Translate this page into:
Antioxidant activity of flavonoid compounds isolated from the petals of Hibiscus rosa sinensis
⁎Corresponding author. vijayavedam2014@gmail.com (Vijayalakshmi Melanathuru)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Hibiscus rosasinensis, medicinal plant known for its various medicinal properties. The present study was to identify and characterize the flavonoid compound from the petals of Hibiscus rosa sinensis. The antioxidant compounds were isolated by Silica Gel G Column chromatography. From the fractions, C5 shows the highest antioxidant activity compared to C3 & C4 compounds. DNA damage protection activity results indicated that two major bands super coiled DNA and open circular DNA was protected by the presence of different concentration (5 µg, 10 µg, 15 µg & 20 µg) of C5 compound isolated from the petals of Hibiscus rosa sinensis. IR absorptions peaks reveals the presence of functional groups alcohols, phenols, α,β unsaturated aldehyes, ketones and alkanes. The structure of compound was characterized as Hibiscetin-3-glucoside (C21H20O14) by Mass spectroscopy and NMR. The study emphasized flavanoid compounds have effective scavenging activities which may be due to its phenolics and flavonoid contents and can be used as anticancer agents.
Keywords
Flavanoid
Antioxidant
DNA protector activity
IR
NMR
Hibiscetin-3-glucoside
1 Introduction
Over the past centuries, the use of medicinal plants and herbal drugs plays an important role in day to day life. About 25–30% of prescribed drugs is produced from the medicinal plants and plays a vital role in treating various types of infectious disease. Around 3000 plants species with medicinal properties are identified in India (Prakasha et al., 2010).
Flavonoids are an abundant class of low molecular weight phytocompounds which form an essential part of the human nutrition (Rice-Evans, 2001). The antioxidant and chelating properties of flavonoids helps to prevent the chronic and age-related diseases (Schroeter et al., 2002).
Hibiscus rosa sinensis which belongs to Malvaceae family is regarded both as an ornamental and medicinal plant with a variety of therapeutic properties. Researchers had isolated and characterized the different phytochemical constituents including flavanoids, alkaloid, saponins, tannins and polyphenols (Ghani 2003). From deep yellow flowers, the following compounds like Quercetin-3-diglucoside, 3,7-diglucoside, cyanidin-3,5-diglucoside and cyanidin-3-sophoroside-5-glucoside have been isolated (Rastogi and Mehrotra 1991). The defensive effect of H. rosa-sinensis extracts against the cancer development’s tumour promotion phase have been studied (Sharma and Sultana, 2004).
2 Materials and methods
2.1 Procurement and authentication of plant sample
Flowers of Hibiscus rosa sinensis were collected from Herbal Garden at Sri Sairam Siddha Medical College, West Tambaram, Tamil Nadu, India. It is an evergreen woody glabrous shrub of 1.5–2.4 m in height. The morphology characteristics of plant include axillary, single, bell-shaped flowers that are red, blue, yellow or white in colour. Usually, the flowers are 10.2 to 15.2 cm in diameter. The capsule of the flower is round in shape and has numerous seeds (Upadhyay et al. 2011). Based on the morphological study, the flower samples were identified and authenticated by Dr. S. Sankaranarayanan, Head of the Department, Department of Medicinal Botany, Government Siddha Medical College, Chennai.
2.2 Extraction of flavonoid from petals of Hibiscus rosa sinensis
The petals were shade and then finely powdered and sieved. The aqueous extract solution was filtered, centrifuged at 5000 rpm for 10 min and extracted successively with petroleum ether, chloroform and ethanol. The flavonoids in the extracts were determined by a standard procedure (Harborne and Mabry, 1992) and used for further studies.
2.3 TLC bioautography analysis
The flavonoid extract of H. rosa sinensis petals was screened for its antioxidant capacity by TLC bioautography method (Bahman et al., 2014) using Toluene: Acetone: Formic acid in the ratio of 6:6:1 as the developing solvent mixture.
