Translate this page into:
Antioxidant and anti-diabetic activities of bioactive fractions of Carica papaya seeds extract
⁎Corresponding authors. agabaidu73@gmail.com (Reuben Agada), armankhan0301@gmail.com (Ameer Khusro)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Carica papaya has tremendous medicinal applications against diverse diseases since ancient period. Considering this, the present study was aimed to assess in vitro antioxidant and anti-diabetic activities of bioactive fractions of C. papaya seed extracts. Initially, the shade-dried ripen seeds of C. papaya were powdered and extracted via soxhlet protocol to obtain crude extracts. The crude extracts were then fractionated using different solvents viz. hexane, ethyl acetate, methanol, and water. Fractions obtained were assessed for α-glucosidase and amylase inhibitory activities using standard methodologyies. Results revealed that ethyl acetate fraction C (83.66 ± 0.10%) and D (84.56 ± 0.23%) showed maximum inhibition of α-amylase with an IC50 value of 36.84 and 36.86 mg/ml, respectively. Likewise, ethyl acetate fraction C (48.72 ± 0.10%) and D (51.81 ± 0.45%) exhibited α-glucosidase inhibition with an IC50 value of 83.54 and 82.33 mg/ml, respectively. The ethyl acetate fractions C and D were further purified to obtain sub-fraction K, L, M, and N and evaluated for in vitro antioxidant and anti-diabetic activities. The sub-fraction K demonstrated maximum antioxidant activity against DPPH and TBA radicals (IC50 = 41.37 and 53.98 mg/ml) respectively. However, the sub-fraction K elicited the most conspicuous anti-diabetic activity towards α-amylase (IC50 = 156.15 mg/ml) and intestinal α-glucosidase (IC50 = 70.89 mg/ml). The mode of inhibition was determined using the Lineweaver- Burk plot. Sub-fraction K exhibited a competitive mechanism of inhibition on both α-amylase and α-glucosidase. The GC-MS profile confirmed the presence of diverse metabolites in the sub-fraction and the major bioactive compounds detected were hexadecanoic acid, methyl ester, 11-octadecenoic acid, methyl ester, N, N-dimethyl, n-hexadecanoic acid, and oleic acid. Our findings suggested the folklore medicinal use of C. papaya seeds as an effective antioxidant and anti-diabetic agents.
Keywords
Antioxidant
Carica papaya seeds
Diabetes mellitus
Gas chromatography-mass spectrometry
1 Introduction
Medicinal plants have been utilized as natural prophylactic and therapeutic agents since ancient periods due to the presence of enormous bioactive components, easy availability, and low toxicity (Bhatia et al., 2019). Currently, the demand for natural therapeutic agents is increasing because these substances are being utilized as foods and drugs owning to their therapeutic efficacy via phytochemical constituents to replace the synthetic compounds (El Omari et al., 2019). It has necessitated the search for medicinal plants as a natural antioxidant because of its ability to scavenge free radicals. Previous reports revealed that oxidative stress participates in the turn of events and pathogenesis of diabetes mellitus (Oboh et al., 2014; Sabiu and Ashafa, 2016). The annihilation of oxidative stress by medicinal plant extracts preserves the pancreatic β-cells, attenuates lipid peroxidation, and insulin sensitivity improvement (Maritim et al., 2003), consequently halting development and movement to diabetes complexities (Sabu and Kuttan, 2009). To date, diabetes mellitus remains one of the most prevailing and devastating metabolic ailments known to mankind. Type 1 diabetes mellitus results in insulin deficiency because of the loss of β-cells of the pancreatic islets that produce insulin. While type 2 diabetes mellitus is characterized by loss of peripheral responses to insulin. If left untreated, type 1 diabetes can degenerate into fatal ketoacidosis. Type 2 diabetes represents the prevalent form of the disease (Gita, 2013). Interestingly, one effective treatment option for type 2 diabetes is to diminish postprandial hyperglycemia by delaying the uptake of glucose via the inhibition of α-amylase and intestinal α-glucosidase activities. In this regard, enzymes inhibitors can slow down the uptake of carbohydrates, suppress postprandial hyperglycemia, thereby treating diabetes and/or obesity (Song et al., 2016).
