Translate this page into:
Antioxidant and anti-inflammatory potentials of aerial and floral parts of Neurada procumbens extracts: In vitro and in vivo studies
⁎Corresponding authors. mirza.imran@iub.edu.pk (Mirza Imran Shahzad), khalid.iqbal@bzu.edu.pk (Rana Khalid Iqbal), sd96850@gmail.com (Subhan Danish)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
Neurada procumbens (Neuradaceae) is an importent plant of the Cholistan desert and is traditionally been used for the treatment and control of diabetes, fever, inflammations and jaundice. The aim of the current project is to investigate the bioactive compounds, free radical scavenging capacity and the anti-inflammatory potential of Neurada procumbens separately for its aerial and floral parts in six different extracts. The phytochemical profile (total bioactive contents, HPLC polyphenolic quantification), anti-oxidant (DPPH and FRAP assays), anti-inflammatory (HRBC stabilization) activities of the Aqu, MetOH, n-But, EtAc, n-Hex, and DCM extracts from the aerial and floral parts of Neurada procumbens were quantified. Based on the current results, the aerial and floral parts of N. procumbens extracts have found to contain a significant amount of active metabolites including polyphenolic compounds such as 2,3 di MeO benzoic acid, p-coumaric acid, chlorogenic acid, quercetin dihydrate and t-ferrulic acid. The total phenolic and total flavonoid contents of the plant were found to range from 28.13 to 78.9 GAE mg/g of plant and 17.23 to 68.23 RE mg/g of plant, respectively. DCM and n-But extracts of floral part exhibited comparatively higher antioxidant potential in DPPH (IC50 < 100 μg/ml) and FRAP (IC50 < 55 μg/ml) assays compared to aerial part. The dichloromethane floral extract demonstrated impressive anti-inflammatory activity in the hemolytic red blood cell lysis assay, with 83 % protection of HRBC lysis at an IC50 of 469.6 µg/ml (p < 0.01). In vivo, a 300 mg/kg body weight of DCM floral extract reduced carrageenan-induced paw oedema by 8.51 ± 0.35 mm to 7.65 ± 0.38 mm, a highly significant difference (p < 0.001).The toxicity studies revealed that the floral-DCM dose extract was found to be safe up to 2000 mg/kg BW, while its lethal dose (LD50) was found to be 4472.13 mg/kg BW in rats. Due to quite low toxicity effects, the floral part of the plant could be recommended as a safe pharmacological agent for various therapeutic applications.
Keywords
Anti-inflammatory
Anti-oxidant
Cholistan desert
Neurada procumbens
Phyto-chemicals
Plant extracts
1 Introduction
Reactive oxygen species (ROS) play a vital role in oxidative stress (Alam et al. 2021; Hassan et al., 2022; Hussain and Shah 2023) and can lead to severe diseases including inflammation, diabetes, anaemia, ischemia, cancer, cardiovascular and degenerative diseases. Inflammation is a complicated pathophysiological response of the body, comprised of a variety of signalling molecules produced by the activation of mast cells, leukocytes, macrophages and members of complement system that cause oedema formation at the site of infection (Amin et al., 2020). Inflammation has the beneficial effects as it protects the body from chemical, allergens, burns or other noxious stimuli in an acute response (Borquaye et al., 2020). However, if the inflammation persists in the acute phase for a longer period, it shifts towards the chronic phase leading to numerous disease complications (Seddighfar et al., 2020). Many synthetic steroidal and non-steroidal drugs have being employed to alleviate the inflammation and oxidative stress. However, due to high cost of production, adverse side effects and lack of accessibility of these synthetic drugs, the trend for new alternative treatments derived from medicinal plants have gained more attention (Mohammadi and Saghaian 2022). Medicinal plants are a rich source of life-saving drugs due to presence of secondary metabolites having active compounds of anti-oxidant, anti-bacterial, anti-viral, anti-inflammatory, anti-hypertensive, anti-carcinogenic, anti-allergic, anti-mutagenic and cardio- and gastro-protective potentials. These plant based compounds have a vital role to manage the oxidative stress by minimizing the production of free radicals and to control and cure these microbial and inflammatory disease condtions (Murtaza et al., 2021).
Pakistan has a wonderful number of characteristic plant assets according to each biological pyramid (Khan et al., 2022). The Cholistan desert has a variety of desert plants that have been utilized for various therapeutic purposes by local individuals (Benarba and Pandiella, 2020). Neurada procumbens (Neuradaceae) commonly known as “Chipri Booti” (Zareen et al., 2018) is one of the desert plants that has been selected in this study due to its traditional usage in the treatment of diabetes, jaundice, fever, inflammation and viral infections (Khurshid et al., 2019). Only the floral part of this plant is already used as a food source and a cooling agent; locally called as “Thaadal” in summer in the Cholistan desert. Hence, it could be hypothesized that the plant might contain significant amounts of bioactive compounds, might exhibit strong antioxidant and anti-inflammatory activities due to its extensive use as a therapeutic agent and could be safe to be used in different pharmaceutical applications. Despite the extensive use of this plant in the desert regions, there is still a lack of scientific evidence regarding its bioactive compounds and the potential health benefits of this plant. In order to explore its biologically active compunds, previously in a limited study, the active polyphenols and flavonoids have been investigated from the whole part of N. procumbens and anti-oxidant and anti-viral activities of only the methanol extract have been reported (Qureshi et al., 2010). Therefore, the current project was planned with an aim to extensively investigate the plant for its bioactive compounds, its free radical scavenging capacity, anti-inflammatory potentials and the toxicity effects of N. procumbens extracts. To the best of our knowledge, this is the first comprehensive study to investigate the aforementioned compounds and to explore different activities of the plant separately for its aerial and floral parts in six different plant extracts. The results of the current study will provide a significant insight into the potential and therapeutic applications of the plant.
2 Material and methods
2.1 Plant collection and extraction
The floral and aerial parts of N. procumbens were collected from the Cholistan desert at Bahawalpur region. The plant was identified by a taxonomist of department of Botany (voucher number Np-707). Then both parts were washed, shade dried and ground into fine powder. The dried powder of aerial and floral parts of the plant (50 mg each) was separately soaked in each of 500 ml of six different solvents: n-hexane, n-butanol, dichloromethane, methanol, ethyl acetate and aqueous for 72 h. After occasional shaking, each mixture was filtered and each extract was concentrated by a rotary evaporator and yield was calculated (Aslam et al., 2021).