2.4 GC–MS analysis
The flavonoid extract was chemically profiled by GC–MS analysis. Interpretation on mass spectrum of GC–MS was done using the database of National Institute Standard and Technology (NIST). The mass spectrum of the unknown component was compared with the known components stored in the NIST library. The molecular weight of components can be determined.
2.5 Isolation of flavonoid compounds from H. rosa sinensis by silica gel G-60 column chromatography
The flavanoid compounds were isolated from H. rosa sinensis by Silica gel- G chromatography by the standard procedure of Sasidharan et al, 2011. The individual fractions (C3, C4 and C5) were collected and concentrated. The desired fractions were tested for its purity by TLC and kept for further use.
2.6 Antioxidant assays for compounds (C3, C4 and C5) from Hibiscus rosa sinensis
Various antioxidant assays were done on the isolated compounds using standard procedures (Re et al., 1999, Athukorala et al., 2006, Dinis et al., 1994, Tripathi and Pandey, 1999; Ruch et al., 1989).
2.7 DNA damage protector activity
The protective efficacy of the extracts against DNA damage was assessed on pBR322 plasmid DNA. The plasmid DNA was first oxidized with hydrogen peroxide and further treated with UV rays in presence of extracts and tested on 1% agarose gel according to a standard protocol (Russo et al., 2000).
2.8 General sophisticated analytical experimental methods for the characterization of isolated compounds
The characterization of Isolated compound can be determined using IR and NMR spectroscopy. The molecular weight of compound can be identified using Mass Spectroscopy.
3 Results and discussion
3.1 TLC bioautography of flavonoid extract from the petals of Hibiscus rosa sinensis
The antioxidant potential of H. rosa sinensis, was assessed by TLC bioautography method. The compounds with radical scavenging capacity were determined in situ with ABTS reagent, after separation on TLC plates and was visualized with visible light. The results revealed that yellowish bands which appeared on the green background were represented as antioxidants with Rf values 0.83, 0.64 and 0.61 respectively. In this method, antioxidant molecules present in the extract can effectively scavenge ABTS•+ radical cations by providing hydrogen atom or electron donation (Re et al., 1999).
3.2 Analysis of flavonoid rich fractions by GC–MS
The GC–MS analysis of the extract further evaluates its chemical profile and showed the presence of phenolic compounds (Table 1). Most cinnamic acid derivatives with phenolic hydroxyl groups were reported with their free radical scavenging properties and have several health benefits (Sova 2012).
S. No.
Compound
Retention Time (min)
Molecular weight
Major peaks
1
2,6 di hexadecanole
12.12
652
256, 239, 213, 153
2
Geranyl isovalerate
13.38
196
238, 183, 168
3
2,6,dimethoxy phenol
14.95
198
407, 224, 143
4
4-Propylphenol
18.97
136
326, 279, 183
3.3 Isolation of compounds from Hibiscus rosa sinensis by silica gel G-60 column chromatography
The dried petals of H. rosa sinensis was extracted with an aqueous MeOH and partitioned with ethyl acetate and then concentrated. The residue was suspended in ethyl acetate, and further partitioned with n-hexane, CHCl3 and n-butyl alcohol. The fractions were collected individually and tested for its purity by TLC. The C3, C4 and C5 compounds were isolated successfully. A correlated finding with present study was found with previous report on isolation of naringenin flavonoid compound from H. rosa-sinensis flower extract which exhibited cytotoxic activity against Human lung non-small cell adenocarcinoma (A549) cell line (Ragasa and Leslie 2011).
3.4 Antioxidant potential of the isolated compounds from H. rosa sinensis
The antioxidant activity of the isolated compounds from the petals of Hibiscus rosa sinensis are carried out by various in vitro antioxidant systems as described below.
3.4.1 ABTS free radical scavenging activity of isolated compounds
ABTS assay revealed the inhibitory effect of isolated compounds C3, C4, C5 and ascorbic acid (Table 2). The EC50 values of these isolated C3, C4 and C5 compounds and the standard were 4.34 μg/ml, 3.30 μg/ml, 2.82 μg/ml and 6.98 μg/ml respectively which specify the effective ABTS scavenging activity. The isolated compounds exhibited radical scavenging activity in the order C5 > C4 > C3. The glycosides can effectively generate hydrogen radicals due to the presence of free hydroxyl groups and it has the ability to quench free radicals (Rice-Evans et al., 1996).