Carica papaya (Family – Caricaceae) is a medicinal plant that is generally distributed in the tropical regions of Africa. Seeds of C. papaya are usually regarded as waste materials after processing and consumption of the edible fruits. These waste materials incorporate unlimited opportunities for new medicine. Therapeutic applications related to the antioxidant activity were attributed to enormous essential micronutrients and diverse secondary metabolites embedded in the C. papaya seeds with no elicit signs of toxicity (Delphin et al., 2014; Zhou et al., 2011; Mohamed et al., 2014). Although several methods for antioxidant activity have been reported, only 2,2-diphenyl-1-picrylhydrazyl (DPPH), Ferric reducing antioxidant power (FRAP), and Thiobarbituric acid (TBA) are fast and reliable (El Omari et al., 2019; Ruoying et al., 2016). Limited studies have been reported in the past regarding the anti-diabetic properties of C. papaya seeds (Adeneye and Olagunju, 2009; Oboh et al., 2014). Hence, this study was investigated to evaluate in vitro antioxidant activity as well as α-amylase and α-glucosidase inhibitory traits of different fractions of C. papaya seeds. Part of the study also focuses on the identification of major therapeutic active compounds in the potent fraction using Gas chromatography-mass spectrometry (GC-MS) analytical techniques.
2 Materials and methods
2.1 Plant collection and preparation of extract
The ripen fruits of C. papaya were collected from farmland during the dry season, identified, and validated by a botanist at the branch of Plant Sciences, School of Life Sciences, Moddibo Adama University of Technology Yola, Nigeria. The seeds were dried and pummeled into coarse powder using lab blender and subjected to soxhlet extraction in hexane, ethyl acetate, methanol, and distilled water sequentially to obtain the crude extracts.
2.2 Fractionation
Solvent extracts (hexane, ethyl acetate, methanol, and aqueous) of C. papaya seeds were separated by column chromatography with silica gel (60–120 mesh) and eluted with methanol:ethyl acetate:hexane (6:2:2), followed by methanol:ethyl acetate:chloroform:n-hexane (3:2:2:3). Altogether 8 fractions were collected and every fraction was screened for α-amylase and α-glucosidase inhibitory activities (Mohammad et al., 2015).
2.3 Alpha-amylase (α-amylase) inhibitory assay
C. papaya seed fractions were assessed for α-amylase inhibitory traits according to the methodology of McCue and Shetty (2004). Enzyme inhibition property was determined as per the formula mentioned below and 50% inhibition concentration (IC50) value was estimated from the regression curve.
Abs = absorbance of Blank and absorbance of the fraction
For the mode of inhibition, the potent sub-fraction K was taken at its IC50 value and incubated with α-amylase. On the other hand, the starch solution was used at varied concentrations (0.125–1% w/v) for determining the inhibition trait. The production of reducing sugars was measured spectrophotometrically using maltose standard curve and changed over to response speeds (V). A two folds corresponding plot (1/V versus 1/S) was plotted. The type of inhibition of the fraction on α-amylase activity was controlled by examining Lineweaver-Burk two fold corresponding plot (Lineweaver and Burk, 1934).
2.4 Alpha-glucosidase (α-glucosidase) inhibitory assay
Fractions were assessed for α-glucosidase inhibitory properties as per the methodology of Kim et al. (2005). Twenty microlitres of varied concentrations (20–100 mg/ml) of fractions, acarbose, and 50 μl of an α-glucosidase solution in 0.1 M phosphate support (pH 6.9) were incubated at 25⁰C for 10 min. Thirty microliters of 5.0 mM (pNPG) solution in 0.1 M phosphate buffer (pH 6.9) were added into it. Test tubes containing the reaction mixture were incubated at 37 °C for 1 h and the reaction was stopped by adding 1 ml of 0.1 M Na2CO3. The inhibitory trait was estimated according to the formula mentioned below and the IC50 value was derived from the regression graph.
For the mode of inhibition, the potent sub-fraction K at its IC50 concentration was incubated with 50 μl of alpha-glucosidase solution (prepared in 0.1 M phosphate support; pH 6.9) for 10 min at 25 °C. Further, 30 μl of pNPG solution (0.625 to 5 mM) was added to all the test tubes of the reaction mixture, incubated for 60 min at 37 °C, and then 1 ml of Na2CO3 was precisely introduced to terminate the reaction. The para-nitrophenol standard curve was resolved from the measure of diminishing sugars discharged utilizing spectrophotometric analysis. A two folds corresponding plot (1/V versus 1/[S]) was plotted to decide the type of inhibition of the sub-fraction on α-glucosidase utilizing Michaelis-Menten kinetics (Lineweaver and Burk, 1934).
2.5 Purification of the potent fraction
Column chromatography and thin layer chromatography (TLC) were used to purify the fraction showing potential inhibitory traits. The potent fraction was subjected to silica gel (60–120 mesh) and eluted with a mixture of methanol, ethyl acetate, chloroform, and hexane with increasing polarity (6:4:4:6, 4:1:1:4, and 3:2:2:3 v/v). The fractions obtained were further separated through repeated column chromatography on silica gel (60–200 mesh) and eluted with methanol: ethyl acetate: hexane gradients (4:2:4) to yield the sub-fractions.