2.2 Phytochemical evaluation
The phytochemical screening of extracts was performed by standard methods as described previously (Mapfumari et al., 2022).
2.3 Total bioactive contents
The total phenolic contents (TPC) of different extracts were evaluated by Folin-Ciocalteu reagent protocol with slight modifications and results were expressed as milligrams of gallic acid equivalent (GAE) per gram of extract (mg GAE/g extract). The total flavonoid contents (TFC) of extracts were determined according to the previously published method (Akhtar and Mirza, 2015) and results were presented as equivalent of milligrams of rutin equivalent (RE) per gram of extract (mg RE/g extract).
2.4 HPLC conditions and polyphenol quantification
The HPLC-PDA quantification of 22 important polyphenolic compounds were done in MetOH, n-But and DCM extracts of both aerial and floral parts of plant by using standard protocol of the Department of Pharmacy, Via deiVestini, Chieti, Italy (Locatelli et al., 2017).
An HPLC analysis of a plant extract was performed using a Waters liquid chromatograph equipped with a 600 solvent pump model and a 2996 photodiode array detector. Separation was achieved using a C18 reversed-phase packing column (Phenomenex, Prodigy ODS (3), 4.6 × 150 mm, 5 μm; Torrance, CA, USA) thermostated at 30 ± 1 °C with a Jetstream2 Plus column oven. The UV/Vis wavelength was adjusted in the range of 200–500 nm, and quantitative analyses were attained at the maximum wavelength of each compound. A 20 μl injection volume was used and the mobile phase was degassed on-line using a DEGAS Biotech Compact Model (LabService, Anzoladell’Emilia, Italy). A gradient elution was then performed using a mobile phase consisting of water-acetonitrile (93:7 v:v, 3 % acetic acid). Subsequently, the concentrations of the objective compounds were assessed applying the calibration curve.
2.5 Anti-oxidant assay
2.5.1 DPPH revolutionary searching action (RSA)
The anti-oxidant potential of the plant extract was determined by utilizing their capability to search free radicals of 1,1-diphenyl-2-picrylhydrazyl (DPPH). Briefly, DPPH and extract with different concentrations were added to 96 wells microplate, nurtured for 30 min at 37 °C in the dark, the purple colour of the DPPH dye was reduced into yellow due to its interaction with antioxidant agent of the plant extract. The optical density at 517 nm (OD517) was recorded (Amin et al., 2020; Truong et al., 2019) and RSA was calculated by following formula:
2.5.2 The FRAP a method for measuring the reducing power of antioxidants
The FRAP assay was performed to assess the antioxidant potential of the plant extracts. Briefly, 20 µl of plant extract, 90 µl of 0.2 M phosphate buffer, and 30 µl of 1 % w/v potassium ferricyanide were added to a microplate well and incubated for 20 min at 50 °C. Subsequently, 30 µl of 10 % w/v trichloroacetic acid and 30 µl of 0.1 % w/v ferric chloride were added, and the plate was left at ambient temperature for 10 min. Absorbance readings were obtained at OD700, and ascorbic acid assisted as a position standard (Chaves et al., 2020).
2.6 In-vitro anti-inflammatory activity
The HRBC membrane stabilization method was used to assess the anti-inflammatory potential of aerial and floral extracts of N. procumbens. Collected blood was mixed in a 1:1 ratio with Alsever solution and centrifuged for 10 min at 3000 rpm. The packed cells were washed with iso saline solution and a 10 % cell suspension was prepared. Serial dilutions of the extracts (4000, 2000, 1000, 500 and 250 µg/ml) were prepared and 1 ml of PBS, 2 ml of hyposaline, and 0.5 ml of HRBC suspension were added to each concentration. It was incubated at 37 °C for 30 min, centrifuged at 3000 rpm for 20 min and haemoglobin contents of the supernatant solution were estimated at 560 nm. Diclofenac was used as a standard reference (Truong et al., 2019) and the percentage (%) protection was calculated as:
2.7 In vivo acute toxicity assay
At animal residence constitutes of the experimental center of a Pharmacy Department, which is portion of the, Faculty of Pharmacy as well as Alternative Medicine of The Islamia University of Bahawalpur, albino rats including two very different sexes having body weights varying from 170 to 250 g were kept. The creatures were raised in typical different laboratories with a 12 h light/dark cycle, a temperature of 22 oC, as well as a moisture of 35 to 60 percent. They also had free availability to a regular meal with beverage. The Pharmacy Animal Ethics Committee (PAEC) of the Department of Pharmacy, The Islamia University of Bahawalpur (PAEC/20/18, Dated: 15–09-2020) gave its moral blessing to all animal experiments and medical interventions.
The rats were grouped among six sections with four animals another to evaluate the acute lethality of the plant's floral-DCM extraction. According to the actual experiment, the animals being denied food but not water for an extended period. Utilizing typical OECD recommendations, 0.5, 1, 2, 3, 4 and 5 g/kg body mass of the extraction were orally provided to every other group. The only thing given to the comparison group was pure, purified water to drink. Several variables studied tracked for 48 h following decentralized autonomous, and a lethal dosage (LD50) was computed. (Ugwah-Oguejiofor et al., 2019).
2.8 In vivo anti-inflammatory activity
The anti-inflammatory ability of the effective remove was assessed in the Albino rats considering among 200–250 g of each sex as earlier depicted (Jisha et al., 2019). Five animals were assigned in every one of the 6 categories, which included the rats. The usual controlled study, group I, did not have any oedema. As both negative and positive treatments, groups II and III received the usual medication diclofenac (15 mg/kg BW) and normal saline (5 ml/kg) to produce or alleviate oedema, accordingly. N. procumbens DCM solutions were given intraperitoneal injection to the rats in sections IV, V, and VI at doses of 100 mg/kg BW, 200 mg/kg BW, as well as 300 mg/kg BW, accordingly. Following 30 min of dosage delivery, 0.1 ml of carrageenan (1% solution in normal saline solution) being injected into the planters tissue of the right hind paw of every rat to cause swelling. At 1 h, 2 h, 3 h, then 4 h post-carrageenan infusion, the length of every rat's foot was assessed using a digital vernier in accordance with the recommended technique.
2.9 Statistical analysis
A mean as well as a standard deviation of the mean (SEM) of three independent measurements are used to show the data. With the use of SPSS 20, one-way variation assessment (ANOVA) was used to compare various interventions. With the use of GraphPad Prism, IC50 was determined. A statistically meaningful level was established at p 0.05.3.