Anti-oxidant assays
Concentration (µg/ml)
Vitamin C
Compound C3
Compound C4
Compound C5
ABTS scavenging activity
5
44.09 ± 1.59
55.55 ± 4.83
59.20 ± 3.13*
63.19 ± 3.13
10
52.77 ± 5.06
64.23 ± 2.34
69.78 ± 4.16
76.55 ± 3.75
15
71.52 ± 1.30
77.94 ± 3.39
79.94 ± 5.85*
83.67 ± 4.21
20
81.42 ± 4.72
83.21 ± 4.61
85.81 ± 6.82*
87.32 ± 5.51
EC50 Value
6.98
4.34
3.30
2.82
Lipid peroxidation Scavenging activity
5
29.88 ± 5.15
32.90 ± 3.66
36.59 ± 4.59
52.89 ± 13.41
10
43.43 ± 5.64
49.63 ± 4.22#
62.95 ± 4.03
73.43 ± 5.76
15
52.83 ± 6.30
60.67 ± 3.55#
71.04 ± 13.52
79.41 ± 5.00
20
71.81 ± 7.32
82.64 ± 5.34
87.14 ± 2.48
91.32 ± 3.56
EC50 Value
11.32
9.08
7.25
4.70
Metal Chelating activity
5
21.22 ± 2.36*
35.05 ± 2.59
39.30 ± 3.06
48.89 ± 3.06**
10
40.09 ± 3.09
58.64 ± 2.12
66.97 ± 4.32
70.12 ± 2.59**
15
58.33 ± 2.59*
70.27 ± 1.70
72.32 ± 4.53
77.98 ± 2.88*
20
68.07 ± 2.42
81.91 ± 1.66
82.38 ± 2.59
87.10 ± 3.13*
EC50 Value
12.24
7.78
6.65
5.22
Nitric Oxide scavenging activity
5
23.13 ± 0.80
25. 58 ± 1.81*
31.63 ± 1.94
36.19 ± 3.50*
10
45.43 ± 2.55*
56.68 ± 1.91
67.72 ± 6.69*
70.59 ± 3.02*
15
60.93 ± 1.11*
74.73 ± 2.64*
78.02 ± 3.13
85.34 ± 2.08*
20
80.25 ± 0.96
84.92 ± 1.60
85.13 ± 2.07*
92.14 ± 3.71*
EC50 Value
10.47
8.63
7.32
6.57
Superoxide scavenging activity
5
19.98 ± 0.97
24.43 ± 1.70*
28.39 ± 1.58
30.58 ± 1.94*
10
38.83 ± 3.28**
44.74 ± 1.82
50.32 ± 1.70
56.63 ± 1.94*
15
56.63 ± 1.09**
62.45 ± 1.82
66.36 ± 1.70*
71.11 ± 2.31*
20
77.94 ± 2.97
86.88 ± 1.35*
90.44 ± 2.06*
93.52 ± 1.57*
EC50 Value
11.67
9.94
8.97
8.10
Hydrogen Peroxide Scavenging activity
5
27.66 ± 2.38
43.98 ± 3.57*
46.59 ± 5.47
50.10 ± 3.76
10
33.10 ± 5.28*
50.79 ± 4.42*
60.65 ± 3.91*
70.29 ± 6.33
15
61.41 ± 4.22
70.62 ± 3.26
75.39 ± 2.55*
80.83 ± 4.25*
20
65.86 ± 6.56*
82.99 ± 3.11
81.06 ± 5.16
91.13 ± 2.02*
EC50 Value
12.32
7.16
5.96
5.19
3.4.2 Reducing power assay of isolated compounds
Reducing power of isolated compounds was determined spectrophotometrically results in the formation of Perl’s Prussian blue colored complex. Fig. 1 showed the reductive ability of the isolated compound in a dose dependent manner. Reducing power of the C5 compound was significantly (p < 0.05) higher than the Compounds C4 and C3 (Table 2).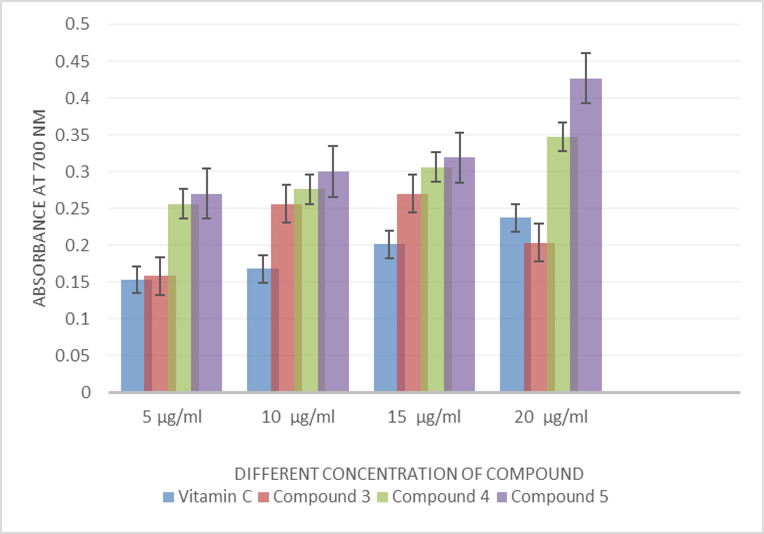
