC cells exhibited membrane and nuclear fragmentation a hall mark of apoptosis (Fig. 3B). These results indicate that the extract could induce apoptosis of EAC cells.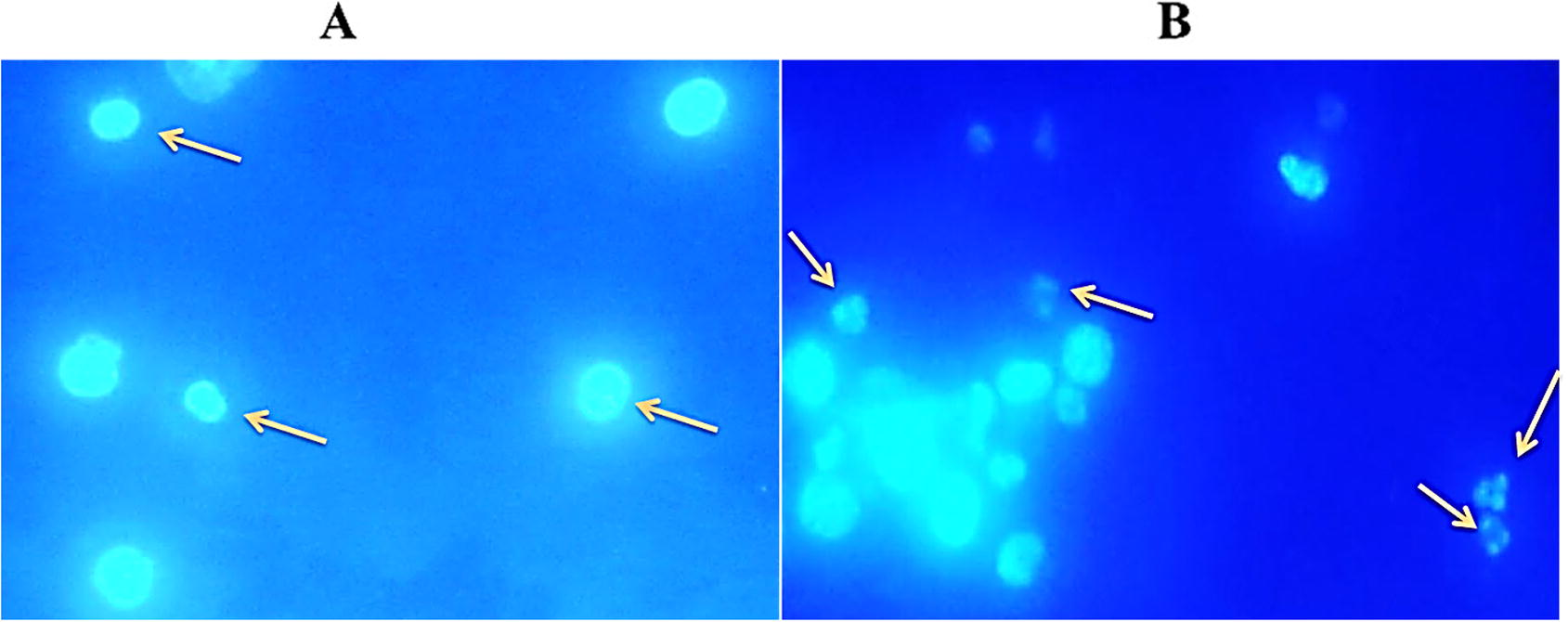
MEKGR induced apoptosis is in Ehrlich ascites carcinoma (EAC) cell. EAC cells were treated for 24 h then cells were collected from the treated and non-treated EAC-bearing mice and stained with Hoechst 33342 and observed by fluorescence microscopy. Left panel (A) indicates control and right panel (B) indicates MEKGR (10 mg/kg/day) noted that apoptotic characteristics e.g. nuclear condensation and fragmentation are seen in figure B.