3 Results
3.1 Phytochemical investigation
Preliminary phytochemical investigations of both aerial and floral parts of N. procumbens showed the abundance of phenols, flavonoids, tannins, terpenoids, coumarins and quinines in MetOH, n-But, EtAc and DCM extracts, while the the maximum amount of sterols and steroids were observed in n-Hex extracts (Table 1). In terms of total bioactive contents, the TPC and TFC of aerial and floral extracts ranged from 28.19 to 127.13 GAE mg/g and 33.20–78.23 RE mg/g of extract, respectively (Table 2). In case of floral extracts, the promising amount of TPC was observed in MetOH (78.14 ± 0.91 GAE mg/g) and DCM (68.23 ± 1.98 GAE mg/g) extracts, while the significant amount of TFC was calculated in DCM (68.23 ± 0.78 RE mg/g) and MetOH (67.21 ± 2.34 RE mg/g) extracts. In the aerial part, the maximum TPC was observed in DCM (65.50 ± 1.38 GAE mg/g of extract) and MetOH (61.34 ± 1.34 GAE mg/g) extracts, followed by EtAc (58.90 ± 2.16 GAE mg/g) extract. However the significant mount of TFC was observed in MetOH (57.42 ± 2.43 RE mg/g) and DCM (54.13 ± 1.83 RE mg/g) extracts. The trend of TPC in different extracts of aerial part was as follows: DCM > MetOH > EtAc > n-But > Aqu > n-Hex, while the trend of TFC in different extracts was MetOH > DCM > n-But > EtAc > Aqu > n-Hex. Likewise, in the floral part, the trend of TPC in different extracts was MetOH > DCM > EtAc > Aqu > n-But > n-Hex, while the trend of TFC in different extracts was DCM > MetOH > n-But > EtAc > Aqu > n-Hex. The terpenoids, phenols, flavonoids and tannins were found in all extracts while increased quantity was observed in MetOH, n-But and DCM extracts of both aerial and floral parts of N. procumbens. +=slightly present, ++=moderately present, +++=strongly present, -=absent. Aqu = aqueous; MetOH = methanol, n-But = n-butanol, EtAc = ethyl acetate, n-Hex = n-hexane, DCM = dichloromethane. The maximum yield was obtained from Aqu and MetOH floral extracts of N. procumbens. While maximum TPC and TFC were recorded in MetOH and DCM floral extracts of the plant. Data (mean ± standard error of mean) is the mean of three repetitions. GAE = gallic acid equivalent, RE = rutin equivalent, Aqu = aqueous, MetOH = methanol, n-But = n-butanol, EtAc = ethyl acetate, n-Hex = n-hexane, DCM = dichloromethane, TPC = total phenolic contents, TFC = total flavonoid contents. According to the DPPH assay,floral extracts showed better scavenging potential compared to aerial extracts. While among the floral extracts, maximum scavenging potential was observed by DCM extract with least IC50, followed by n-But and MetOH extracts.
Preliminary phytochemical
tests
N. procumbens
(Aerial parts)
N. procumbens
(Floral parts)
Aqu
MetOH
n-But
EtAc
n-Hex
DCM
Aqu
MetOH
n-But
EtAc
n-Hex
DCM
Molisch
+
+
+
+
–
++
+
+
+
–
+
+
Benedict
+
+
+
–
–
–
–
+
+
–
+
+
Barfoed
+
–
+
–
–
–
–
+
+
–
+
+
Ninhydrin
–
–
–
–
–
–
+
+
–
–
–
–
Terpenoid
++
+
++
++
+
++
+
+++
++
++
+
+++
Salkowski
+
++
+
+
+
+
+
+
+
++
+
+
Saponins
++
++
+
+
–
++
+
++
+
+
+
++
Libermann
+
+
+
++
+
++
+
++
+
+
++
+
Phenols
++
++
+
++
–
++
++
+++
++
++
–
+++
Flavonoids
++
++
++
++
–
++
+
+++
++
++
–
+++
Anthocyanin / betacyanin
–
–
–
–
–
–
–
–
–
–
–
–
Coumarins
++
–
–
+
–
–
–
–
–
–
–
–
Sterols
++
–
–
–
–
–
–
–
++
+
++
+
Quinines
–
++
–
–
++
–
–
–
–
–
–
Cardiac glycosides
+
+
–