Effect of Isolated compounds on reducing power activity of petals of Hibiscus rosa sinensis.
3.4.3 Lipid peroxidation assay of isolated compounds
The isolated compounds of H. rosa sinensis tested for inhibition of the lipid peroxidation using standard method. The EC50 values for Lipid peroxidation of Isolated compounds C3, C4, C5 and ascorbic acid were found to be 9.08 μg/ml, 7.25 μg/ml, 4.70 μg/ml and 11.32 μg/ml which indicates the efficient lipid peroxidation activity (Table 2).
3.4.4 Metal chelating activity of isolated compound
Metal chelating activity of isolated compounds was determined using standard procedure. Table 2 shows the chelating effect of the isolated compounds of H. rosa sinensis on ferrous ions. At 20 μg/ml, the chelating effect of the C5 compound could reach to 87.10%. However, vitamin C and other compound C3, C4 showed lowest. The results inferred that the C5 compound has excellent Fe2+ chelating effect and has the ability to protect against oxidative damage. A phenolic constituent has ability to inhibit free radicals, is mainly due to the presence of their phenolic hydroxyl groups (Marijana et al., 2016).
3.4.5 Nitric oxide radical scavenging assay of isolated compounds
The nitric oxide radical scavenging activity of the isolated compounds of H. rosasinensis were detected and compared with the standard ascorbic acid. The highest activity was to be found in C5 92.14% with an EC50 value of 6.57 μg/ml. However, ascorbic acid exhibited maximum percent inhibition of 80.25%, with an EC50 value of 10.47 μg/mL (Table 2). The scavenging activity of the isolated compounds against nitric oxide was detected by its ability to inhibit the formation of nitrite through direct competition with oxygen and oxides of nitrogen in the reaction mixture (Pacher et al., 2007).
3.4.6 Superoxide scavenging activity of isolated compounds
Superoxide scavenging activity of isolated compounds was performed using ascorbic acid as standard. The EC50 for Compound C3, C4 and C5 were 9.94 μg/ml, 8.97 μg/ml and 8.10 μg/ml respectively and ascorbic acid is 11.67 μg/ml (Table 2). The secondary metabolites possess wide spectrum of biological activities and also protects the cells from colossal oxidative damage (Ghafar et al., 2010).