2.6 α -amylase and α -glucosidase inhibition assays of sub-fractions
α-amylase and α-glucosidase inhibitory traits of sub-fractions were assessed following the methodologies of McCue and Shetty (2004) and Kim et al. (2005), respectively as described above.
2.7 Antioxidant activity of sub-fraction-
2.7.1 DPPH radical scavenging assay
DPPH radical scavenging effect of the sub-fractions was determined following the methodology of Sayah et al. (2017). The absorbance was recorded at 523 nm against the blank. DPPH scavenging activity was estimated according to the equation given below and IC50 value was determined.
2.7.2 Thiobarbituric acid (TBA) method
About 2 ml of a mixture containing 20–100 mg/ml of sub-fractions was mixed with 2 ml of 20% trichloroacetic acid and 2 ml of 0.67% thiobarbituric acid solutions. This mixture was kept in a water bath (100 °C) for 10 min and allowed to cool. Subsequent to cooling, it was centrifuged at 3000 rpm for 20 min and the absorbance of the reaction was read at 532 nm (Sayah et al., 2017). TBA antioxidant activity was calculated adopting the equation as given below:
2.7.3 FRAP assay
The FRAP property was determined as per the method of Sayah et al. (2017). One milliliter of sub-fraction (20–100 mg/ml) was added into 1 ml of 0.2 M sodium phosphate buffer (pH 6.6) and 1 ml of 1% potassium ferricyanide in isolated test tubes. After incubation at 50 °C for 20 min, 1 ml of 10% trichloroacetic acid was supplemented to the reaction mixture, and the mixture was then centrifuged at 3000 rpm for 10 min. Thereafter, 1 ml of supernatant was added into 1 ml of distilled water and 200 μl of 0.1% FeCl3. Absorbance was estimated at 700 nm spectrophotometrically and FRAP assay was calculated as follows:
2.8 GC-MS analysis of the sub-fraction
The bioactive metabolites present in the sub-fraction K were determined according to the methodology of Venkatadri et al. (2017) using GC-MS (SHIMADZU QP2010).
2.9 Statistical analysis
Data were analyzed utilizing the Statistical Package for Social Sciences (SPSS, version 28.0). The outcomes were expressed as mean ± standard error of the mean (SEM) and further analyzed employing one-way Analysis of Variance (ANOVA). Significant impacts were reported at (P < 0.05).
3 Results
3.1 α-amylase and α-glucosidase inhibition of fractions
C. papaya seed extracts (hexane, ethyl acetate, methanol, and aqueous) were exposed to TLC and column chromatography to obtain two fractions from each solvent extract and designated as hexane fractions (A and B), ethyl acetate fractions (C and D), methanol fractions (E and F), and aqueous fractions (G and H). Fig. 1 shows the inhibition of α-amylase activity by the seed fractions. Fraction D significantly restrained the activity of pancreatic α-amylase conferring 84.56 ± 0.23% inhibition at 100 mg/ml. This auspicious effect was comparable to other fractions (A − 82.07 ± 0.21%, B − 83.56 ± 0.06%, C − 83.66 ± 0.1%, E − 27.30 ± 0.23%, F − 44.15 ± 1.81%, G − 18.21 ± 0.33%, and H − 33.05 ± 0.3%) and acarbose (standard drug) which offered 58.69 ± 4.1% of α -amylase inhibition. The IC50 values of fraction A, B, C, D, and acarbose were estimated as 37.3, 37.53, 36.84, and 36.86 mg/ml, respectively.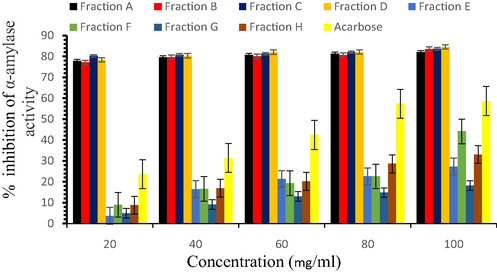
α-amylase inhibition properties of fractions.
