Translate this page into:
Antimicrobial potencies of selected native African herbs against water microbes
⁎Corresponding author. firstrebby@gmail.com (Adeyemi O. Adeeyo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Purpose
Phyto-active components of Zanthoxylum zanthoxyloides and Gongronema latifolium was investigated against microbial contaminants in water for possible novel antimicrobial usage in water treatment.
Method
Crude ethyl acetate and chloroform fractions of Zanthoxylum zanthoxyloides and Gongronema latifolium were prepared and screened for antimicrobial and phytochemical profiles using standard methods.
Results
Crude extracts of the different plant examined selectively comprised saponins, tannins, reducing sugars, anthraquinones, flavonoids, terpenoids, phlobatanins and alkaloids. All plant extracts showed broad spectrum antibiosis against E. coli, P. aeruginosa, Klebsiella sp, S. pneumoniae and B. cereus, as well as A. niger, A. flavus, Trichoderma sp and Candida sp. Chloroform extracts compared well than ethyl acetate extracts.
Conclusion
This work represents the first report to direct the possible application of Zanthoxylum zanthoxyloides and Gongronema latifolium to potential application in water. Overall results revealed that antimicrobial activities are dose and plant dependent. Noteworthy is the comparatively greater antimicrobial activity of crude extracts over commercial antibiotics used at the concentration of extracts tested. These plants can therefore serve as a potent source of natural water disinfectant.
Keywords
Antimicrobial
Bio-disinfection
G. latifolium
Water
Z. zanthoxyloides
1 Introduction
The importance of water as source of life and purification is well inscribed in history; Hindus, sprinkle water on new born child and believed that “The iota of life is created in water” (Hinduism: Asthagarideyam, Atharvaveda); Muslims take drop of holy water from Mecca and hold the view that God gives life to every living thing by means of water (Islam: Quran 21:30); Christians perform baptism and water is usually a fundamental symbol of faith, “Whoever believes in me, stream of living water will pour from within him” (Christianity: John 7:38). Excavations from the Neolithic time have found a striking correspondence between settlements and wells (Skandhan et al., 2011). The importance of ample water and quality is thus apparent in human history and beliefs (Jadhav, 2014). The Biblical ancient book of Exodus (15:23–27) is the oldest historical reference to phyto-purification of water; “So the people grumbled against Moses, saying, “What are we to drink?” “Then Moses cried out to the Lord; and the Lord showed him a piece of wood. He threw it into the water, and the water became fit to drink” (Jadhav, 2014). Human settlements, civilisation and religion have all evolved round water (Jadhav, 2014).
Potable water remains a fundamental human need and right (Megersa et al., 2014) with Individuals entitled to sufficient, safe, accessible and affordable water (WHO, 2003; Skandhan et al., 2011). Adequate treatment is important to prevent the occurrence of harmful contaminants in water (Hyung and Kim, 2009). However, in most developing nations, processing of water in disinfection units is rarely adequate resulting to increased exposure to waterborne diseases and fatal consequences in many rural societies in developing African nations (Shaheed et al., 2009; Dubreuil, 2013; Megersa et al., 2014; Jones and Bridgeman, 2017) with children and the elderly mainly affected (WHO, 2006). It has been recommended that household water treatment (WHO, 2007) is a way forward to combatting drinking water problems (WHO, 2011). Plant-based water treatment solutions is evolving as a feasibly cheap technology (Table 1). Since Africa produces good percentage of medicinal plants (Megersa et al., 2014), it is vital to understand the application of African indigenous plant extracts in water treatment, antimicrobials and as disinfectants. Two essentially important native African plants worthy of further investigations are Zanthoxylum zanthoxyloides and Gongronema latifolium (Jones and Bridgeman, 2017).
Plant
Part
Description
Reference
Application
M. oleifera.
Seed
Tree
Koehn and Carter (2005)Gebremichael et al. (2005)
Coagulation and disinfection
Opuntia ficus indica.
D. Lablab.
LeavesFruit
Shrub
HerbShilpa et al. (2012)Zhang et al. (2006)
Coagulation and disinfection
Cicer arietinum L.
Seeds
Herb
Choubey et al. (2012)
Coagulation and disinfection
Aloe barbadensis
Hibiscus sabdarifa L.
Jatropha curcas L.Citrus aurantifoliaGarcinia kola Heckel
Carica papaya L.
SeedsCalyxSeedFruitSeedSeed
HerbHerbTreeTreeHerbTree
Yongabi et al. (2011a)Yongabi et al. (2011b)
Coagulation and disinfection
Parkinsonia aculeate
Vigna unguiculata
Seed
SeedTree
HerbMarobhe and Gunaratna (2012)
Coagulation and disinfection
Cyamopsis tetragono
Seed
Herb
Pritchard et al. (2009)
Coagulation and disinfection
Manihot esculenta
Root
Shrub
Vara (2012)
Coagulation and disinfection
Solanum incunum L.
Leaf
Shrub
Kihampa et al. (2011)
Disinfection and Coagulation
P. vulgaris L.
Seed
Herb
Sciban et al. (2006)
Coagulation
Zanthoxylum species are deciduous shrubs and trees of the family Rutaceae which comprise 250 species used as sources of pharmaceutical and cosmetics raw materials. It is externally applied to abscesses, ulcers, swellings, haemorrhoids and wounds. Rheumatic pain, fever, malaria, tuberculosis, oedema, sickle cell anaemia and paralysis have all been maintained using the plant material (Nacoulma, 1996). Other traditional uses include mouth freshening and tooth care (Gaur, 1999; Negi et al., 2011). Bark sap, root and stem bark powder are applied in eye infection and whooping cough treatment (Arbonnier, 2004). Water preparation of the stem, root and bark is also applied for cancer treatment and probably in psycho-active intervention for numerous magico-religious uses (Arbonnier, 2004). Diverse phytochemicals have been reported as constituents of the plants (Ngassoum et al., 2003; Ouattara et al., 2004; Fogang et al., 2012; Wouatsa et al., 2013).