3.4.7 Hydrogen peroxide scavenging activity of isolated compounds
In this assay, the isolated compounds C3, C4, and C5 exhibited dose-dependent hydrogen peroxide scavenging activities at concentrations ranging from 5 to 20 μg/mL. The scavenging effects of the isolated compounds were shown in Table 2, and the EC50 values of the samples were ranged from 7.16 to 5.19 μg/ml. Based on the comparison of the EC50 values, the order of the hydroxyl radical-scavenging activity was found to be C5 > C4 > C3.The similar result has been reported in the methanol extract of Muccuna pruriens seeds (Gulcin et al., 2004).
3.5 DNA protector activity of C5 compound isolated from petals of H. rosa sinensis
DNA damage protection activity of the isolated compound C5 was done using the modified method of (Russo et al., 2000). The result shows the electrophoretic pattern of DNA after UV-photolysis of H2O2 in the absence and presence of isolated compound C5 from Hibiscus rosa sinensis. DNA derived from pBR322 plasmid showed two bands on agarose gel electrophoresis; the scDNA (supercoiled circular DNA) ocDNA (open circular form). The UV irradiation of DNA in the presence of H2O2 results in the cleavage of ocDNA to a faint linear DNA on the agarose gel. The results indicated that two major bands super coiled DNA and open circular DNA was protected by the presence of different concentration of compound C5 isolated from the petals of Hibiscus rosa sinensis (Fig. 2).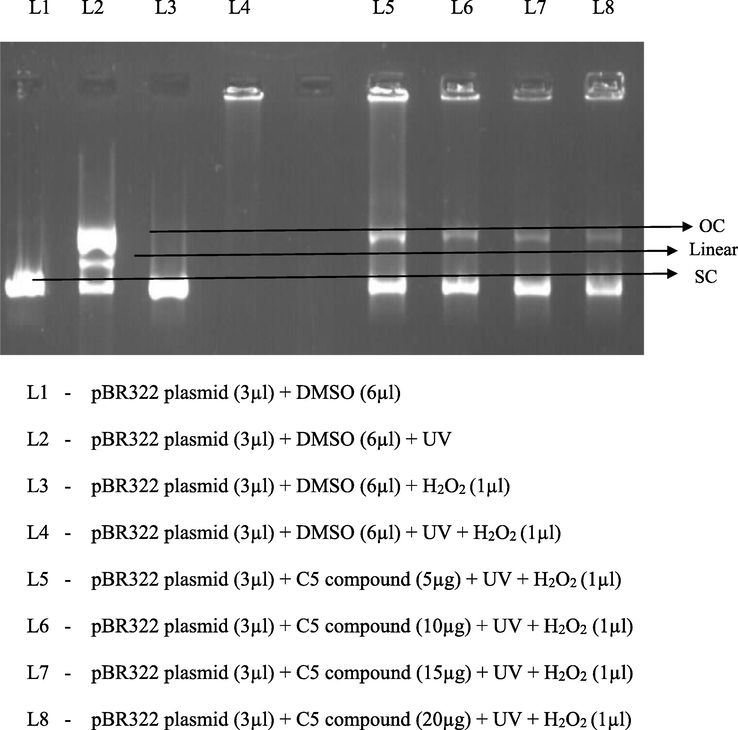
DNA Protector Activity of C5 Compound isolated from petals of Hibiscus rosa sinensis.
The UV-photolysis of H2O2 generates hydroxyl (OH) radicals, which causes severe oxidative damage. The hydroxyl radical (OH) bound to DNA leads to deoxysugar fragmentation, base modification and strand breakage (Chaudhary et al., 1996). The result shows the electrophoretic pattern of DNA after UV-photolysis of H2O2 in the absence and presence of isolated compound C5 from Hibiscus rosa sinensis.
3.6 Characterization of C5 compound from Hibiscus rosa sinensis by infrared spectroscopy
The isolated C5 compound structure was determined by spectral analysis such as IR, NMR and Mass spectroscopy. IR has proven to be a valuable tool for the characterization and identification of functional groups present in the isolated compound C5. The isolated compound C5 had IR absorptions at 3495, 3389, 3182 cm−1 (OH), 2959, 2881 cm−1 (C–H), 1697, 1687 cm−1 (C=O), 1649 cm−1 (–C=C–), 1462 cm−1 (C–H) and 1438 cm−1 (C–C) (Table 3). The peaks revealed the number of functional groups that have a great significant towards medicinal prospects of Hibiscus rosa sinensis (Fig. 3).