+
–
+
++
++
–
++
–
++
Fats / Oils
–
–
+
+
++
+
–
–
++
+
++
++
Steroids
–
–
++
+
++
+
–
–
++
+
++
+
Tannins
+
++
+
++
++
++
++
+++
+
++
+
+++
N. procumbens
Plant extract
Extraction yield (%)
TPC
(GAE mg/g extract)
TFC
(RE mg/g extract)
IC50
(μg/ml)
Aerial
Aqu
4.42
37.13 ± 1.12
27.85 ± 0.96
331.30 ± 1.21
MetOH
3.53
61.34 ± 1.34
57.42 ± 2.43
111.20 ± 2.34
n-But
3.20
51.80 ± 1.17
47.38 ± 1.91
137.20 ± 2.76
EtAc
2.50
58.90 ± 2.16
42.18 ± 1.87
237.10 ± 2.29
n-Hex
2.10
28.19 ± 1.21
17.23 ± 0.75
–
DCM
1.98
65.50 ± 1.38
54.13 ± 1.83
110.00 ± 2.89
Floral
Aqu
5.60
63.23 ± 1.21
48.65 ± 1.92
149.30 ± 1.61
MetOH
5.20
78.14 ± 0.91
67.21 ± 2.34
99.10 ± 2.01
n-But
3.20
57.80 ± 0.21
58.80 ± 0.81
97.10 ± 0.91
EtAc
4.10
63.32 ± 1.63
52.23 ± 1.96
123.20 ± 0.63
n-Hex
2.70
33.20 ± 1.07
37.80 ± 0.65
643.40 ± 2.19
DCM
3.10
68.23 ± 1.98
68.23 ± 0.78
86.10 ± 0.21
Ascorbic acid
–
–
–
17.32 ± 0.18
3.2 HPLC-PDA polyphenolic quantification
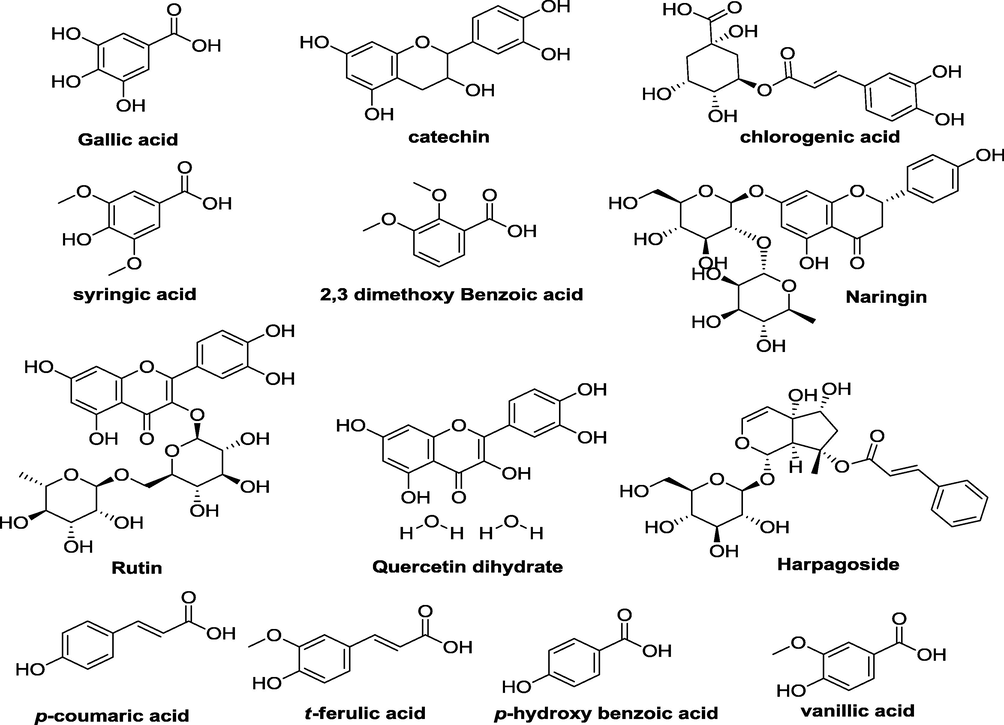
In HPLC-PDA polyphenolics quantification, the p-coumaric acid, chlorogenic acid, quercetin dihydrate, sinapinic acid and t-ferulic acid were quantified in n-But extract, while syrengic acid, 2,3 di MeO benzoic acid, quercetin dihydrate and t-ferulic acid have been quantified in DCM aerial extract. While in case of floral extracts, 2,3 di MeO benzoic acid, p-coumaric acid, chlorogenic acid, quercetin dihydrate and t-ferulic acid were identified and quantified in the n-But floral extract and quercetin dihydrate, p-hydroxybenzoic acid, p-coumaric acid, vanillic acid and harpagoside were majorly quantified in DCM floral extract (Table 3 & Fig. 1) (see Figs. 2a and 2b). By HPLC quantification, 5 polyphenols were identified with maximum quantity of chlorogenic acid in n-But aerial extract when compared to standard library of 22 polyphenols. In DCM aerial extracts, 4 polyphenols were identified with inceased level of 2,3 di Meo Benzoic acid. In n-But floral extract, chlorogenic acid and 2,3 di Meo Benzoic acid were found maximium in 5 identified polyphenols. In DCM floral extracts, among the 5 identified polyphenols, vanillic acid and quercetin dihydrate were quantified in maximum amount. RT = retention time, BLQ = below limit of quantification.
Plant part
Extract
RT (min)
Compounds identified
Concentration (µg/mg)
Compound class
Aerial
n-But
13.969
Chlorogenic acid
0.47 ± 0.03
Phenolic acid
20.521
p-coumaric acid
BLQ
Phenolic acid
23.076
Sinapinic acid
BLQ
Phenolic acid
25.284
t-ferrulic acid
BLQ
Phenolic acid
35.549
Quercetin dehydrate
BLQ
Flavonoid
DCM
17.850
Syrengic acid
BLQ
Phenolic acid
24.851
t-ferrulic acid
BLQ
Phenolic acid
29.104
2,3 di Meo Benzoic acid
0.28 ± 0.02
Phenolic acid
37.718
Quercetin dihydrate
BLQ
Flavonoid
Floral
n-But
12.451
Chlorogenic acid
0.40 ± 0.03
Phenolic acid
21.142
p-coumaric acid
BLQ
Phenolic acid
25.791
t-ferrulic acid
BLQ
Phenolic acid
28.163
2,3 di MeOBenzoic acid
0.42 ± 0.02
Phenolic acid
35.998
Quercetin dihydrate
BLQ
Flavonoid
DCM
12.719
p-hydroxy benzoic acid
BLQ
Phenolic acid
15.197
p-coumaric acid
BLQ
Phenolic acid
21.150
Vanillic acid
0.22 ± 0.01
Phenolic acid
37.921
Quercetin dihydrate
0.42 ± 0.03
Flavonoid
42.964
Harpagoside
BLQ
Flavonoid
Key polyphenolic metabolites extracted in high (%) yield.
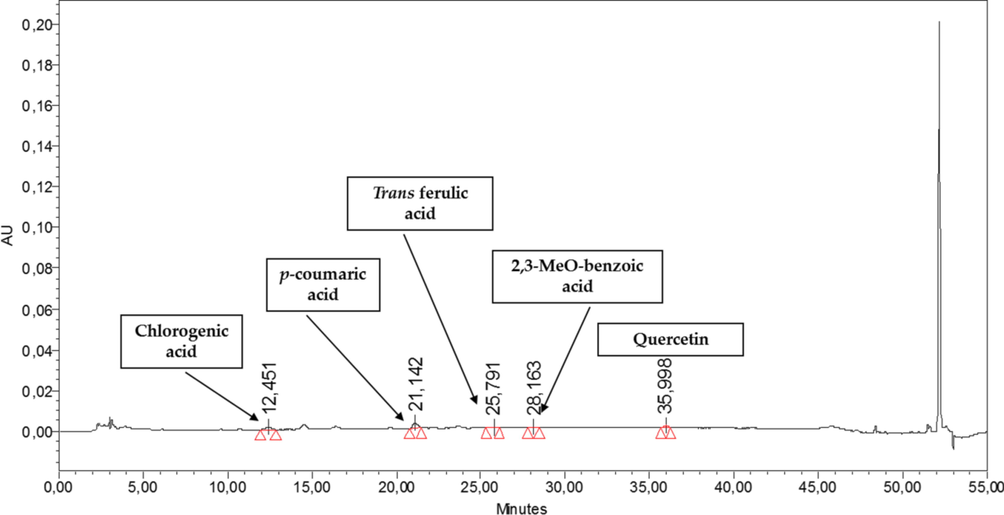
HPLC chromatograph of N. procumbens (floral) n-But extract.