G. latifolium (Asclapiadaceae) is a soft-stemmed palatable perennial plant (Okafor, 2005). It contains saponins, essential oils, alkaloids, tannins as well as other phytochemicals (Schneider et al., 1993; Morebise and Fafunso, 1998; Morebise et al., 2002; Essien et al., 2007). Water and alcoholic extracts from this plant exhibit hypoglycemic, hypolipidemic, antioxidative and anti-inflammatofry properties (Morebise et al., 2002; Ogundipe et al., 2003; Ugochukwu and Babady, 2003; Ugochukwu et al., 2003). Hot water treated fruits of Gongronema latifolium are eaten as laxative in soup preparation (Akuodor et al., 2010). Extract of the leaf in ethanol assists in treatment of viral hepatitis, bilharziosis, and as an all-purpose antimicrobial agent (Eja et al., 2011). Numerous 17β-marsdenin derivatives, and several other phytochemicals have been found in the plant. Essential oil of the leaves is mainly composed of linalool, (E)-phytol and aroma dendrene hydrate (Oshodi et al., 2004; Nenaah and Ahmed, 2011). No water treatment application of the above plants have been reported till date.
The aim of this report is to assess the disinfection potential of the two selected native African plant species on inherent bacterial and fungal water contaminants in order to have insight into their properties, particularly, in water purification in African countries where clean water accessibility is of major concern.
2 Materials and methods
2.1 Plant sampling and microbial culture collections
Zanthoxylum zanthoxyloides and Gongronema latifolium samples were collected from plant material market (8.1294oN, 4.2005oE) Ogbomoso, Oyo State, Nigeria. Plants were washed, air dried and grinded to powder, then sieved to get a fine powder. Dry running through published literatures was used in identification of emerging microbial contaminants in water and identified bacteria species (P. aeruginosa, Klebsiella sp., E. coli, S. pneumonia, B. cereus) and fungal isolates (A. flavus, A. niger, Trichoderma sp and Candida sp.) were sourced from local strains collection from Culture Bank, Microbiology Unit, Pure and Applied Biology Department, LAUTECH, Ogbomoso, Nigeria.
2.2 Preparation of samples
50 g of dried and grinded samples of Zanthoxylum zanthoxyloides and Gongronema latifolium were extracted separately by maceration using 150 ml of ethyl acetate and chloroform as extraction solvent for 48 hrs. The solvent fractions were separated using vacuum filtration aided by a sterile Whatmann No 1 filter paper and filtering crucible. The filtered extracts were then dried with the help of a freeze drier and stored in sterile polypropylene airtight container at 4 °C or dispensed in appropriate sterile dissolving solution when required.
2.3 Antimicrobial activity, preparation of antimicrobial disc and sensitivity test
Crude extracts of G. latifolium and Z. zanthoxyloides eluted by ethyl acetate and chloroform and freeze dried were prepared into solutions of different concentrations ranging between 25 and 500 mg/ml by dissolving appropriate amount of crystallised extracts in sterile deionised water. About 20 µl of extract solution corresponding to a range of (0.5 mg – 10 mg) was then transferred on sterile 6 mm diameter sterile paper discs prior to antimicrobial sensitivity testing by disc diffusion method as described by (Adebayo et al., 2014; Lateef and Adeeyo, 2015). Briefly, 1 to 2 colonies of each bacteria culture were introduced into nutrient broth, and incubated at 37 °C for 24 hrs. A 1 to 50 dilution of the stock culture was then adjusted to tune of 0.5 McFarland standard (1.0 × 106 cfu/ml). About 100 µl of the test microbes were then swabbed over the agar plate surface using surface spread method. Treated paper discs were afterward arranged serially and radially but firmly unto seeded agar plates. The plates were afterward incubated at 37 °C for 24 hrs. Fungal plates were incubated at room temperature (27 ± 2 °C) for 48 hrs. The degree of microbial susceptibility was determined by measuring the visible diameter of inhibition on microbial growth after incubation. Test concentrations of crude extract ranged from 500 and 25 mg/ml. Standard reference commercial antibiotic disc for Gram-negative (streptomycin), Gram-positive (gentamycin) and fungi (nystatin) were used for a comparative study.
2.4 Phytochemical analyses
The screening for plant extract composition was assayed according to standard methods and as modified by Adeeyo et al., 2018. Screening for tannins, saponnin, reducing sugars, alkaloids, terpenoids, flavonoids, steroids, anthraquinones and phlobatannins were carried out on extracted crude products.
2.5 Data analysis
For Data analysis, SPSS, version 19.0 was used in analysing the different means of data obtained. ANOVA test was used to determine the level of significance of the result at various concentrations. Also, comparison of the different solvent extracts (chloroform and ethyl acetate) were made statistically. Antimicrobial efficacy of the extracts were examined against that of standard antibiotics. Zones of inhibition was compared with reference standard to assign the level of activities of extracts.
3 Result and discussion
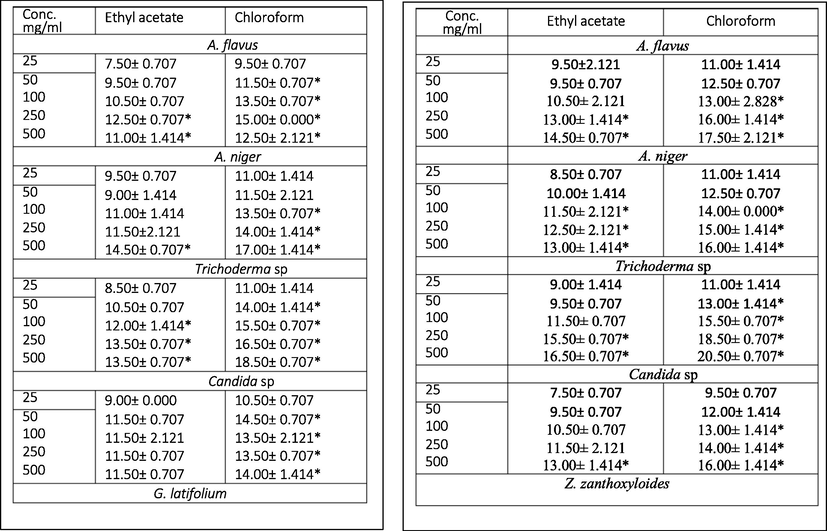
The antimicrobial activities of extracted phytobiotics against bacterial and fungal isolates tested were investigated with extract doses ranging from 25 to 500 mg/ml. Figs. 1–5 shows the zone of inhibition of G. latifolium and Z. zanthzyloides against selected Gram-negative (P. aeruginosa, Klebsiella sp., E. coli), Gram-positive (S. pneumonia, B. cereus) and Table 2 shows the inhibition of the plant materials against fungal isolates (A. flavus, A. niger, Trichoderma sp and Candida sp.) using ethyl acetate and chloroform extracts.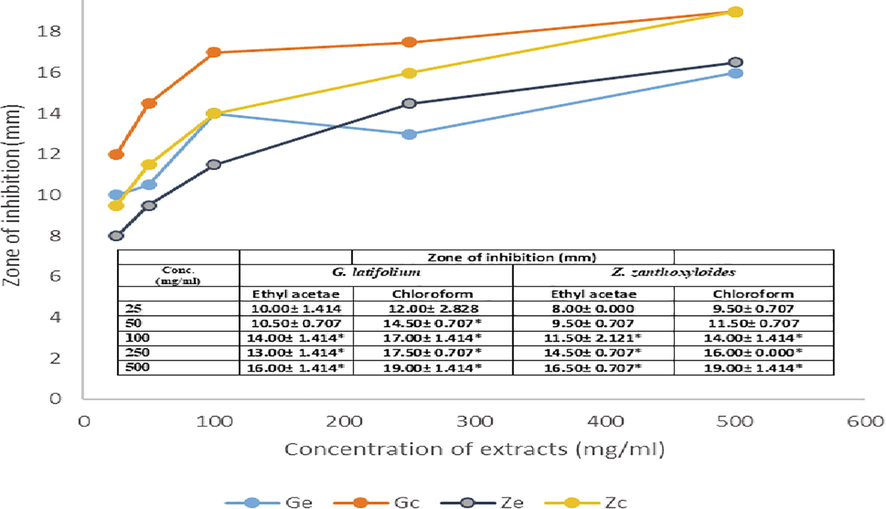
Antibacterial activities of G. latifolium and Z. zanthoxyloides Chloroform and Ethyl acetate extract on P. aeruginosa.