Frequency cm−1
Bond
Functional groups
3495 (S)
3389 (b)
3182 (b)OH Stretch, H bonded
Alcohols, Phenols
2959 (m)
2881 (m)C–H Stretch
Alkanes
1697 (s)
1687 (s)C=O Stretch
α,β unsaturated aldehydes, ketones
1649 (m)
–C=C– Stretch
Alkenes
1600
C–C Stretch
Aromatics
1462 (m)
C–H bend
Alkanes
1438 (m)
C–C Stretch
Aromatics
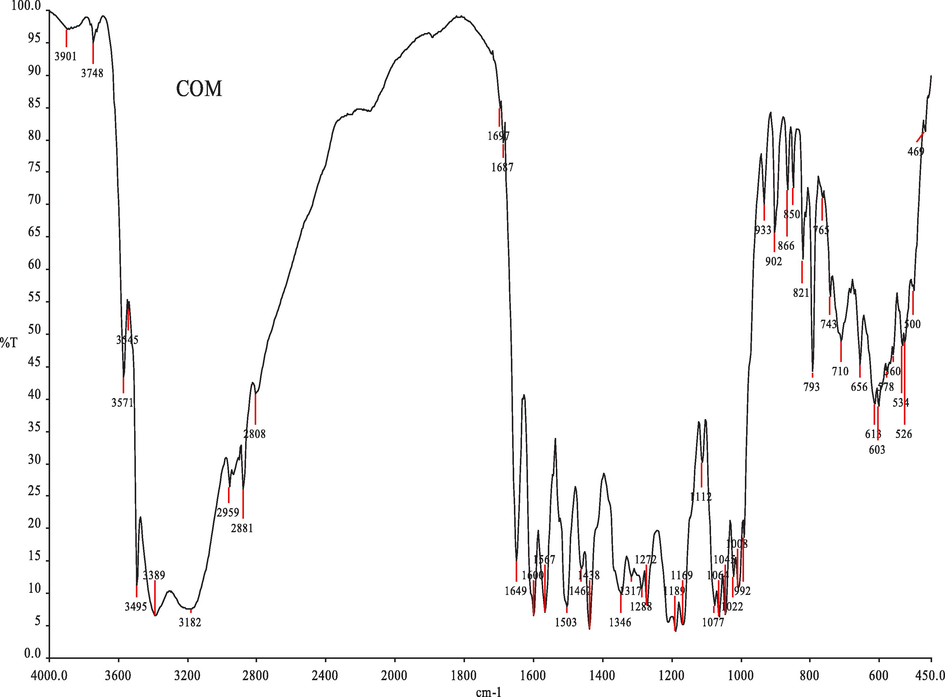
Characterization of C5 compound from Hibiscus rosa sinesis by Infrared Spectroscopy.
3.7 Characterization of C5 compound from Hibiscus rosa sinensis by NMR spectroscopy
The NMR spectra of the isolated compound recorded at 400 MHz on Bruker AV400 spectrometer in DMSO solvent using TMS as internal standard. The molecular formula of the compound is C21H20O14. The proton Nuclear Magnetic Resonance (1H NMR) spectrum of compound exhibits the signals at (δ ppm) value as singlet at 6.53(s, 2H), 5.89(s, 1H) corresponds to aromatic C–H protons of the C-16, C-12, C-2 and C-25 protons respectively. A singlet indicated 5.60 (s, 1H) related to methane proton of the C-25. The six singlet peaks appears at the δ ppm 4.07, 4.03, 3.79, 3.70, 3.36 (6H) corresponds to C-19, C-24, C-20, C-18, C-29 and C-27 hydroxyl protons respectively. The two doublets appear at 3.71 and 3.46 (d, 2H) related to methylene protons of C-34. The three singlet appears at 1.89, 1.58 and 1.45 (s, 3H) related to the hydroxyl group of C-32, C-31 and C-30 respectively. The singlet observed at 0.66 and 0.62 corresponds to C-21 and C-35 (s, 2H) of hydroxyl protons respectively due to the presence near to electron withdrawing groups of oxygen atoms. The two singlets appear at 0.10 and 0.01 (s, 2H) related to hydroxyl groups of the C-22 and C-23 respectively.
