Translate this page into:
Antimicrobial effects of Ferula species- an herbal tactic for management of infectious diseases
⁎Corresponding author. m.alruways@su.edu.sa (Mashael W. Alruways)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Drug resistance and new diseases are becoming serious threat for flora and animals. Scientists are developing more effective amalgams to thwart millions of demises. Traditional medication uses Ferula spp. in being conferred in this framework. We used Scientific direct, Google Scholar, PubMed, to research Ferula's antimicrobial profile amongst which we found five of Ferula spp. are antibacterial. Moreover, ferulenol from Ferula communis unveiled decent action. Four novel thiophene amalgams were attained from Ferula foetida roots (foetithiophenes C-F [3–6]). Foetithiophene F (6) showed toxicity to Gram-positive bacteria. Foetithiophene F from Ferula foetida has antibacterial and antifungal activities. Alkaloids, coumarins, flavonoids, saponins, opines, ergosterols, steroids, and terpenes overflow. Ferula spp. can be used for drug development after thorough follow-up investigations. Medicinal plant extracts cannot kill all pathogens. Chemicals can be extracted more effectively and selectively using plant-specific extraction approaches. Plant extracts' antimicrobial susceptibility is tested—capricious test discoveries. Ferula asafoetida L. is the keyderivation of Asafoetida, which has a pungent, persistent, and sulphurous odour and oleo-gum resin, which plays a vital protagonist in both medicine and nutrition. Asafoetida has been a spice and cure since ancient times. Recent study has shown relaxing, neuroprotective, memory-enhancing, digestive enzyme, antioxidant, antispasmodic, hypotensive, hepatoprotective, antibacterial, anticarcinogenic, anticancer, anticytotoxicity, anti-obesity, anthelmintic, and antagonistic properties. This paper discusses Asafoetida's pharmacology, therapy, and phytochemistry. Discovering novel antimicrobials from plant extracts is difficult despite efforts to improve antibacterial activity. Medicinal plant extracts must be researched for their mechanisms of action, chemical interactions, pharmacokinetics, and pharmacodynamics before being considered antibacterial. In this review, we explored Ferula spp.-based components' antimicrobial properties, processes, and chemical possibilities.
Keywords
Drug resistance
Ferula species
Antimicrobial
Inhibition
Toxicity
Phytochemicals
Essential oils
1 Introduction
More than 170 species make up the genus Ferula (family Apiaceae), found in much of central Asia, North Africa, and the Mediterranean region. The appearance of new infectious diseases, along with an increase in the prevalence of medication resistance among microorganisms, has made it increasingly likely that there will be a demand for innovative antimicrobials soon (Kumar et al., 2014). One of the most significant problems that needs to be addressed everywhere is drug resistance (Balkrishna et al., 2021). In continuation, germs are the root cause of an alarmingly high annual rate of hospitalizations as well as fatalities (Freeman, 1997; Balkrishna et al., 2021). Even though we have modern treatments such as antibiotics, microbes are acquiring resistance to them at an alarmingly fast rate. Furthermore, existing treatments are limited, ineffective, or inefficient; hence, there is an immediate need to address these challenges by looking for new drugs or modifying existing ones. When they are 4–5 years old, ferula plants have large taproots, often carrot-shaped roots, which measure approximately 15 cm in diameter at the crown. The living rhizome root's top is stripped bare and the stem is broken at the crown before the plants bloom. A dome-shaped structure made of twigs and earth covers the exposed surface. Eventually, a milky liquid drip extrude out from the cut surface. The exudates are scraped off when more latex flows, and a new slice of the root is cut; occasionally, the resin is taken along with the slice. Until exudation stops, the resin collection and root cutting are repeated (Duan et al., 2002).
The medicinal plants could be used as alternative therapeutic options in this situation. Traditional medicine (TM) relies heavily on medicinal plants, which have been utilized to treat a variety of infectious disorders (Kumari et al., 2018). According to the WHO, TM is used by 80% of the world's population for primary health care, while commercial medications are used by the remaining 20%. Medicinal plants have long been employed in TM and the pharmaceutical industry as a rich source of bioactive components (Paul and Debnath, 2018; Sharma et al., 2021).Various names of Asafoetida are represented in Table 1.