Furthermore, the inhibitory effects of seed fractions on in vitro α-glucosidase activities are presented in Fig. 2. Among various fractions, ethyl acetate fraction D at higher concentrations exhibited 51.81 ± 0.45% inhibition of α -glucosidase as compared to the acarbose (62.38 ± 3.47%). The ethyl acetate fraction D was observed as the most potent fraction (IC50 - 82.33 mg/ml).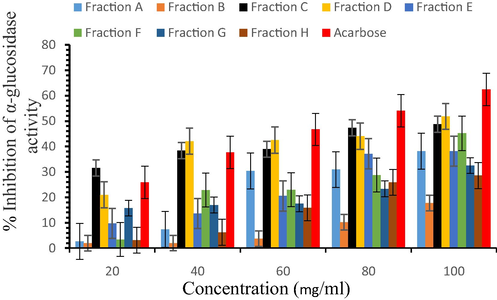
α-glucosidase inhibition properties of fractions.
3.2 α-amylase and α-glucosidase inhibition of sub-fractions
Since the ethyl acetate fractions (C and D) of seed extract showed better inhibition of the enzymes (α-amylase and α-glucosidase), it was further purified to obtain two sub-fractions from each ethyl acetate fraction and designated as sub-fraction K, L, M, and N). All the sub-fractions inhibited α-amylase activity but sub-fraction K showed maximum inhibition property of 30.23 ± 1.77% at the highest concentration (100 mg/ml) as presented in Fig. 3. The IC50 values for sub-fraction K, L, M, N, and acarbose were estimated at 156.15, 173.37, 207.04, 178.32, and 74.64 mg/ml, respectively. Although the effect was concentration-dependent, the percentage inhibition of the sub-fractions was lower in correlation to acarbose but the sub-fraction K exhibited significant (P < 0.05) inhibition of α-amylase with respect to the other sub-fractions.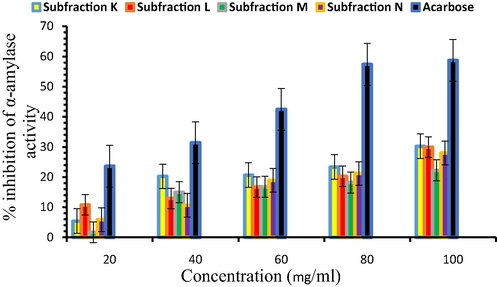
α-amylase inhibition properties of sub-fractions.
Likewise, α-glucosidase inhibition properties of sub-fractions such as K, L, M, and N were observed at varying concentrations (Fig. 4). All the sub-fractions showed increased inhibition of α-glucosidase in a concentration-dependent manner (20–100 mg/ml) but sub-fraction K significantly inhibited the activity of α-glucosidase (57.01 ± 1.44–87.04 ± 0.66%). The activity was further supported by its lower IC50 value (70.89 mg/ml) as compared to other sub-fractions (L- 82.16 mg/ml, M − 96.08 mg/ml, and N − 96.54 mg/ml) and acarbose (71.47 mg/ml).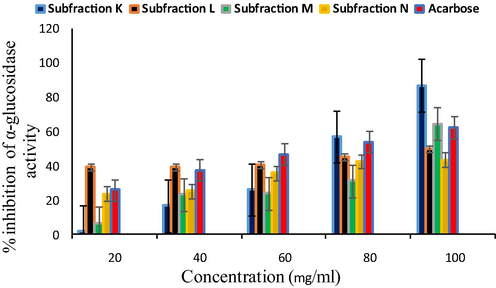
α-glucosidase inhibition properties of sub-fractions.
To show the mechanism of inhibition of the sub-fraction K on the enzymes (α-amylase and α-glucosidase), the Lineweaver-Burk plot was determined from the sub-fraction K. This suggests that the sub-fraction K showed a competitive type of inhibition on both enzymes (Figs. 5 and 6). The Vmax values were unaffected while the Km increased in the presence of the inhibitors.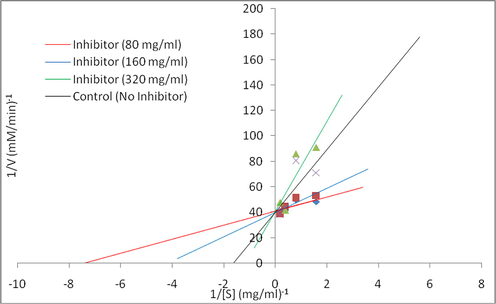
Lineweaver-Burk plot of the reaction of α-amylase in the presence of subfraction K.
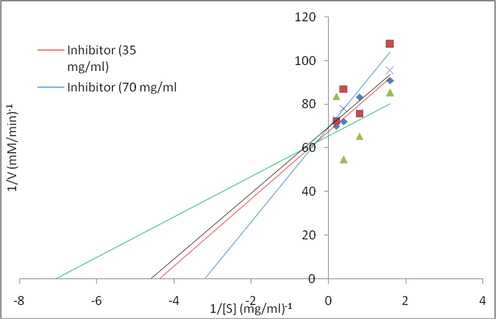
Lineweaver-Burk plot of the reaction of α-glucosidase in the presence of subfraction K.