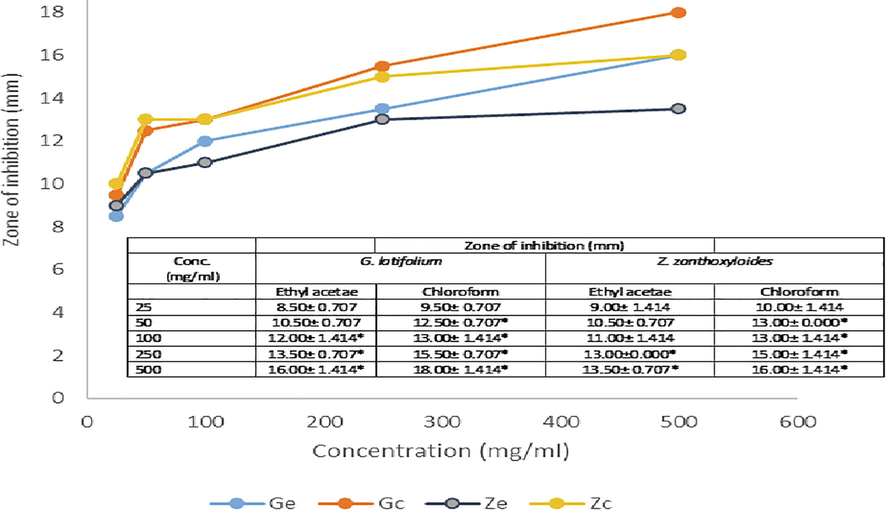
Antibacterial activities of G. latifolium and Z. zanthoxyloides Chloroform and Ethyl acetate extract on Klebsiella sp,
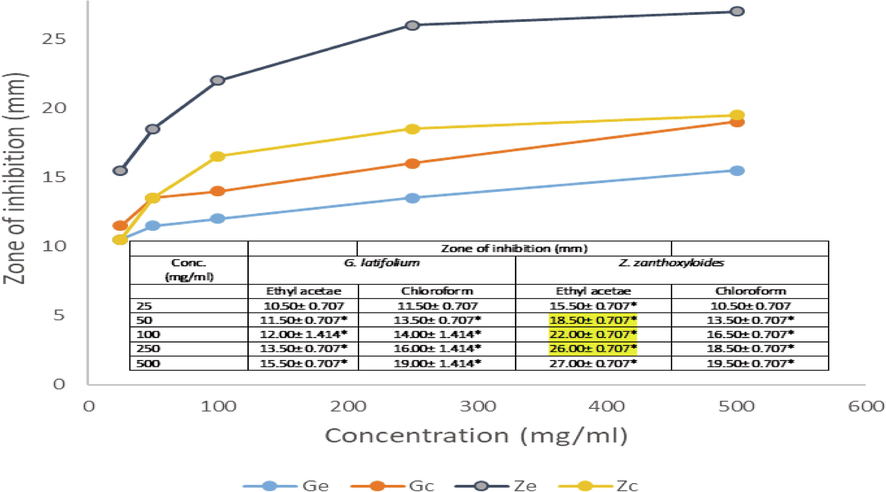
Antibacterial activities of G. latifolium and Z. zanthoxyloides Chloroform and Ethyl acetate extract on E. coli.
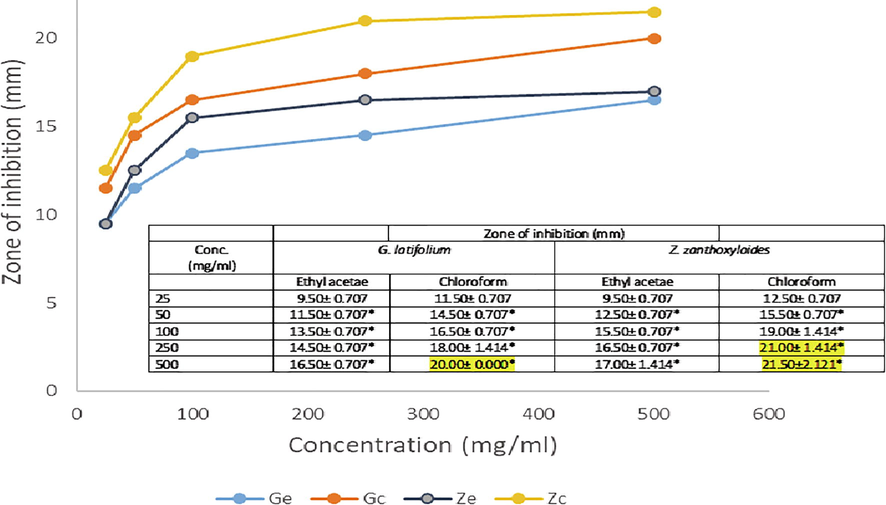
Antibacterial activities of G. latifolium and Z. zanthoxyloides Chloroform and Ethyl acetate extract on S. pneumonia.