3.8 Hematological studies
Effects of the methanol extract on the hematological profile of the EAC cell bearing mice were examined and the results were presented in table 3. Administration of EAC cells resulted in the reduction of Hb content and RBC counts, whereas an increase was found in WBC counts. Interestingly, the EAC-bearing mice restored their hematological profiles moderately after administration of the extract. Data are expressed as mean ± SD for six animals in each group.
Parameters
Normal
EAC + Control
EAC + MEKGR (5 mg/kg)
EAC + MEKGR (10 mg/kg)
Hgb (g/dL)
14.48 ± 0.52
10.5 ± 1.08
12.65 ± 0.65*
14.35 ± 0.25*
RBC(×109 cells/mL)
6.67 ± 0.23
1.59 ± 0.12
2.82 ± 0.31*
4.10 ± 0.41*
WBC(×106 cells/mL)
8.75 ± 0.53
39.5 ± 4.4
28.5 ± 3.10*
20.00 ± 1.82*
3.9 Brine shrimp lethality bioassay
The brine shrimp lethality bioassay was carried out to assess the in vitro cytotoxic effect of MEKGR. Percent of mortality of nauplii were increased with the increasing concentration of MEKGR and the medium lethal concentration (LC50) was found to be 11.37 µg/ml.
3.10 Chemical profile of MEKGR analyzed by GC-MS
The chemical profile of MEKGR extract that was identified by GC–MS spectrum, are summarized in table 4. A total of eight components, 2-propenoic acid (10.18%), phthalic acid (3.37%), palmitic acid (35.17%), sandaracopimaradiene (8.20%), oleic acid (22.15%), octadecanoic acid (10.10%), 2-[2-(4-nonylphenoxy)ethoxy]ethanol (3.57%) and glycidyl stearate (7.27%) were identified in the methanol extract and the presence of these compounds had a significant correlation with the inhibition of EAC cell growth (R2 = 0.078, p < 0.05) in vitro.
Peak#
Name of compound
Retention Time
(%) percentage composition
1
2-Propenoic acid
18.456
10.18
2
Phthalic Acid
18.532
3.37
3
Hexadecanoic acid
21.598
35.17
4
Sandaracopimaradiene
21.786
8.20
5
Oleic acid
24.027
22.15
6
Octadecanoic acid
24.274
10.10
7
2-[2-(4-Nonylphenoxy)ethoxy]ethanol
24.625
3.57
8
Glycidyl stearate
26.280
7.27
4 Discussion
Plant secondary metabolites have vital roles in balancing the intracellular redox status and in antioxidant function. Polyphenolic compounds are thought to be the most important and abundant antioxidants found in the plant kingdom and have been claimed to possess anticancer activities (Mates et al., 2008). Flavonoids have been shown to possess antimutagenic and anti-malignant effect (Mates et al., 2009). The present study showed that MEKGR contain a good amount of phenolic and flavonoid compounds which fascinating its free radical scavenging capability. The results of this study are in good contract with previous studies on other plant materials (Islam et al., 2013).
Several in vitro assay models were employed in the present investigation to assess the antioxidant activity of MEKGR. DPPH is stable free radical which undergoes reaction with a hydrogen donor and gets reduced. In DPPH assay, antioxidants present in the extract are thought to act as hydrogen donor and responsible for the reduction of DPPH. By scavenging free radical antioxidants play a preventive role in different diseases including cancer (Pisoschi and Negulescu, 2011). DPPH activity of MEKGR exhibited a strong and positive correlation with its total phenolics (R2 = 0.932, p < 0.05) and flavonoid (R2 = 0.955, p < 0.05) contents suggesting the probable reduction of the DPPH radicals by the hydrogen donating ability of phenolic and flavonoid rich MEKGR (Islam et al., 2013).