3.3 In vitro antioxidant effect of sub-fractions
Fig. 7a shows the DPPH radical scavenging abilities of K, L, M, and N sub-fractions. Results revealed that all the sub-fractions such as L (27.91 ± 1.54–74.28 ± 0.41%), M (61.56 ± 0.67–85.82 ± 0.05%) and N (58.03 ± 0.90–83.17 ± 0.04%) scavenged DPPH free radical in a dose-dependent manner but the sub-fraction K showed a significant (P < 0.05) scavenging activity (70.82 ± 0.33–91.66 ± 0.02%) in comparison to ascorbic acid (8.18 ± 0.34–58.62 ± 0.69%).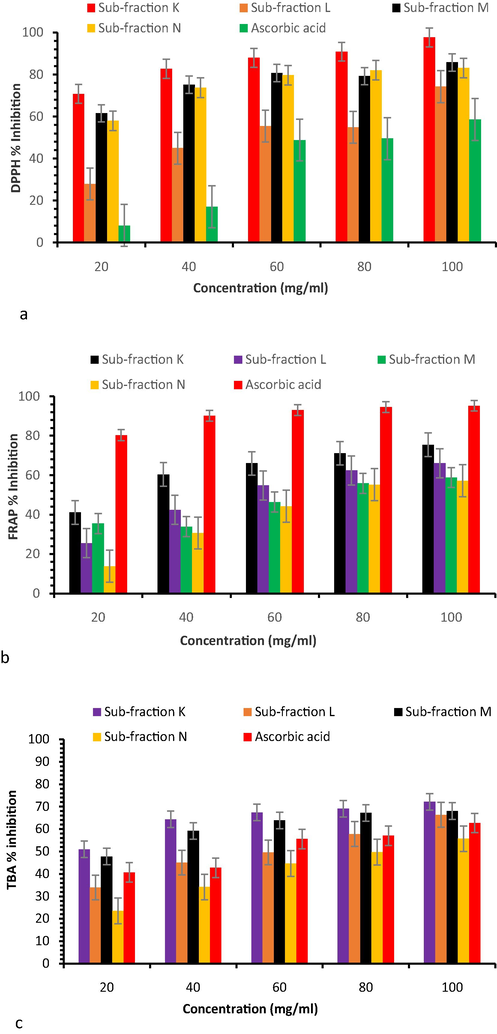
(a) DPPH radical scavenging, (b) FRAP assay, and (c) TBA radical scavenging activities of sub-fractions.
The TBA scavenging ability of the sub-fractions is exhibited in Fig. 7b. Results showed significant (P < 0.05) inhibition of TBA by sub-fraction K (50.96 ± 0.73–72.13 ± 0.09%) as compared to sub-fractions L (33.92 ± 0.39–66.38 ± 0.53%), M (47.77 ± 0.4–68.03 ± 0.46%), and N (23.43 ± 0.59–55.67 ± 0.2%).
The FRAP assay of the sub-fractions is shown in Fig. 7c. As the concentration increased (20–100 mg/ml), FRAP trait of sub-fractions also increased (sub-fraction K − 41.12 ± 0.35–75.43 ± 0.36%; L − 25.52 ± 1.43–66.05 ± 0.05%; M − 35.49 ± 0.56–58.83 ± 0.19; and N − 13.77 ± 0.5–57.23 ± 0.23%) inhibition compared to ascorbic acid (90.33 ± 0.04–95.24 ± 0.02%).