The 13CNMR shows that the C-9 appears at 157.05 deshielded to near to high electro negative atom of oxygen atom. The peaks appear at 153.30, 152.08 and 147.08 corresponds to the carbons of C-3, C-1 and C-5 respectively. C-13 and C-15 signals appear at values 146.57 and 146.57 respectively due to hydroxyl carbon atoms. C-14 and C-6 hydroxyl carbon appears at 136.34 and 129.65 respectively. The peaks correspond to 108.35, 108.35 and 102. 09. The carbon atom of the C-12, C-16 and C-4 respectively. C-25, C-2 shielded to chemical shift values corresponds to 101.59 and 97.83 respectively. 76.54, 75.24, 73.51 and 70.33 corresponding the chemical shift values of C-27, C-29, C-24 and C-28 respectively. Methylene carbon highly shielded at 62.01 related to C-34 carbon atom.
3.8 Characterization of C5 compound from H. rosa sinensis by mass spectroscopy
The chemical ionization technique is used to record Mass spectra on SHIMADZU spectrometer. The molecular formula of the compound is C21H20O14. The Calculated and Observed molecular weight of the compound shows MS (m/z) 496.48 respectively. All the above FTIR, 1H NMR, 13C NMR and Mass spectra of the compound which clearly proved the structure of the isolated compound (Hibiscetin-3-glucoside) (Fig. 4).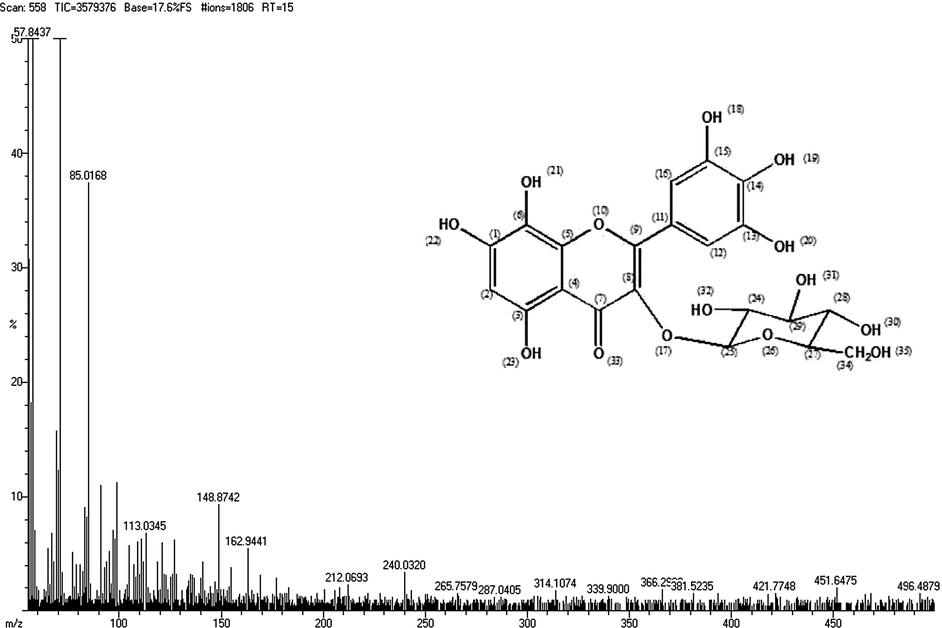
Characterization of C5 compound from Hibiscus rosa sinesis by Mass Spectroscopy.