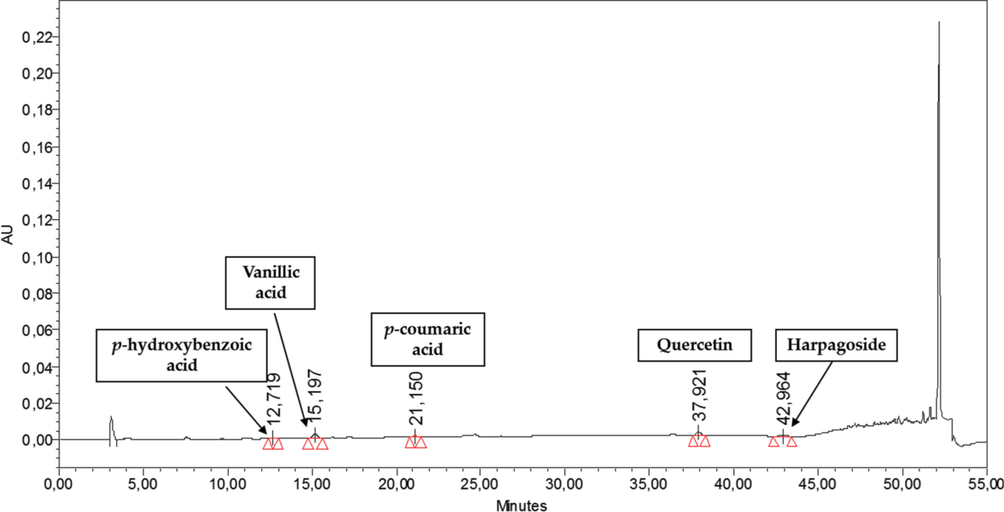
HPLC chromatograph of N. procumbens (floral) DCM extract.
3.3 Anti-oxidant activity
Accoring to DPPH assay, the floral extract has shown more scavenging potential as compared to aerial extracts. Among floral extracts, the maximum percentage of radical scavenging assay (RSA) was shown by DCM and n-But extracts with significant least IC50 at 86.1 μg/ml and 97.1 μg/ml, respectively followed by MetOH extract with IC50 of 99.3 μg/ml. The trend of DPPH scavenging activity of N. procumbens floral extracts was DCM > n-But > MetOH > EAc > Aqu > n-Hex. In case of aerial extracts, the maximum anti-oxidant potential was given by DCM and MetOH extracts with significant least IC50 < 112 μg/ml. The DPPH scavenging activity of N. procumbens aerial extracts was ranked as DCM > MetOH > n-But > EtAc > Aqu > n-Hex (Table 2). According to antioxidant potential by FRAP assay, the floral extract also shown strong reducing power as compared to aerial extracts. Among the floral extracts, n-But and DCM extracts exhibited the strongest reducing power, even better than ascorbic acid at 1000 µg/ml, followed by EtAc and MetOH floral extracts with significant reducing ability. The trend of reducing power by N. procumbens floral extracts was n-But > DCM > EtAc > MetOH > n-Hex > Aqu (Fig. 3a). In case of aerial extracts, DCM and n-But extracts showed reducing power more than ascorbic acid at 1000 µg/ml. The reducing power of N. procumbens aerial extracts was ranked as DCM > n-But > EtAc > MetOH > Aqu > n-Hex (Fig. 3b).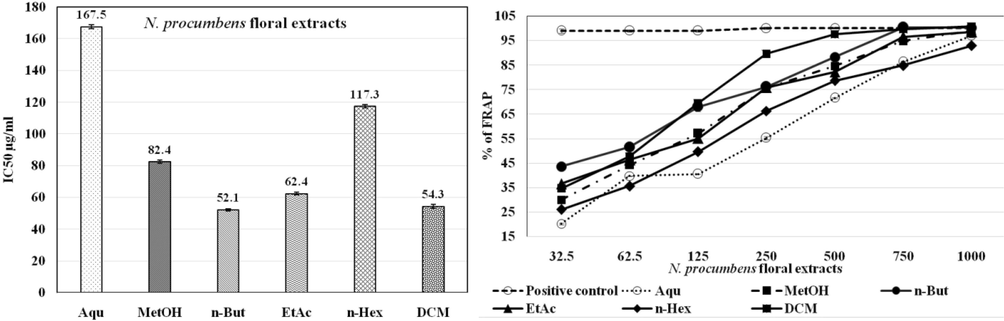
The IC50 μg/ml (left panel) and relative % of FRAP (right panel) of N. procumbens aerial extracts in anti-oxidant assay. n-But and DCM extracts of aerial parts showed the strongest reducing power and least IC50. The values are represented as Mean ± SEM of triplicate in each group. The results are analyzed using one-way ANOVA and IC50 was calculated by Graph Pad Prism. Ascorbic acid was used as a standard drug (positive control).
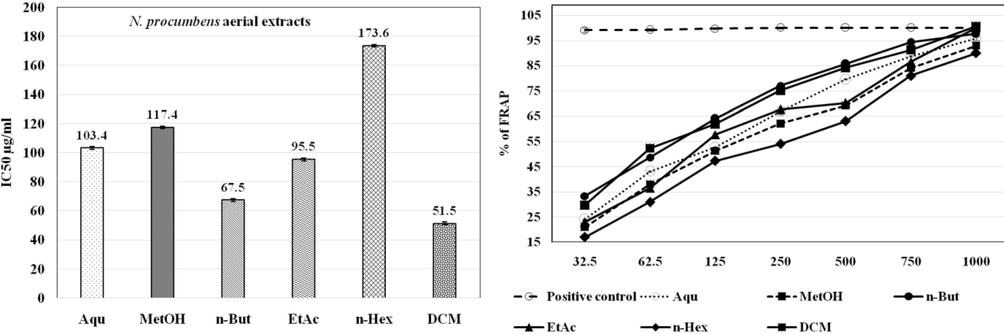
The IC50 μg/ml (left panel) and relative % of FRAP (right panel) of N. procumbens floral extracts in anti-oxidant assay. n-But and DCM extracts of floral parts showed the strongest reducing power and least IC50. The values are represented as Mean ± SEM of triplicate in each group. The results are analyzed using one-way ANOVA and IC50 was calculated by Graph Pad Prism. Ascorbic acid was used as a standard drug (positive control).