3.4 GC-MS analysis of sub-fraction K
The GC-MS chromatogram of the sub-fraction K is shown in Fig. 8. The study identified the presence of 18 bioactive compounds in the sub-fraction K and is represented with their GC retention time, relative abundance (area), and compounds names in Table 1. Results of GC-MS profile marked hexadecanoic acid, methyl ester (9.18%), 11-Octadecenoic acid, methyl ester (10.12%), N, N-dimethyl (13.50%), n-Hexadecanoic acid (14.60%), and oleic acid (23.22%) as the main bioactive metabolites in the sub-fraction. The structures of these predominant compounds are shown in Fig. 9.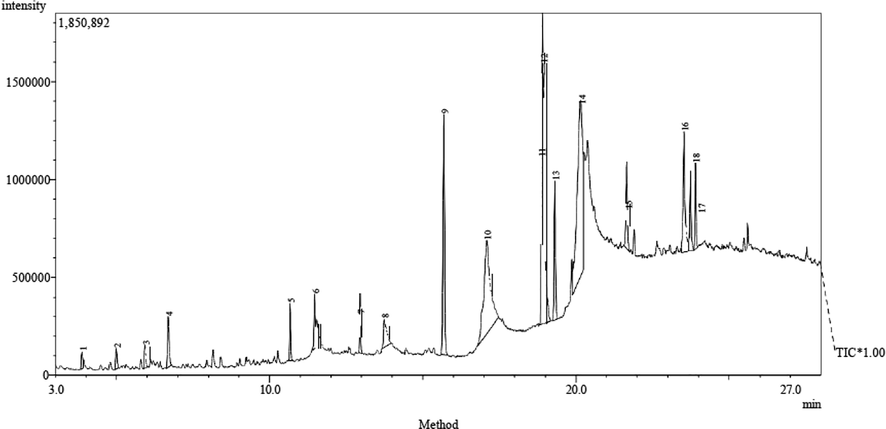
GC-MS chromatogram of sub-fraction K.
S/N
Retention time (s)
Area (%)
Molecular formula
Molecular Weight (g/mol)
Compound name
1
3.868
0.61
C7H12O
112.17
2-Heptenal, (E)-
2
4.988
0.77
C10H14
134.21
4,7-Methanoindene, 3a,4,5,6,7,7a-hexahydro-, endo-
3
5.922
0.79
C11H16
148.24
9-Methylene-tricyclo[4.2.1.1(2,5)]decane
4
6.675
2.08
C10H8
128.17
4-Phenylbut-3-ene-1-yne
5
10.658
1.38
C11H22O2
186.28
Decanoic acid, methyl ester
6
11.466
1.56
C12H24O2
200.31
Decanoic acid, ethyl ester
7
12.955
1.44
C18H36O2
284.48
Hexadecanoic acid, 15-methyl-, methyl ester
8
13.735
2.23
C11H22O2
186.29
Octanoic acid, 2,6-dimethyl-, methyl ester
9
15.683
9.18
C17H34O2
270.45
Hexadecanoic acid, methyl ester
10
17.082
14.60
C16H32O2
256.41
n-Hexadecanoic acid
11
18.911
13.50
C3H7NO
73.09
N,N-dimethyl-
12
18.966
10.12
C19H36O2
296.5
11-Octadecenoic acid, methyl ester
13
19.316
4.58
C19H38O2
298.5
Octadecanoic acid, methyl ester
14
20.151
23.22
C18H34O2
282.47
Oleic Acid
15
21.657
3.08
C39H76O5
624
Octadecanoic acid, 2-hydroxy-1,3-propanediyl ester
16
23.528
5.97
C17H36O
256.5
6,10,13-Trimethyltetradecanol
17
23.739
2.70
C13H13N3O
227.26
1-(6-Methyl-2-pyridyl)-3-phenylurea
18
23.901
2.19
C23H46O2
354.61
Docosanoic acid, methyl ester
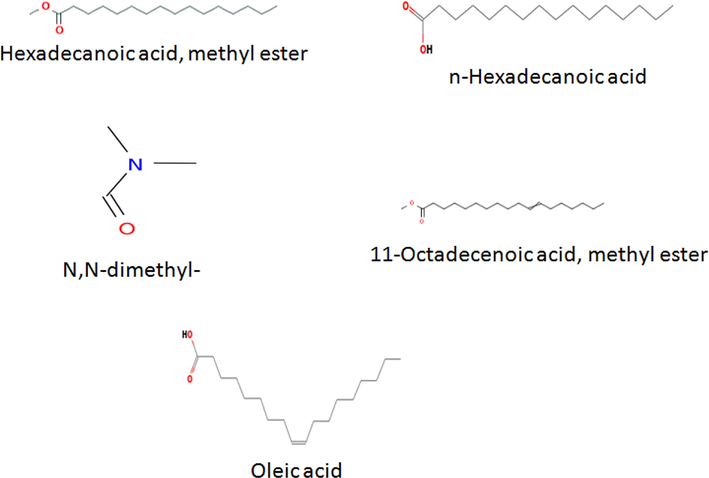
Major bioactive compounds present in the sub-fraction K.