MEKGR can react with free radicals to generate more stable products, ceasing radical chain reactions. The ABTS cation radical reacts with a hydrogen donating antioxidant and therefore the solution is decolorized. The assay is commonly used to measure the radical scavenging activity of hydrogen donating and chain breaking antioxidants in plant extracts (Pisoschi and Negulescu, 2011). ABTS scavenging activity of this extract was also found to be highly correlated with the content of phenolics (R2 = 0.978, p < 0.05) and flavonoid (R2 = 0.994, p < 0.01). Biological tissues generate NO in a biochemical reaction catalyzed by specific nitric oxide synthases (Alam et al., 2013). In buffered saline, sodium nitroprusside reacts with oxygen to give rise to nitrite ions that can be measured by using Griess reagent (Sre et al., 2012). A high value of Pearson’s correlation coefficients (R2 = 0.999, p < 0.01) indicates a strong relationship between nitric oxide activity and phenolics. Flavonoids also showed a strong correlation (R2 = 0.985, p < 0.05) with nitric oxide scavenging activity. This study demonstrated the potency of MEKGR as significant source of natural phytoconstituents and antioxidant supplements, indicating their strong potential to be used in traditional medicine system. Compounds of natural origin containing antioxidant properties have been reported to be the important therapeutic intervention for cancer (Lamoral-Theys et al., 2010; Fotsis et al., 1997). From this view point, we further extended our study to examine the anticancer activity of MEKGR.
EAC cells offer special benefits for anticancer drug test due to their suitability to study in almost any mouse host. Moreover, EAC cells lack H-2 histocompatibility antigens, which is the probable reason for their quick proliferation (Chen and Watkins, 1970).
MTT assay was performed in the present study where MEKGR was found to inhibit EAC cells growth in a dose-dependent manner. Reduction of average tumor weight, cell growth inhibition and enhancement of life of the EAC-cell bearing mice are measured for the judgment of potency of a certain compound as anticancer agent (Price and Greenfield, 1958). EAC cell bearing mice were found to increase tumor weight swiftly, but it was observed that the treatment of the EAC-cell bearing mice with the methanol extract reduced tumor burden significantly, inhibited cell growth sufficiently and increased the life span remarkably. The data are also consistent with those reported in the literature (Perveen et al., 2012).
For many years, chemotherapy has been used as a principle mode of treatment for cancer, but the major problems with this therapy are myelosuppression and anemia. Anemia in EAC-bearing mice is mainly observed due to breakdown of red blood cell (Kabir et al., 2012) and this may result from deficiency of iron in hemolytic or myelopathic condition. The treatment with methanol extract under investigation can also reverse all the depleted hematological parameters back towards almost normal level and similar results were also observed in EAC-bearing mice treated with plant extract (Perveen et al., 2012).
Apoptosis, an intrinsic cell-suicidal mechanism regulated by various cellular signaling pathways, is characterized by cell shrinkage, condensation of chromatin and apoptotic body formation (Kabir et al., 2016). Ability of inducing apoptosis is a highly desired aspect of an anticancer drug since this process selectively removes cancer or malignant cells without damaging normal cells. Fluorescence microscopic analysis of cells stained with Hoechst 33342, a blue fluorescing dye that stains chromatin DNA, is a rapid and convenient way to observe cell morphological features such as nuclear fragmentation, chromatin condensation etc. Apoptosis in EAC cells by methanol extract was confirmed by the study of changes in nuclear component and cell shape which was compared with that of control EAC cells suggesting that MEKGR can play significant role in cancer prevention by inducing apoptosis. A number of studies conducted previously also reported the induction of apoptosis in EAC cells during the treatment with different plant extracts (Islam et al., 2014b).
2-Propenoic acid, 3-(4-methoxyphenyl)-, ethyl ester (10.18%), phthalic acid, 6-ethyloct-3-yl2-ethylhexyl ester (3.37%) palmitic acid (35.17%), sandaracopimaradiene (8.20%), oleic acid (22.15%), octadecanoic acid (10.10%), 2-[2-(4-nonylphenoxy)ethoxy]ethanol (3.57%) and glycidyl stearate (7.27%) were identified as the major constituents of MEKGR by GC–MS analysis. In previous studies, oleic acid (Carrillo et al., 2012), phthalic acid (Hayshi et al., 1998) and octadecanoic acid (Lee et al., 2006) were found to possess antitumor activity suggesting that the potent anticancer activity of methanol extract of MEKGR may be responsible for the presence of these active components. Hexadecanoic acid was found to have potential antioxidant activity (Rajalakshmi and Mohan, 2016).