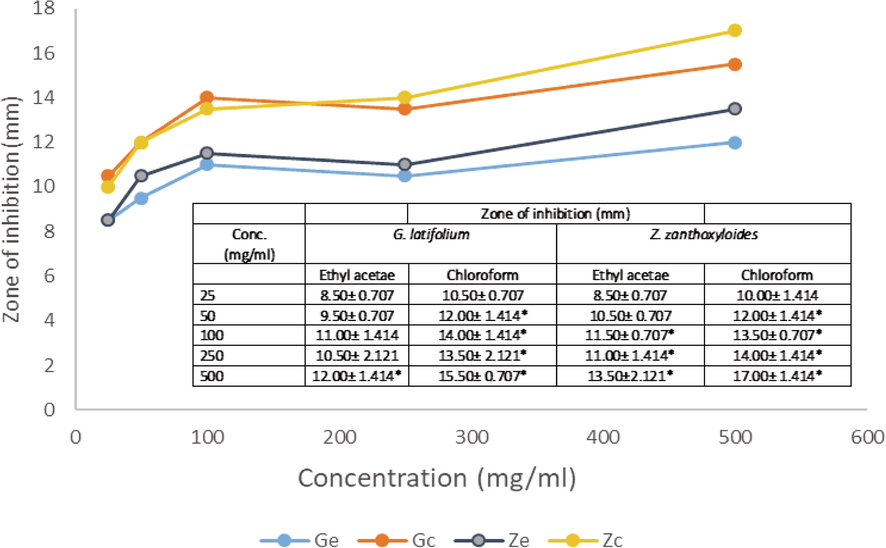
Antibacterial activities of G. latifolium and Z. zanthoxyloides Chloroform and Ethyl acetate extract on B. cereus.
3.1 Dose dependent activities
The degree of antimicrobial activity by measuring the visible diameter of inhibition on microbial growth has been described as strong (23–38 mm), moderate (19–22 mm) and low (12–18 mm) (Ahmad et al., 1999). The dose response to plant extracts can be observed in Figs. 1–5 and Table 2. At the lower doses (25–50 mg/ml), the extracts show low to moderate antimicrobial activities across the various plant extracts irrespective of the extraction solvent. The range of inhibition zone is between 7.5 and 18.50 mm. Noteworthy is the activity of ethyl acetate extract of Z. zythoxyliodes at this dose (18.50 mm; Fig. 1C) against E. coli. At the higher dosage, extracts showed moderate to high antibiosis with a range of 10.5 to 27.0 mm. Noteworthy is the activities of Z. zythoxyloide’s ethyl acetate extract against E. coli (Fig. 1C; 27.0 mm), as well as its chloroform extract against S. pneumoniae (21.50 mm) (Fig. 1D) and Trichoderma sp (Table 2; 20.50). G. latifolium chloroform extract also exhibited appreciable activity against all tested microbes and its activity against S. pneumonia (Fig. 1D; 20.00 mm) is remarkable. These effects are qualitatively alike to that of Shaheed et al. (2009) and Jones and Bridgeman (2017) who reported increase in performances of herbal extracts at higher dosages against microbial pollutants of water.
3.2 Comparative phytobiotic activities (with commercial antibiotics)
The abilities of phytoactive extracts of plants materials investigated as compared with commercial antibiotics were investigated. Plant extracts investigated performed beyond the activities of commercial streptomycin, gentamycin and nystatin within selected concentration range investigated. Statistical analysis revealed notable significant differences from 25 mg/ml and above, but mostly at higher concentrations. The comparative antibiosis of commercial antibiotics, as well as minimum and maximum extract concentrations against tested microbes were presented in Tables 3 and 4. The results revealed that potent phytoactive formulations of the plant materials tested can be developed within the range researched in this work, and with better performance than available commercial antibiotics. The advent of such formulations as envisaged in this work may be the dawn of novel technology in achievement of the World Health Organisation (WHO) recommendation of zero faecal count/100 ml water sample (WHO, 1998) especially when developed for water disinfection. Potent antimicrobial activities of plant materials have been reported for different formulations such as antipsoriatic cream with varied combinations of neem, bakuchi, sarsaparilla and daruhaldi which resulted in good anti-inflammatory and antimicrobial results (Mundada et al., 2009); herbal gel comprising Curcuma- longa, Aloe-vera and A. indica resulted in a good antimicrobial activity against the tested microbes in comparison to commercially available antimicrobials (Pandey et al., 2010). In a study where polyherbal ointment was made using methanolic extracts of M. pudica, S. indica A. indica, C. odorata, and assessed for bioactivities, the results showed better antibacterial properties (Rajasree et al., 2012). These reported research findings supported phytoactivities of plant materials as indicated in this work. Herbal extracts of Ocimum samctum and Eucalyptus globules as anti-microbial agents were found to exhibit major activity against P. aeuroginosa, B. subtilis, Escherichia. coli, S. aureus, S. cerevisiae as well as C. albicans and were safer for human usage when compared to reference standards Amphoterecin and Ampicillin respectively (Wani et al., 2013). + values with significant difference from the standard; A, Streptomycin; B, Gentamycin; Min, minimum; Max, maximum. +, values with significant difference; NIS, Nystatin, Min, minimum; Max, maximum.