4 Discussion
Many medicinal plants have been reported to exhibit blood-glucose-diminishing effects through diverse mechanisms that are directly or indirectly used for the preparation of many modern drugs. However, these mechanisms are similar to those of the synthetic oral anti-diabetic agents but these plants have not been accepted as valid medicinal agents in annihilating diabetes mellitus due to documented scientific evaluation (Campbell-Tofte et al., 2012). In the current study, the extracts of C. papaya seeds were purified to obtain bioactive fractions as an antioxidant and anti-diabetic agent. Among those fractions, ethyl acetate fraction showed the most prominent in vitro inhibitory activities against α-amylase and intestinal α-glucosidase. These enzymes inhibition allows a minimal quantity of glucose to be absorbed into the blood circulation, hence the plasma glucose will not spike after a meal (Chipiti et al., 2015).
Since the fractions (C and D) obtained from ethyl acetate extract of C. papaya seeds demonstrated the most active inhibitory potential against α-amylase and intestinal α-glucosidase, they were further purified to obtain sub-fractions K, L, M, and N. Among those, sub-fraction K moderately inhibited α-amylase and significantly inhibited α-glucosidase activity with low IC50 values. Our findings agree with the report of Krentz and Bailey (2005) who proposed that an effective Type 2 diabetes mellitus drug model mildly inhibits pancreatic α-amylase with a potent inhibitor of intestinal α-glucosidase. Further, the competitive mechanism of inhibition on both enzymes by the sub-fraction K indicated that the inhibitor ties to the free enzyme retarding the formation of the enzyme-substrate complex.
Free radicals are a direct consequence of hyperglycemia in diabetes mellitus, which inappropriately enhance cellular and enzyme damage, increase lipid peroxidation, and develop insulin resistance (Brownlee, 2001; Maritim et al., 2003). Consuming plants with excellent antioxidant attributes has been aggressively explored to treat diseases globally and to ameliorate the ravaging activity of free radicals (Sharma et al., 2013). C. papaya seeds have been reported to possess strong antioxidant properties such as the reduction of DPPH radicals, ABTS, NO2, and ferric ion reducing power due to rich diverse bioactive secondary metabolites (Sagbo et al., 2017; Zhou et al., 2011). In this context, sub-fraction K demonstrated high antioxidant ability which might be due to the presence of bioactive metabolites in the seed. Previous studies on free radical-scavenging activities of C. papaya seeds indicated that it exhibited a conspicuous activity compared to the standard ascorbic acid (Kothari and Seshadri, 2010; Norshazila et al., 2010; Contreras-Calderón et al., 2011).
The GC-MS chromatogram uncovered the presence of distinct bioactive compounds in the ethyl acetate extract fraction of C. papaya seeds. The major compounds found in this study have been known to possess a lot of pharmacological activities in the past. Several studies have documented n-hexadecanoic acid and oleic acid as a promising antioxidant, anti-inflammatory, and hypocholesterolemic, and 5-α reductase inhibitor activities agents (Rajeswari et al., 2012; Anyasor et al., 2014; Omotoso et al., 2014; Gnanavel and Saral, 2013). Therefore, findings suggested that these active metabolites in C. papaya seeds might have preserved the beta-cell function and inhibited the activity of α-amylase and intestinal α-glucosidase in vitro.
5 Conclusions
The present study identified hexadecanoic acid, methyl ester, 11-octadecenoic acid, methyl ester, N, N-dimethyl-, n-hexadecanoic acid, and oleic acid as the predominant anti-diabetic compounds in C. papaya seeds. It is suggested that these compounds may exert a synergistic effect towards anti-diabetic properties. This is also supported by strong antioxidant activity and competitive mode of α-amylase and α-glucosidase inhibitions, thereby interfering with the conversion of carbohydrates into glucose. In summary, the outcomes of this study suggest further in vivo validation of C. papaya seeds in ameliorating diabetes and other related metabolic illnesses.
Acknowledgements
Authors gratefully acknowledge the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-1441-329.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preliminary hypoglycemic and hypolipidemic activities of the aqueous seed extract of Carica papaya Linn. in Wistar rats. Biol. Med.. 2009;1:1-10.
- [Google Scholar]
- Anyasor, G.N., Onajobi, F.D., Osilesi, O., Adebawo, O.O., 2014. Phytochemical constituents in hexane fraction of Costusafer Ker Gawl. Stem. Vedic Res. Int. Phytomed. 2, 66–72.
- In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complement Altern. Med.. 2019;19(1)
- [CrossRef] [Google Scholar]
- Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813-820.
- [Google Scholar]
- Harnessing the potential clinical use of medicinal plants as anti-diabetic agents. Botanics: Targets Therap.. 2012;2:7-19.
- [Google Scholar]
- In vitro alpha-amylase and alpha-glucosidase inhibitory effects and cytotoxic activity of Albizia antunesiana extracts. Pharmacogn. Mag.. 2015;11:231-236.