4 Conclusion
In this study the flavonoid compounds of Hibiscus rosa sinensis petals were investigated with various antioxidant systems. The results indicated that C5 compound of H. rosa sinensis possessed phenolic and flavonoid contents and showed excellent antioxidant activities comparing to standard ascorbic acid. The isolated compound can be used to scavenge free radicals and also prevent formation of toxic products as well as maintain the shelf life of food and pharmaceuticals. From the spectral analysis study, clearly proved the structure of the flavanoid compound Hibiscetin-3-glucoside (C21H20O14) from the petals of Hibiscus rosa sinensis and can be also used as an effective anticancer drug in the field of cancer therapy. Further investigation to be done to study the cytotoxic effect of isolated compound Hibiscetin-3-glucoside against cancer study.
Acknowledgments
The authors are grateful to Indian Institutes of Technology (IIT), Department of Chemistry, Chennai for NMR study and I would like to express my sincere thanks to Gloris Biomed Research Centre for their excellent technical assistance and support throughout my study. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this Research Group No. (RG-1437-024).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol.. 2006;44:1065-1074.
- [Google Scholar]
- TLC-bioautography and GC-MS analyses for detection and identification of antioxidant constituents of Trachyspermum copticum essential oil. Iran. J. Pharm.. 2014;13:127-133.
- [Google Scholar]
- Characterization of an N6-oxopropenyl-2'-deoxyadenosine adduct in malondialdehyde-modified DNA using liquid chromatography/electrospray ionization tandem mass spectrometry. Carcinogenesis. 1996;17:1167-1170.
- [Google Scholar]
- Action of phenolic derivatives (acetaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys.. 1994;1994(315):161-169.
- [Google Scholar]
- Medicinal Plants of Bangladesh with Chemical Constituents and Uses (second ed.). Dhaka: Asiatic Society of Bangladesh; 2003.
- Flavonoid, hesperidine, total phenolic contents and antioxidant activities from Citrus species. Afr. J. Biotechnol.. 2010;9:326-330.
- [Google Scholar]
- Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J. Ethnopharmacol.. 2004;90:205-215.
- [Google Scholar]
- The Flavonoids Advances in Research. London, New York: Chapman and Hall; 1992.
- Evaluation of metal concentration and antioxidant, antimicrobial and anticancer potentials of two edible mushrooms Lactarius deliciosus and Macrolepiota procera. J. Food Drug. Anal.. 2016;3:477-484.
- [Google Scholar]
- Nitric oxide and peroxynitrite: in health and disease. Physiol. Rev.. 2007;87:315-424.
- [Google Scholar]
- Folk medicine of NR Pura Taluk in Chikamaglur district of Karnataka. Indian J. Tradit. Know.. 2010;9:55-60.
- [Google Scholar]
- Compendium of Indian Medicinal Plants. Lucknow and New Delhi: Central Drug Research Institute and Publications and Information Directorate; 1991. p. :833.
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med.. 1996;20:933-956.
- [Google Scholar]
- Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003-1008.
- [Google Scholar]
- Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol.. 2000;16:91-98.
- [Google Scholar]
- Extraction, isolation and characterization of bioactive compounds from plants' extracts. African J. Trad., Complement. Alter. Med.. 2011;8:1-10.
- [Google Scholar]
- MAPK signaling in neurodegeneration: influences of flavonoids and of nitric acid. Neurobiol. Aging.. 2002;23:861-880.
- [Google Scholar]
- Effect of Hibiscus rosa sinensis extract on hyperprolification and oxidative damage caused by benzoyl peroxide and ultraviolet radiations in mouse skin. Basic Clin. Pharmacol. Toxicol.. 2004;95:220-225.
- [Google Scholar]
- Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini. Rev. Med. Chem.. 2012;12:749-767.
- [Google Scholar]
- Role of alcoholic extract of shoot of Hypercium perforatum (Linn) on LPO and various species of free radicals in Rats. Indian J. Exp. Biol.. 1999;37:567-571.
- [Google Scholar]
- Effect of ethanolic extract of Hibiscus rosa sinensis L., flowers on hair growth in female wistar rats. Der. Pharm. Lett.. 2011;3(4):258-263.
- [Google Scholar]