G. latifolium
Z. zanthoxyloides
Ethyl acetate
Chloroform
Ethyl acetate
Chloroform
P. aeruginosa
STR
7.50 ± 0.707
8.00 ± 1.414
7.00 ± 0.000
8.50 ± 0.707
Min
10.00 ± 1.414
12.00 ± 2.828
8.00 ± 0.000
9.50 ± 0.707
Max
16.00 ± 1.414+
19.00 ± 1.414+
16.50 ± 0.707+
19.00 ± 1.414+
Klebsiella sp
STR
7.50 ± 0.707
8.00 ± 0.000
7.00 ± 0.000
7.50 ± 0.707
Min
8.50 ± 0.707
9.50 ± 0.707
9.00 ± 1.414
10.00 ± 1.414
Max
16.00 ± 1.414+
18.00 ± 1.414+
13.50 ± 0.707+
16.00 ± 1.414+
E.coli
STR
7.50 ± 0.707
9.00 ± 0.000
8.50 ± 0.000
7.00 ± 0.000
Min
10.50 ± 0.707
11.50 ± 0.707
15.50 ± 0.707+
10.50 ± 0.707
Max
15.50 ± 0.707+
19.00 ± 1.414+
27.00 ± 0.707+
19.50 ± 0.707+
S. pneumonia
GEN
7.50 ± 0.707
9.00 ± 0.000
7.50 ± 0.707
8.50 ± 0.707
Min
9.50 ± 0.707
11.50 ± 0.707
9.50 ± 0.707
12.50 ± 0.707
Max
16.50 ± 0.707+
20.00 ± 0.000+
17.00 ± 1.414+
21.50 ± 2.121+
B. cereus
GEN
7.50 ± 0.707
7.00 ± 0.000
7.00 ± 0.000
7.50 ± 0.707
Min
8.50 ± 0.707
10.50 ± 0.707
8.50 ± 0.707
10.00 ± 1.414
Max
12.00 ± 1.414+
15.50 ± 0.707+
13.502.121+
17.00 ± 1.414+
G. latifolium
Z. zanthoxyloides
Ethyl acetate
Chloroform
Ethyl acetate
Chloroform
A. flavus
NIS
7.00 ± 0.000
7.00 ± 0.000
7.50 ± 0.707
8.50 ± 0.707
Min
7.50 ± 0.707
9.50 ± 0.707
9.50 ± 2.121
11.00 ± 1.414
Max
12.50 ± 0.707+
15.00 ± 0.00+
14.50 ± 0.707+
17.50 ± 2.121+
A. niger
NIS
7.50 ± 0.707
7.00 ± 0.000
7.00 ± 0.000
8.50 ± 0.707
Min
9.50 ± 0.707
11.00 ± 1.414
8.50 ± 0.707
11.00 ± 1.414
Max
14.50 ± 0.707+
17.00 ± 1.414+
13.00 ± 1.414+
16.00 ± 1.414+
Trichoderma sp
NIS
7.00 ± 0.000
8.50 ± 0.707
7.50 ± 0.707
8.50 ± 0.707
Min
8.50 ± 0.707
11.00 ± 1.414
9.00 ± 1.414
11.00 ± 1.414
Max
13.50 ± 0.707+
18.50 ± 0.707+
16.50 ± 0.707+
20.50 ± 0.707+
Candida sp
NIS
7.50 ± 0.707
8.50 ± 0.707
7.50 ± 0.707
8.50 ± 0.707
Min
9.00 ± 0.000
10.50 ± 0.707
7.50 ± 0.707
9.50 ± 0.707
Max
11.50 ± 0.707
14.00 ± 1.414+
13.00 ± 1.414+
16.00 ± 1.414+
3.3 Antibacterial activities
The antibacterial activity of the extracts differs with respect to extraction solvents and susceptibility of microbial isolates increases with concentration of extract in solution (Figs. 1–5). Noteworthy is the activities of the extracts against E. coli which is a conventional indicator of water pollution. The activities of extracts tested were all notable against E. coli. Antibiosis of extracts on E. coli showed moderate to high performance at concentrations of 50 to 500 mg/ml. It is, however, notable that significant susceptibility was recorded at a concentration of 25 mg/ml of ethyl acetate extract of Z. zanthoxyloides which was the least concentration tested and may confirm possible potent antimicrobial activities of the investigated plants against groups of coliforms and microbial pathogens in water. The effects of the plant extracts against E. coli, Kelsiella sp and P. aeruginosa indicated the activities of these plant materials against a range of Gram-negative bacteria. For P. aeruginosa, susceptibility to plant extracts increases with concentration of the extracts in solution from 25 to 500 mg/ml. At dosages of 25 and 50 mg/ml, maximum values of 12.0 and 14.50 mm zones of inhibition respectively were observed when compared across all plant materials and extraction solvent use, which are low levels of susceptibility. The susceptibility of P. aeruginosa at this dose is, however, comparable to that of streptomycin. At doses of 100, 250 and 500 mg/ml maximum zones of inhibition recorded with respect to plant material and extraction solvents were 17.00, 17.50 and 19.00 mm respectively. Chloroform extracts of both plants seemed to have effective antimicrobial action against P. aeruginosa. Considering Klebsiella sp., a low level susceptibility to concentrations of extracts at 25 and 50 mg/ml was observed. The minimum inhibition zones recorded at 25 and 50 mg/ml were 8.50 mm and 10.50 mm while the maximum zones were 10 and 13 mm respectively. These were low level antibiosis. However, the activity of chloroform extract of the two plants were significant at 50 mg/ml against Klebsiella sp. All other concentrations of extracts irrespective of solvent material possessed significant antimicrobial activity against Klebsiela sp. Maximum zone was observed at 18.00 mm with chloroform extract of G. latifolium at 500 mg/ml.
S. pneumonia and B. cereus are Gram-positive bacteria, the least antibiosis recorded for S. pneumoniae was 9.50 mm at concentration of 25 mg/ml of G. latifolium ethyl acetate extract while the optimum recorded was 20.00 mm (G. latifolium) and 21.50 mm (Z. zanthozyloides) from chloroform extract. Highest activity of plant extract against B. cereus was found to be 17.00 mm from chloroform extract of Z. zanthoxyloide at concentration of 500 mg/ml. These results also indicates the ability of extract to inhibit the growth of Gram-positive bacteria. The susceptibility of all tested microbial isolates to the plant extracts is therefore a clear indication that plants studied would be good candidates for disinfection product development.
3.4 Antifungal activities
Susceptibility of the fungal isolates to tested plant extracts were observed. The maximum antifungal activity of the extracts recorded were in the order of 20.20 mm (Zanthoxylum chloroform extract against Trichoderma at 500 mg/ml) > 17.50 mm (Zanthoxylum chloroform extract against A. flavus at 500 mg/ml) > 17.00 mm (Gongronema chloroform extract against A. niger at 500 mg/ml) > 16.00 mm (Zanthoxylum chloroform extract against Candida sp at 500 mg/ml). Activities of extracts were significant from a concentration of 50 mg/ml to 500 mg/ml and show low to moderate antimicrobial activities (Table 2).