3.4 Anti-inflammatory activity
The floral extracts have shown better protection of HRBCs lysis in a dose dependant manner as compared to aerial extracts. Among these floral extracts, DCM extract shown the maximum activity at the concentration of 4000 μg/ml with 83.75 % protection (IC50 469.6 µg/ml) against HRBC lysis in a hypotonic solution. The overall trend of in vitro anti-inflammatory activity of N. procumbens floral and aerial extracts was: DCM > MetOH > EtAc > n-But > Aqu > n-Hex (Table 4). The stabilization effect on human red blood cells membrane (HRBC) was evaluated by aerial and floral extracts at different concentrations. The maximum stabilization effect was observed by floral extracts, wherein DCM extract showed maximum protective effect on membrane with significantly low (P < 0.01) IC50. The IC50 values calculated by Graph pad prism and analysed by two-way ANOVA are presented as Mean ± SEM of three independant observations in each group. **=P < 0.01. Aqu = aqueous, MetOH = methanol, n-But = n-butanol, EtAc = ethyl acetate, n-Hex = n-hexane, DCM = dichloromethane extract.
Aerial
Part
% of protection
IC50
(μg/ml)
Floral
part
% of protection
IC50
(μg/ml)
Aqu
31.82 ± 1.48
–
Aqu
55.91 ± 0.50
3321.0 ± 1.00
MetOH
66.00 ± 0.50
1903.0 ± 1.04
MetOH
77.20 ± 0.60
1089.0 ± 1.18
n-But
43.75 ± 0.65
–
n-But
67.45 ± 0.85
1636.0 ± 0.85
EtAc
60.00 ± 0.16
2427.0 ± 0.98
EtAc
79.56 ± 0.76
1147.0 ± 1.24
n-Hex
22.75 ± 0.50
–
n-Hex
47.40 ± 0.80
–
DCM
68.33 ± 0.46
1787.0 ± 1.16
DCM
83.75 ± 0.45
469.6 ± 0.784**
Diclofenac
86.15 ± 0.45
369.30 ± 0.87
Diclofenac
86.15 ± 0.45
369.30 ± 0.87
3.5 Anti-inflammatory potential in carrageenan induced rat paw edema model
At 1 h post-carrageenan induction, the inflammatory control group (-ve control group) showed a marked rise in inflammation compared to normal rats group, peaking at 4th h before declining to baseline levels at 5th h. The standard drug diclofenac (15 mg/kg BW) significantly reduced paw inflammation at 2nd h (16.72 %), 3rd h (24.90 %) and 4th h (29.00 %), compared to -ve control group (p < 0.001). The DCM floral extract displayed significant anti-inflammatory activity, with maximum effects at 3rd h (18 %–22 %; p < 0.01) and 4th h (22 %-27 %; p < 0.001). All three concentrations of DCM extract (100 mg/kg BW, 200 mg/kg BW and 300 mg/kg BW) had shown significantly reduced paw oedema (p < 0.005, p < 0.01 and p < 0.001), respectively. Among all doses of DCM extract, the optimal concentration was 300 mg/kg BW that was providing maximum inhibition (p < 0.001) of about 27.4 % (Table 4 & Fig. 4).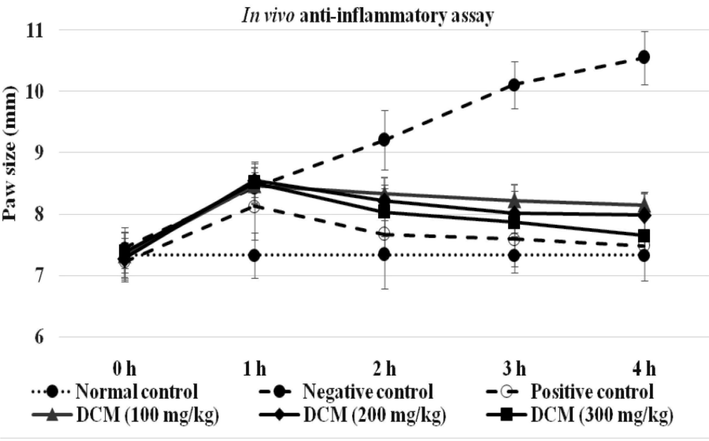
In vivo anti-inflammatory potential of N. procumbens DCM-floral extract. Carrageenan induced inflammation was treated with various concentrations of DCM floral extract and compared with negative (untreated group) and positive (standard drug / diclofenac) controls. The reduction in the paw oedema was analyzed by measuring the paw size (mm) at a time interval of 1 h post-induction. All three concentrations of DCM extract (100 mg/kg BW, 200 mg/kg BW and 300 mg/kg BW) showed significant reduction in paw oedema (p < 0.005, p < 0.01 and p < 0.001), respectively. The values are represented as Mean ± SEM of triplicate readings in each group and the results were analyzed by using one-way Analysis of Variance. DCM = dichloromethane.
3.6 In vivo acute toxicity studies
No toxicity signs were recorded in rats after the oral administration of 0.5–2 g/kg BW doses. However, at the doses of 4 g/kg and 5 g/kg, the animals showed toxicity symptoms with 25 % and 100 % mortality within 2–3 h post-administration, respectively. Thus, the median lethal dose (LD50) was found to be 4472.13 mg/kg BW in rats (Table 5). Six groups of rats were administered with different doses of DCM floral extract. Toxicity signs were observed from the dose of 3000 mg/kg BW. Hence, according to the OECD guidlines, this plant belongs to the the toxicity class-5 (low toxicity class having LD50 < 5000 mg/kg BW). LD50 is lethal dose that cause 50 % mortality on animal trial.
Extract dose
(mg/kg BW)
Toxicity signs
Mortality
(%)
LD50
(mg/kg BW)
500
None
0
4472.12
1000
None
0
2000
None
0
3000
Jerks, fits, writhing
0
4000
Coma, convulsion, salivation
25
5000
Convulsion and expired
100
4 Discussion
In the current era, due to extensive application of medicinal plants for the cure of different diseases in the developing countries, the plants were investigated and a number of phytochemicals have been extracted having anti-oxidant and anti-inflammatory potentials (Achakzai et al., 2021). In the current study, six different (Aqu, MetOH, n-But, EtAc, n-Hex, DCM) extracts separately from aerial and floal parts of N. procumbens were prepared and subjected to phytochemical evaluation and HPLC quantifications and then each extract was tested for its anti-oxidant and anti-inflammatory potential. Since, the extract yield and phytochemical extraction from the same plant highly depend on the nature of the solvent used. It is widely accepted that the varying levels of activity exhibited by extracts of the same plant are a result of the differing concentrations of phytochemicals present, which is dependent on the solvent utilized (Alam et al., 2020).