- [Google Scholar]
- Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int.. 2011;44(7):2047-2053.
- [Google Scholar]
- Phytochemical screening of various ethanolic seed extracts. World J. Pharm. Pharm. Sci.. 2014;7:1041-1048.
- [Google Scholar]
- Hyperglycemia and hyperlipidemia mitigating impact of Catharanthus roseus (Sadabahar) leaves aqueous extract on type 2 diabetes mellitus subjects. J. Plant Sci. Res.. 2013;3:170-174.
- [Google Scholar]
- GC-MS Analysis of petroleum ether and ethanol leaf extracts from Abrus precatorius Linn. Int. J. Pharm. Biosci.. 2013;4:37-44.
- [Google Scholar]
- Inhibitory effects of pine bark extract on alpha-glucosidase activity and postprandial hyperglycemia‖. Nutrition. 2005;21:756-761.
- [Google Scholar]
- Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Nutr. Food Sci.. 2010;40(4):403-408.
- [Google Scholar]
- The determination of enzyme dissociation constants. J. Am. Chem. Soc.. 1934;56(3):658-666.
- [Google Scholar]
- Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65(3):385-411.
- [Google Scholar]
- Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol.. 2003;17(1):24-38.
- [Google Scholar]
- Inhibitory effects of rosmarinic acid extract on porcine pancreatic amylase in vitro. Asian Pac. J. Clin. Nutr.. 2004;13:101-106.
- [Google Scholar]
- Isolation and purification of the antibacterial principle from Avicennia marina l in methanol. Int. J. Pharm. Pharm. Sci.. 2015;7:5-8.
- [Google Scholar]
- Carica papaya as a source of natural medicine and its utilization on selected pharmaceutical applications. Int. J. Pharm. Pharm. Sci.. 2014;6:857-860.
- [Google Scholar]
- Antioxidant levels and activities of selected seeds of Malaysian tropical fruits. Malaysian J. Nutr.. 2010;16:149-159.
- [Google Scholar]
- Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water-extractable phytochemicals from unripe pawpaw fruit (Carica papaya) J. Basic Clin. Physiol. Pharmacol.. 2014;25:21-34.
- [Google Scholar]
- Evaluation of in vitro antioxidant and antidiabetic activities of aristolochia longa extracts. Evidence-Based Complementary Altern. Med.. 2019;2019:1-9.
- [Google Scholar]
- Phytochemical analysis of Cnidoscolus aconitifolius (Euphorbiaceae) leaf with spectrometric techniques. Niger. J. Pharm. Appl. Sci. Res.. 2014;3:38-49.
- [Google Scholar]
- GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae) Res. J. Pharm. Biol. Chem. Sci.. 2012;3:301-308.
- [Google Scholar]
- Antioxidant activities and phenolic compounds analysis of extractions of C. papaya seed residue. Singapore: World Scientific Publishing Company; 2016. p. :596-604.
- Membrane stabilization and kinetics of carbohydrate metabolizing enzymes (α -amylase and α -glucosidase) inhibitory potentials of Eucalyptus obliqua L.Her. (Myrtaceae) Blakely ethanolic leaf extract: an in vitro assessment. South Afr. J. Botany. 2016;105:264-269.
- [Google Scholar]
- Antidiabetic and antioxidant activity of Terminalia bellerica. Roxb. Indian J. Exp. Biol.. 2009;47:270-275.
- [Google Scholar]
- A review of medicinal plants having antioxidant potential. Indian J. Res. Pharm. Biotechnol.. 2013;1:404-409.
- [Google Scholar]
- Antioxidant, antibacterial and phytochemical properties of two medicinal plants against the wound infecting bacteria. Asian Pacific J. Tropical Biomed.. 2017;7(9):817-825.
- [Google Scholar]
- Sayah, K., Marmouzi, I., NaceiriMrabti, H., Cherrah, Y., Faouzi, M.E.A., 2017. Antioxidant activity and inhibitory potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) aerial parts extract against key enzymes linked to hyperglycemia. Biomed. Res. Int. 2017, 2789482.
- Cinnamic acid amides from Tribulus terrestris displaying uncompetitive α-glucosidase inhibition. Eur. J. Med. Chem.. 2016;114:201-208.
- [Google Scholar]
- In vitro assessment on medicinal properties and chemical composition of Michelia nilagirica bark. Asian Pac. J. Trop. Biomed.. 2017;7:782-790.
- [Google Scholar]
- Zhou, K., Wang, H., Mei, W., Li, X., Luo, Y., Dai, H., 2011. Antioxidant activity of papaya seed extracts. Molecules 16, 6179–6192.