The overall results revealed that antimicrobial activities of the plant extracts are dose dependent with comparatively greater efficacy than commercial antibiotics at the concentration of extracts tested. E. coli was the most susceptible microbial isolate tested representing the potentials of the extracts against a group of coliform which are important indicators of microbial pollution in water. Other microbial isolates also recorded susceptibility to extracts tested to varying degrees. It was observed in this finding that microbes tested were most susceptible to chloroform extract of Z. zanthoxyloides and G. latifolium except for the activity of ethyl acetate extract of Z. zanthoxyloide against E. coli. Results of phytochemical screening revealed the varied presence of tannins, flavonoids, terpenoids, anthraquinones, alkaloids and saponins, (Table 5). The observed trends of phytochemicals of Z. zanthoxyloides in this work is similar with the earlier reports on same plant (Adesina, 1986; Chaaib, 2004; Adesina, 2005). Plant phytochemicals have been established to exhibit antimicrobial properties (Iwu et al., 1999; Banso and Adeyemo, 2006). Antimicrobial potency as reported in this work with a broad range of activity against Gram-positive and Gram-negative organisms is similar to that reported in the work of Adegbolagun and Olukemi (2010) when reporting the activity of Z. zanthxyloides against selected microbial isolates. Optimum antimicrobial inhibition reported in this work is, however, greater than that reported by Adegbolagun and Olukemi (2010) for Bacillus, P. aeruginosa and E. coli which may be attributed to the concentrations of extract used. Adesina (2005) has described the broad spectrum antibacterial activity of many species of Zanthoxylum against different microbes, although aqueous extracts was reported to be less effective against microbial sample of P. aeruginosa and E. coli (Ndukwe et al., 2005; Adebiyi et al., 2009). Extracts from Z. zanthoxyloides have previously also been documented for notable activity against bacteria associated with periodontal diseases (Taiwo et al., 1999). In the work of Olila et al. (2001), there was no antimicrobial activity reported from Zanthoxylum chalybeum against E. coli and Candida albicans tested. This was attributed to low dosage and solubility of extract in extraction solvent used (Nair and Chanda, 2006).
Analysis
G. latifolium
Z. zanthoxyloides
Ethyl acetate
Chloroform
Ethyl acetate
Chloroform
Saponins
(−)
(+)
(+)
(−)
Tannins
(+)
(+)
(−)
(−)
Reducing Sugars
(−)
(−)
(−)
(−)
Alkaloids
(+)
(+)
(+)
(+)
Flavonoids
(−)
(+)
(+)
(+)
Terpenoids
(−)
(−)
(+)
(+)
Phlobatannins
(−)
(−)
(−)
(−)
Steroids
(−)
(−)
(−)
(−)
Anthraquinones
(−)
(−)
(+)
(−)
(+), present; (−), absent
The doses of antimicrobial samples used in this work were found to be effective in the treatment of the microbial isolates investigated with effectiveness against all bacterial and fungal isolates. Phytochemical products with minimum inhibitory concentrations (MIC) ranging from 100 to 1000 mg mL−1 are referred to as antimicrobials (Abreu et al., 2012).
The activity of G. latifolium as reported in this work is also similar to other reports on the activity of G. latifolium’s extracts to inhibit different microbes (Morebise et al., 2002; Ugochukwu and Babady, 2002; Farombi, 2003). The presence of saponins and flavonoids in G. latifolium have been attributed to its antimicrobial efficacy (Morebise and Fafunso, 1998). In a similar manner to the findings of this study, Staphylococcus sp., Pseudomonas sp., Shigella sp., K. Pneumonia, Salmonella sp., E. coli and O. anthropi were all inhibited at 100 mg/ml in Adeleye et al. (2011). The presence of essential oil was attributed to high antimicrobial efficacy against tested microbes including C. albicans. Inhibition ranged from 7.5 mm for P. aeruginosa to 11.25 mm for Shigella flexneri, respectively. However, activity of extracts were comparatively lesser than that of commercial antibiotics at the concentration tested. This suggests the inefficiency of active ingredient present at the concentration of extract tested which was not the case in this work. The extracts tested over the range of concentrations considered showed improved antimicrobial activities over all antibiotics used, mostly, with significant differences
Inhibition activities observed against bacterial and fungal cell as exerted by phytochemicals in this study may be due to the collapsing of cell walls and membranes, resulting in leakage of cell component, interruption of proton motive force, dysfunctioning of efflux pump and enzyme, leading to cytosis (Burt, 2004). Phytochemical activities of plant extract against microbes from disruption of nucleic acid synthesis, interfering with intermediate metabolism, initiation of coagulation of cytoplasmic components and disruption of normal cell communication in quorum sensing have also been reported (Sanchez et al., 2010; Chitemerere and Mukanganyama, 2014; Anandhi et al., 2014; Nazzaro et al., 2013; Radulovic et al., 2013; Mogosanu et al., 2015). A common phenomenon is that several compounds in crude plant extract act at different target sites in pathogens and contribute to optimum efficacy of plant extracts (Gupta et al., 2014). Phytochemicals may exhibit antimicrobial effect in microbes not only through direct lethal activity but also by altering key events in pathogenesis (Brijesh et al., 2009). The activity of inhibition as observed in this study can therefore be bactericidal in which the zone of inhibition experienced results from complete lethal action against the microbes or bacteriostatic in which the inhibition zones results in a state of viable but dormant situation referred to as viable but non culturable (VBNC) state. Conventional chemically synthesised antibiotics and phytobiotics significantly differ with respect to frequency in radicals and spatial structure (Koehn and Carter, 2005). The latter is with a fewer nitrogen, sulphur, halogens, phosphorus and exhibit enhanced scaffold variety, stereo chemical abundance, diversity in ring system, molecular complexity and carbohydrate contents (Schmidt et al., 2008). Also, plant products can alter or prevent protein-protein interactions, thus acting as effective modulators of immune response, apoptosis, mitosis and signal transduction (Koehn and Carter, 2005) making them suitable alternatives to synthetic antibiotics.
4 Conclusion
A major conclusion is that plant samples tested revealed potent antimicrobial activities which notably compared with commercial antibiotics at the concentration of extracts tested. Very strong potency of the plant extract was observed against E. coli; a water pollution indicator. The mount of crude extract needed in this work to attain effective inhibition of microbial isolates can be reduced by further identification of active ingredients and subsequent purification. Formulation and development of active component of G. latifolium and Z. zanthoxyloides into biotechnological products for water purification is recommended.
Author contributions
A.O Adeeyo and K.A Odelade conceived this work, J.O Odiyo and T.A.M Msagati assisted in interpretation of data.
Funding
Authors declare no funding for this research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep.. 2012;29:1007-1021.
- [CrossRef] [Google Scholar]
- Effect of radiofrequency radiation from telecommunication base stations on microbial diversity and antibiotic resistance. J. Appl. Sci. Environ. Manag.. 2014;18:669-674.