Preliminary investigations of aerial and floral parts of the plant have confirmed the abundance of phytochemicals in all extracts, except the least quantity of these compounds was found in n-Hex extract (Table 1). The results were found to be in accordance with the previous studies, wherein the maximum phenolic contents were also quantified in MetOH, DCM and n-But extracts of B. ciliata, C. grata and C. viticella plants (Alam et al., 2020). In the same context, maximum phenolic contents in the MetOH, DCM and n-But extracts were observed by previous studies (Gomes et al., 2019). The compounds like coumaric acid, chlorogenic acid, epicatechin, benzoic acid, t-ferulic acid and gallic acid were quantified in MetOH, DCM and n-But extracts. According to several studies, coumaric acid, catechin, epicatechin, benzoic acid, t-ferulic acid and gallic acid are found to be potent anti-oxidant and anti-inflammatory agent(s) (Okafor et al., 2022; Sun et al., 2023). The antioxidant system provides a defensive role by various mechanisms including single electron transfer, donation of hydrogen and metal ion chelation. The hydroxyl group found in these polyphenolic compounds play a vital role in the therapeutic and pharmaceutical chemistry. Since, it is a key factor in biological activities like anti-microbial, anti-viral, anti-oxidant, insecticidal, anti-chlorotic, anti-parasitic and hypoglycemic reactions (Zulhendri et al., 2021).
The promising anti-oxidant potential in these extracts may also be due to the presence of polyphenols or phenolic substituents. The abundance of polyphenols and flavonoids in DCM, MetOH and n-But floral extracts of N. procumbens gives a strong anti-oxidant potential to the plant. Additionally, the existence of terpenoids and flavonoids like luteolin, xanthone and xanthonoids in DCM, MetOH and n-But extracts also gives the anti-oxidant potential to the plant. It has been reported that polyphenol and flavonoids compounds give radical scavenging potential to plants, due to their abilities of hydrogen donation and chelating activity of metal ion (Khatib et al., 2021). Interestingly, the DPPH antioxidant activity of the n-But extract was found to be two-fold higher than what was reported earlier. The presence and richness of TPC/TFC in DCM, n-But and MetOH floral extracts also gives potent anti-oxidant potential to these extracts (Zengin et al., 2023). In FRAP assay, the reducing ability of the compound is also considered to be the characteristic of anti-oxidant potential. In this activity, the Fe+3 are converted to Fe+2 by capturing the electrons released by the test compound (Chaves et al., 2020). The n-But and DCM aerial extracts showed the strongest scavenging potential in DPPH assay and also showed the highest reducing power similarly in the FRAP assay. In the same context, previous studies have also reported the correlation between the reducing power and TFC of plant extracts, representing the involvement of flavonoids in the reducing action (Khatib et al., 2021; Wani et al., 2020). The reducing capacity is usually due to the presence of reductones in the plant that employ anti-oxidant potential by donating the hydrogen atom and breaking of free radical chain (Kumar et al., 2017).
There is also a strong correlation between anti-oxidant and anti-inflammatory potentials. The presence of phenolic compounds prompted us to investigate their in vitro and in vivo anti-inflammatory potential since both pathways are interlinked. In vitro anti-inflammatory assays demonstrated that various aerial and floral extracts had a marked stabilizing effect on the hypo-tonicity-induced lysis of human red blood cells (HRBCs). This effect was attributed to the inhibition of lysozyme, a bactericidal enzyme, and b-glucuronidase; an acid protease, both of which are released extra-cellularly by neutrophils and are involved in tissue inflammation. (Lekouaghet et al., 2020). The lysosomal membrane of activated neutrophils is analogous to the erythrocyte membrane, suggesting that extracts which stabilize the erythrocyte membrane may also be capable of stabilizing the lysosomal components of stimulated neutrophils (Winer et al., 2020). The DCM floral parts exhibited significant in vitro anti-inflammatory potential like that of several studies reporting anti-inflammatory effects of different plants extracts through RBC membrane stabilization (Amini et al., 2021). In case of in vivo studies, the sequence of inflammation progression and regression as observed in the current study is also previously reported (Yang et al., 2020). In the current study, all doses of DCM floral extract of N. procumbens showed a dose-dependent anti-inflammatory potential due to its anti-oxidant activity caused by polyphenols and flavonoids in the extracts, hence rendering the plant a strong anti-inflammatory agent. For the selection of an optimal dose, the toxicity effects of different doses of the plant have been tested in rats and found the LD50 at <2000–5000 mg/kg BW by DCM floral extract, thus, the plant belongs to low toxicity class as per OECD guidelines. Many other plants also belong to this class due to their LD50 of <2000 mg/kg BW (Ugwah-Oguejiofor et al., 2019). Hence, it could be the reason that flowers of N. procumbens are typically used by the desert nomads for the treatment of fever, inflammation and other infections, as they might quite well aware of the safe dose and toxicity effects of the plant due to their life long personal experiences.
5 Conclusions
In conclusion, the current study has established the medicinal importance of N. procumbens through its complete phytochemical profiling and confirmed the presence of various active metabolites having strong anti-oxidant and anti-inflammatory activities. The study provides the therapeutic and pharmacological basis for the traditional applications of the plant due to its least toxicity effects and hence, strongly recommends that the floral part of the plant could be recommended as a safe pharmacological agent for various therapeutic applications.
Acknowledgment
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R677), King Saud University, Riyadh, Saudi Arabia for financial support..
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Functional and Phytochemical potential of Berberis. Pak-Euro J. Med. Life Sci.. 2021;4(SI):S25-S34.
- [Google Scholar]
- Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. Journal of Infection and Public Health. 2020;13(11):1734-1741.
- [Google Scholar]
- 24-epibrassinolide (EBR) reduces oxidative stress damage induced by cadmium toxicity by restricting cd uptake and modulating some key antioxidant enzymes in maize plants. Pakistan J. Bot.. 2021;53:59-66.