5 Conclusion
In this study, the synergistic effect of some the individual compounds (such as phthalic acid, oleic acid, octadecadienoic acid and hexadecanoic acid) confirmed by GC–MS analysis and antioxidative phenolic and flavonoid contents may be responsible for anticancer and antioxidant activity of methanol extract of Kaempferia galanga rhizome. The exact anticancer mechanism of methanol extract is yet to be discovered, hence the present study suggested to isolate active principles from the methanol extract of Kaempferia galanga rhizome and to investigate their effects on cancer signaling pathways.
Conflict of interest statement
We declare that we have no conflict of interest.
Funding
This research did not receive any grant.
References
- Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J.. 2013;21:143-152.
- [Google Scholar]
- Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci.. 2004;74:2157-2184.
- [Google Scholar]
- Antitumor effect of oleic acid; mechanisms of action. A review. Nutr. Hosp.. 2012;27:1860-1865.
- [Google Scholar]
- Evidence against the presence of H2 histocompatibility antigens in Ehrlich ascites tumor cells. Nature. 1970;225:734-735.
- [Google Scholar]
- In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol.. 2011;136:309-315.
- [Google Scholar]
- Application of flow injection-chemilumineacence to the study of radical scavenging activity in plants. Phytother. Res.. 2000;14:250-253.
- [Google Scholar]
- Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Physicians India. 2004;52:794-804.
- [Google Scholar]
- Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem.. 2002;50:3010-3014.
- [Google Scholar]
- Flavonoid, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res.. 1997;57:2916-2921.
- [Google Scholar]
- Inhibition of Ehrlich’s ascites carcinoma by ethyl acetate extract from the flower of Calotropis gigantea L. in mice. J. Appl. Biomed.. 2010;8:47-54.
- [Google Scholar]
- Comparative evaluation of antimicrobial and antioxidant activities of Kaempferia galanga for natural and micropropagated plant. Int. J. Pharm. Pharm. Sci.. 2010;2:72-75.
- [Google Scholar]
- Antioxidant properties of Lactuca sativa leaf extract involved in the protection of biomolecules. Biomed. Prev. Nutr.. 2013;3:367-373.
- [Google Scholar]
- Antitumor activity of (10E, 12Z)-9-hydroxy-10, 12-octadecadienoic acid from rice bran. J. Ferment. Bioeng.. 1998;86:149-153.
- [Google Scholar]
- Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi, Bangladesh. BMC Complement. Altern. Med.. 2013;13:142.
- [Google Scholar]
- Antiproliferative and hepatoprotective activity of metabolites from Corynebacterium xerosis against Ehrlich ascites carcinoma cells. Asian Pac. J. Trop. Biomed.. 2014;4:284-292.
- [Google Scholar]
- Growth inhibition and apoptosis of Ehrlich ascites carcinoma cells by the methanol extract of Eucalyptus camaldulensis. Pharm. Biol.. 2014;52:281-290.
- [Google Scholar]
- A p-Menth-1-ene-4,7-diol (EC-1) from Eucalyptus camaldulensis Dhnh. Triggers Apoptosis and Cell Cycle Changes in Ehrlich Ascites Carcinoma Cells. Phytother. Res.. 2016;29:573-581.
- [Google Scholar]
- Purification and characterization of a Ca(2+)-dependent novel lectin from Nymphaea nouchali tuber with antiproliferative activities. Biosci. Rep.. 2011;31:465-475.