- [Google Scholar]
- Antimicrobial and antioxidant activities of crude extracts of two Nigerian chewing sticks. Pharm. Biol.. 2009;47:320-327.
- [CrossRef] [Google Scholar]
- Chemical profiling and antimicrobial properties of phyto-active extracts from Terminalia glaucescens stem against water microbial contaminants. Open Biotechnol. J.. 2018;12:3-17.
- [Google Scholar]
- Effect of light irradiation on the antimicrobial activity of Zanthoxylum zanthoxyloides (lam) methanolic extract. Afr. J. Pharm Pharmacol.. 2010;4:145-150.
- [Google Scholar]
- Antimicrobial Activity of Essential Oil and Extracts of Gongronema latifolium Decne on Bacterial Isolates from Blood Stream of HIV Infected Patients. J. Pharmacol. Toxicol.. 2011;6:312-320.
- [Google Scholar]
- Further novel constituents of Zanthoxylum zanthoxyloides root and pericarp. J. Nat. Prod.. 1986;69:715-716.
- [Google Scholar]
- The Nigerian Zanthoxylum: Chemical and biological values. Afri J Trad Compl Med.. 2005;2:282-301.
- [Google Scholar]
- Antimicrobial potency of selected medicinal plants with special interest in activity against phytopathogenic fungi. Indian Vet med J.. 1999;23:299-306.
- [Google Scholar]
- Studies on anti ulcer, analgesic and antipyretic properties of the ethanolic leaf extract of Gongronema latifolium in rodents. Afr. J. Biotechnol.. 2010;9:2316-2321.
- [Google Scholar]
- DNA fragmentation induced by the glycosides and flavonoids from C. coriaria. Int. J. Curr. Microbiol. Appl. Sci.. 2014;3(12):666-673.
- [Google Scholar]
- Trees, shrubs and lianas of West African dry zones. CIRAD: Margraf Publishers Gmbh, MNHN, Paris, France; 2004. p. :1-573.
- Phytochemical screening and antimalarial assessment of Abutilon mauritianum, Bacopamonnifera and Datura stramonium. Biokemistri.. 2006;18:39-44.
- [Google Scholar]
- Studies on the antidiarrhoeal activity of Aegle marmelos unripe fruit. BMC Complementary and Alternative Medicine.. 2009;9:47.
- [Google Scholar]
- Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol.. 2004;94:223-253.
- [Google Scholar]
- Phytochemical investigation of an African chewing stick, Zanthoxylum zanthoxyloides (Lam) Zepernick and Timler. Doctorate Thesis. University of Lausanne; 2004. p. :11-31.
- Evaluation of cell membrane integrity as a potential antimicrobial target for plant products. BMC Complementary and Alternative Medicine.. 2014;14:278.
- [CrossRef] [Google Scholar]
- Comparison of some natural coagulants bioremediation. Int J Technol Adv Eng.. 2012;2:429-434.
- [Google Scholar]
- Antibacterial and antidiarrheal activities of plant products against enterotoxinogenic Escherichia coli. Toxins.. 2013;5:2009-2041.
- [Google Scholar]
- Chemical composition and antibacterial activity of Ocimum gratissimum. Pharm. 2011;30:723-834.
- [Google Scholar]
- Antioxidant and antitussive properties of Gongronema latifolium leaves used locally for the treatment of fowl cough in Nigeria. J. Appl. Sci. Environ. Manage. 2007;11:47-50.
- [Google Scholar]
- African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. Afr. J. Biotechnol.. 2003;2:662-671.
- [Google Scholar]
- Characterization and biological activity of essential oils from fruits of Zanthoxylum xanthoxyloides Lam. and Z. Leprieurii Guill. & Perr., two culinary plants from Cameroon. Flavour Fragr J.. 2012;27:171-179.
- [Google Scholar]
- Flora of the District Garhwal North West Himalaya: With Ethnobotanical Notes. TransMedia, Srinagar 1999:1-811.
- [Google Scholar]
- A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res.. 2005;39:2338-2344.
- [Google Scholar]
- Approaches in fostering quality parameters for medicinal botanicals in the Indian context. Indian J. Pharmacol.. 2014;46:363-371.
- [Google Scholar]
- Dispersion of C 60 in natural water and removal by conventional drinking water treatment processes. Water Res.. 2009;43:2463-2470.
- [Google Scholar]
- New Antimicrobial of plant origin. In: Janick J., ed. Perspectives on new crops and new issues. Alexandria, V.A.: ASHS Press; 1999. p. :457-462.
- [Google Scholar]
- Advancement in drinking water treatments from ancient time. Int. J. Sci. Environ. Technol.. 2014;3:1415-1418.
- [Google Scholar]
- Jones, A.N., Bridgeman, J., 2017. Disinfection ability of hibiscus seeds in water treatment. P I Civil Eng. 2017, Dx.doi.org/10.1680/Jwama.16.00106.
- Performance of Solanum incunum L. as natural coagulant and disinfectant for drinking water. Afr. J. Environ. Sci. Technol.. 2011;5:867-872.
- [Google Scholar]
- The evolving role of natural products in drug discovery. Nat. Rev. Drug Discovery. 2005;4:206-220.
- [Google Scholar]
- Green synthesis and antibacterial activities of silver nanoparticles using extracellular laccase of Lentinus edodes. Not. Sci. Biol.. 2015;7:405-411.
- [CrossRef] [Google Scholar]
- Effect of coagulant protein from Parkinsonia aculeata and Citrus juice on bacteria isolated from Ruvu River in Tanzania. Int. J. Appl. Sci. Eng. Res.. 2012;1:714-724.
- [Google Scholar]
- The use of indigenous plants for drinking water treatment in developing countries: a review. J. Bio. Env. Sci.. 2014;5:269-281.
- [Google Scholar]
- Prevention of microbial communities: novel approaches based natural products. Curr. Pharmcautical Biotechnol.. 2015;16:94-111.
- [Google Scholar]
- Antimicrobial and phytotoxic activities of saponin extracts from medicinal plants. Biokemistri.. 1998;8:69-77.
- [Google Scholar]
- Awe EO: Antiinflammatory property of Gongronema latifolium. Phytother. Res.. 2002;16:S75-S77.
- [CrossRef] [Google Scholar]
- Formulation and evaluation of polyherbal antipsoriatic cream. Pharmacologyonline. 2009;2:1185-1191.