- [Google Scholar]
- A comparative assessment of anti-inflammatory, anti-oxidant and anti-bacterial activities of hybrid and indigenous varieties of pumpkin (Cucurbita maxima Linn.) seed oil. Biocat. Agri. Biotechnol.. 2020;28:101767
- [Google Scholar]
- Important insights from the antimicrobial activity of Calotropis procera. Arabian Journal of Chemistry. 2021;14(7):103181
- [Google Scholar]
- Medicinal plants as sources of active molecules against COVID-19. Frontiers in Pharmacology. 2020;11:1189.
- [Google Scholar]
- Anti-inflammatory and anti-oxidant activities of ethanolic extracts of Tamarindus indica L. (Fabaceae). Cog. Chem.. 2020;6(1):1743403.
- [Google Scholar]
- Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants. 2020;9(1):76.
- [Google Scholar]
- Anti-biofilm activity of hydromethanolic plant extracts against Staphylococcus aureus isolates from bovine mastitis. Heliyon. 2019;5(5):e01728.
- [Google Scholar]
- Oxidative stress alleviation as indicated by enzymatic and non-enzymatic antioxidants and osmoregulators in barley (Hordeum vulgare L.) under salt (NaCl) stress by ascorbic acid (ASA) Pakistan J. Bot.. 2022;54:7-15.
- [Google Scholar]
- Organic amendments mitigate drought stress-induced oxidative changes in synthetic cultivars of maize. Pakistan J. Bot.. 2023;55:429-436.
- [Google Scholar]
- Anti-inflammatory efficacy of methanolic extract of Muntingia calabura L. leaves in Carrageenan induced paw edema model. Pathophysiol.. 2019;26(3–4):323-330.
- [Google Scholar]
- Evaluation of the characteristics of native wild Himalayan fig (Ficus palmata forsk.) from Pakistan as a potential species for sustainable fruit production. Sustainability. 2022;14(1):468.
- [Google Scholar]
- HPLC–DAD profiling of a phenolic extract from Moroccan sweet Basil and its application as oxidative stabilizer of sunflower oil. Chemical Papers. 2021;75(5):1907-1917.
- [Google Scholar]
- Phytochemical composition and in vitro pharmacological investigations of Neurada procumbens L. (Neuradaceae): A multidirectional approach for industrial products. Indus. Crops Prod.. 2019;142:111861
- [Google Scholar]
- Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res. Notes. 2017;10:60.
- [Google Scholar]
- In vitro evaluation of antioxidant and anti-inflammatory activities of the hydroalcoholic extract and its fractions from Leuzea conifera L. roots. South African Journal of Botany. 2020;132:103-107.
- [Google Scholar]
- Optimization of aqueous extraction and biological activity of Harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytotherapy Research. 2017;31(6):937-944.
- [Google Scholar]
- Phytochemical Screening, Antioxidant and Antibacterial Properties of Extracts of Viscum continuum E. Mey. Ex Sprague, a South African Mistletoe. Plants. 2022;11:2094.
- [Google Scholar]
- Factors affecting consumption of different forms of medicinal plants: the case of licorice. Agriculture. 2022;12:1453.
- [Google Scholar]
- Pomegranate Peel Extract and Quercetin Possess Antioxidant and Hepatoprotective Activity against Concanavalin A-induced Liver Injury in Mice. Pak. Vet. J.. 2021;41(2):197-202.
- [Google Scholar]
- Flavonoid and phenolic acid profiles of dehulled and whole Vigna subterranea (L.) verdc seeds commonly consumed in South Africa. Molecules. 2022;27:5265.
- [Google Scholar]
- Ethnomedicinal uses of herbs from northern part of Nara desert. Pakistan. Pak. J. Bot.. 2010;42(2):839-851.
- [Google Scholar]
- Analgesic and anti-inflammatory properties of hydroalcoholic extracts of Malva sylvestris, Carum carvi or Medicago sativa, and their combination in a rat model. J. Integ. Med.. 2020;18(2):181-188.
- [Google Scholar]
- Truong, D.-H., Nguyen, D.H., Ta, N.T.A., Bui, A.V., Do, T.H., Nguyen, H.C., 2019. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 8178294.
- Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii NE Brown in mice and rats. Heliyon. 2019;5(1):e01179.
- [Google Scholar]
- Phytochemical analysis and evaluation of antibacterial activity of different extracts of soil-isolated fungus chaetomium cupreum. Biologie et Médecine. 2020;11(1):72.
- [Google Scholar]
- Nuphar lutea Extracts Exhibit Anti-Viral Activity against the Measles Virus. Molecules. 2020;25(7):1657.
- [Google Scholar]
- In vitro and in vivo anti-inflammatory effects of different extracts from Epigynum auritum through down-regulation of NF-κB and MAPK signaling pathways. Journal of Ethnopharmacology. 2020;261:113105
- [Google Scholar]
- In-Vitro Propagation of Neurada procumbensl L (Chipri Booti): An Endangered Medicinal Plant from Cholistan Desert. Pak. J. Agri. Res.. 2018;31(1)
- [Google Scholar]
- Phytochemical Profile and Biological Activities of Different Extracts of Three Parts of Paliurus spina-christi: A Linkage between Structure and Ability. Antioxidants. 2023;12(2):255.
- [Google Scholar]
- Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods. 2021;10(6):1360.
- [Google Scholar]
Further reading
- Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Con.. 2016;61:156-164.
- [Google Scholar]
- Anti-biofilm activity against Staphylococcus aureus MRSA and MSSA of neolignans and extract of Piper regnellii. Revista Brasileira de Farmacognosia. 2017;27(1):112-117.
- [Google Scholar]
- Anti-inflammatory activity of leaves of Jatropha gossypifolia L. by HRBC membrane stabilization method. J. Acute Dis.. 2013;2(2):156-158.
- [Google Scholar]
- Anti–inflammatory activity of the leaf extacts of Gendarussa vulgaris Nees. Asian Pacific J. Trop. Med.. 2011;1(2):147-149.
- [Google Scholar]
- Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. International Journal of Molecular Sciences. 2010;11(6):2362-2372.
- [Google Scholar]
- Therapeutic Potential of Phenolic Compounds in Medicinal Plants - Natural Health Products for Human Health. Molecules. 2023;28:1845.
- [Google Scholar]
- In vitro and in vivo anti-inflammatory activity of leaves of Symplocos cochinchinensis (Lour) Moore ssp Laurina. Bangladesh J. Pharmacol.. 2008;3(2):121-124.
- [Google Scholar]
- Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Applied and Environmental Microbiology. 2010;76(1):243-253.
- [Google Scholar]