- [Google Scholar]
- Purification and characterization of a snake guard seed lectin with antitumor activity against Ehrlich ascites carcinoma cells in vivo in mice. Protein Pept. Lett.. 2012;19:360-368.
- [Google Scholar]
- Solanum tuberosum lectin inhibits Ehrlich ascites carcinoma cells growth by inducing apoptosis and G2/M cell cycle arrest. Tumor Biol.. 2016;37:8437-8444.
- [Google Scholar]
- Antineoplastic activity of acetone semicarbazone (ASC) against Ehrlich ascites carcinoma (EAC) bearing mice. J. Natl. Sci. Found. Sri Lanka. 2010;38:225-231.
- [Google Scholar]
- Indian Medicinal Plants. Vol vol. VI. Dehradun, India: Bishen Singh Mahendra Pal Singh; 1997.
- Natural polyphenols that display anticancer properties through inhibition of kinase activity. Curr. Med. Chem.. 2010;17:812-825.
- [Google Scholar]
- Antitumor and antiangiogenic activities of phthalic acid derivative polymers with medium-molecular-weight. Mol. Cryst. Liq. Cryst. Sci. Technol.. 2006;354:287-301.
- [Google Scholar]
- Antioxidant activities of methanolic extracts from four different rose cultivars. J. Food Nutr. Res.. 2014;2:69-73.
- [Google Scholar]
- The nitric oxide scavenging properties of Gingo biloba extract EGb 761. Biochem. Biophysics. Res. Commun.. 1994;15:748-755.
- [Google Scholar]
- Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch. Toxicol.. 2008;82:273-299.
- [Google Scholar]
- Natural antioxidants: therapeutic prospects for cancer and neurological diseases. Mini Rev. Med. Chem.. 2009;9:1202-1214.
- [Google Scholar]
- Studies on products of browning reactions: antioxidant activities of products of browning reaction prepared from glucose amine. Jap. J. Nut.. 1986;44:307-315.
- [Google Scholar]
- Screening for Antioxidant Activity in Eighteen Local Northeastern vegetables using Silica Gel Thin-layer Chromatography followed by spraying with DPPH. NU Sci. J.. 2008;5:1-6.
- [Google Scholar]
- Preventive effect of ethanol extract of Alpinia calcarata Rosc. on Ehrlich’s ascitic carcinoma cell induced malignant ascites in mice. Asian Pac. J. Trop. Med.. 2012;5:121-125.
- [Google Scholar]
- Methods for total antioxidant activity determination: a review. Biochem. Anal. Biochem.. 2011;1:106.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Bio.. 1999;269:337-341.
- [Google Scholar]
- Evaluation of anticancer, antioxidant, and possible anti-inflammatory properties of selected medicinal plants used in Indian traditional medication. J. Tradit. Complement. Med.. 2014;4:253-257.
- [Google Scholar]
- GC–MS analysis of bioactive components of Myxopyrum serratulum A.W. Hill (Oleaceae) Int. J. Pharm. Sci. Rev. Res.. 2016;38:30-35.
- [Google Scholar]
- Polyphenolic content and antioxidant activity of Eugenia pollicina leaf extract in vitro and in model emulsion systems. Food Res. Int.. 2011;44:1190-1196.
- [Google Scholar]
- Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn. in experimental animals. J. Ethnopharmacol.. 2008;118:225-230.
- [Google Scholar]
- In vitro plantlet production system for Kaempferia galanga, a rare Indian medicinal herb. Plant cell tissue. org.. 2000;63:193-197.
- [Google Scholar]
- Antineoplastic potential of medicinal plants. Recent Pat. Biotechnol.. 2011;5:85-94.
- [Google Scholar]
- Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. and Viticult.. 1965;16:144-158.
- [Google Scholar]
- Phytochemical screening and “in-vitro” anti-oxidant activity of methanolic root extract of Erythrina indica. Asian Pac. J. Trop. Biomed.. 2012;2:1696-1700.
- [Google Scholar]