- [Google Scholar]
- Plantes médicinales et pratiques médicales traditionnelles au Burkina Faso Cas du plateau central. TOME II. Thèse d’Etat. Univ Ouaga. 1996:1-332.
- [Google Scholar]
- Activity of some medicinal plants against certain pathogenic bacterial strains. Ind. J. Pharmacol.. 2006;38:142-144.
- [Google Scholar]
- Effect of essential oils on pathogenic bacteria. Pharmaceuticals.. 2013;6:1451-1474.
- [Google Scholar]
- Antibacterial activity of aqueous extracts of selected chewing sticks. J. Contemp. Dent. Pract.. 2005;8:1-7.
- [Google Scholar]
- Chemical constituents and biological activities of the genus Zanthoxylum: A review. Afr. J. Pure Appl. Chem.. 2011;5:412-416.
- [Google Scholar]
- Antimicrobial Activity of Effects and Latex of Caiotropis procera (Ait) and synergistic Effect with Reference to Antimicrobials Research. J. Med. Plant. 2011;5:706-716.
- [Google Scholar]
- Antimicrobial study of essential oils of Ocimum gratissimum leaves and Zanthoxylum xanthoxyloides fruits from Cameroon. Fitoterapia. 2003;74:284-287.
- [Google Scholar]
- Hypoglycemic potentials of methanolic extracts of selected plant foods in alloxanized mice. Plant Foods Hum. Nutr.. 2003;58:1-7.
- [CrossRef] [Google Scholar]
- Okafor, J.C., 2005. Conservation and the use of traditional vegetables from woody forest species in Southeastern Nigeria. In the International Plant Genetic Resources Institute workshop on Genetic Resource of Traditional Vegetables in Africa Conservation and Use. ICRAF – HQ, Nairobi, Kenya.
- Antibacterial and antifungal activities of extract of Zanthoxylum chalybeum and Warburgia ugandensis, Ugandan medicinal plants. Afr J Health Sci.. 2001;1:66-72.
- [Google Scholar]
- Antimicrobial activity of aqueous extracts of Vernonia amygdalina, Garcinia kola and Gongronema latifolium and their blends on some beer spoilage organisms. Tech. Q. Master Brew. Assoc. Am.. 2004;41:398-402.
- [Google Scholar]
- LC/MS/NMR analysis of isomeric divanilloylquinic acids from the root bark of Fagara zanthoxyloides Lam. Phytochemistry. 2004;65:1145-1151.
- [Google Scholar]
- Formulation and evaluation of anti-bacterial and anti-fungal activity of a herbal ointment containing Aloe-vera, Azadirachta indica and Curcuma- longa. J Chem Pharm Res.. 2010;2:182-186.
- [Google Scholar]
- Potential of using plant extracts for purification of shallow well water in Malawi. J Phys Chem Earth.. 2009;34:799-805.
- [Google Scholar]
- Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr. Med. Chem.. 2013;20:932-952.
- [Google Scholar]
- Formulation and evaluation of antiseptic polyherbal ointment. Int J Pharm and Life Sci.. 2012;3:2021-2031.
- [Google Scholar]
- Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Environ. Microbiol.. 2010;76:6888-6894.
- [Google Scholar]
- 4 new pregnane glycosides from Gongronema latifolium (Asckepiadaceae) Liebigs Ann. Chem.. 1993;10:1057-1062.
- [Google Scholar]
- Extraction and partial purification of coagulation active components from common bean seeds. Acta Periodica Technologica. 2006;37:37-43.
- [Google Scholar]
- Disinfection of waterborne coliform bacteria using Luffa cylindrica fruit and seed extracts. Environ. Technol.. 2009;230:1435-1440.
- [Google Scholar]
- Evaluation of Cactus and Hyacinth Bean peels as natural coagulants. Int. J. Chem. Eng.. 2012;3:187-191.
- [Google Scholar]
- Antibacterial activities of extracts form Nigerian chewing sticks. Phytother. Res.. 1999;13:675-679.
- [Google Scholar]
- The effect of Gongronema latifolium leaf extract on serum lipid profile and oxidative stress of hepatocytes of diabetic rats. J. Biosci.. 2003;28:1-5.
- [Google Scholar]
- Antioxidant effects of Gongronema latifolium in hepatocytes insulin dependent diabetes mellitus. Filoterapia. 2002;73:612-618.
- [Google Scholar]
- Antihyperglycemic effect of aqueous and ethanolic extracts of Gongronema latifolium leaves on glucose and glycogen metabolism in livers of normal and streptozotocin-induced diabetic rats. Life Sci.. 2003;73:1925-1938.
- [Google Scholar]
- Screening and evaluation of innate coagulants for water treatment: a sustainable approach. Int. J. Energy Environ. Eng.. 2012;3:1-29.
- [Google Scholar]
- W.H.O, (World Health Organization)., 1998. Guidelines for Drinking-Water Quality. Vol. 2: Health Criteria and Other Supporting Information: Addendum. WHO, Geneva, Switzerland.
- W.H.O, (World Health Organization), 2003. The right to water. Geneva: WHO Library Cataloguing-in publication data. 2003.
- W.H.O, (World Health Organization)., 2006. Guidelines for Drinking Water Quality, First Addendum to 3rd Edition Recommendations 1. (Accessed 22.02.12). http://www.Who.int/watersanitationhealth/dwq/gd wq0506.pdf.
- W.H.O, (World Health Organization)., 2007. The World Health Report – a Safer Future: Global Public Health Security in the 21st Century, Geneva.
- W.H.O, (World Health Organization)., 2011. Evaluating household water treatment options: Health-based targets and microbiological performance specifications, Geneva.
- Formulation and Evaluation of Herbal Sanitizer. Int. J. Pharm. Tech. Res.. 2013;5:40-43.
- [Google Scholar]
- Aromatase and glycosyl transferase inhibiting acridone alkaloids from fruits of Cameroonian Zanthoxylum species. Chem. Cent. J.. 2013;7:125.
- [Google Scholar]
- Application of phytodisinfectants in water purification in rural Cameroon. Afr. J. Microbiol. Res.. 2011;5:628-635.
- [Google Scholar]
- Indigenous plant based coagulants/disinfectants and sand filter media for surface water treatment in Bamenda, Cameroon. Afr. J. Biotechnol. 2011 2011b, 10:8625–8629
- [Google Scholar]
- A preliminary study on cactus as coagulant in water treatment. Process Biochem.. 2006;41:730-733.
- [Google Scholar]