Country
Name
Afghanistan
Kama, Anguza
Bangladesh
Hing
China
A-wei
Denmark
Dyvelsdrak
England
Asafetida
Finland
Asafetida, Hajupihka, Pirunpaska, Pirunpihka
France
Asafetide, Assa foetida, Ferule persique, Merde du diable
Germany
Asafetida, Asafotida, Asant, Stinkasant, Teufelsdreck
Greece
Aza
Hungary
Ordoggyoker
India
Hengu, Hing, Hingu, Ingu, Inguva, Kayam, Perungayam, Perunkaya, Raamathan
Iran
Rechina fena, Zaz
Italy
Assafetida
Myanmar
Sheingho
Netherlands
Asafetida, Duivelsdrek, Godenvoedsel, Sagapeen
The aroma of asafoetida is pungent, persistent, and noticeably sulphurous. Since it has a flavour redolent of meat and nascent vegetables, onion and garlic, it is an ingredient frequently used in modern Indian cuisine, this is probable the motive for this. Asafoetida has a long history of use as a medicinal remedy for a wide variety of conditions, including but not limited to whooping cough; asthma; ulcers; epilepsy; stomach-ache; flatulence; bronchitis; intestinal parasites; antispasmodic; poor digestion; and influenza. Asafoetida has been shown to treat a wide variety of stomach conditions successfully. The favourable physiological impact observed most frequently is the capacity of asafoetida to speed up digestion. This is accomplished through increasing salivary production and the activity of salivary amylase. To accomplish this, it boosts the production of bile acid, improves bile flow, and stimulates pancreatic and small intestine digestive enzyme activity. In this way, dietary lipids are metabolised more quickly and efficiently.
Moreover, it is used to treat conditions such as high blood pressure, low stomach acid, gas, and loose stools. It is hypothesised that women are more likely to be affected by this illness. It is utilised to treat various problems, including leucorrhea, aunique pain, problematic and frequent menstruation, unintentional abortion, and sterility, amongst others. Recent pharmacological and biological research has shown that asafoetida has several medicinal properties, including antioxidant, antibacterial, antiviral, antifungal, cancer chemo-preventive, anti-diabetic, anti-carcinogenesis, antispasmodic, hypotensive, relaxing action, neuroprotective, and molluscicidal.
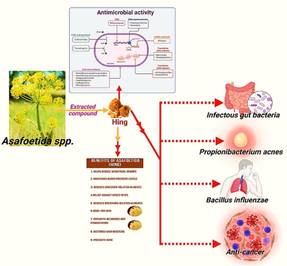
In continuation, plants and their bioactive composites are forerunners in the discovery of new medicines. The phytochemicals exert antimicrobial action by different mechanisms and this effect is attributed to substantialquantities of secondary metabolites alike tannins, alkaloids, phenolics, and flavonoids (Duraipandiyan et al., 2006; Djeussi et al., 2013; Gantait et al., 2014). In this context, Ferula is an imperative genus of the family Apiaceae with 212 taxonomically accepted species. The genus is characterized by perennial herbs and is distributed from Macaronesia to West Himalaya and Ethiopia, East Central Europe to Mongolia and has been introduced into Bangladesh and Laos (KewScience-Plants of the World Online, 2021). Traditionally, the plants of the genus can be utilized in the treatment of various diseases (Kahraman et al., 2019). Moreover, they also display anti-convulsant, stimulant and expectorant property (Razavi et al., 2016).The genus Ferula is categorized by the prevalence of oleo-gum-resins (Asafoetida) and their rehearsal in natural and conventional medications (Sonigra and Meena, 2021).Asafoetida has been used as a tranquillizer in the past and decreases blood pressure, as well. It is commonly used in Indian food and as a medicine in Indian medical structures such as Ayurveda (Mahendra and Bisht, 2012). Asafoetida is a spice that has been used as a medicine and therapy for hundreds of years. It is imperative that the data required to evaluate potential medicinal plants' efficacy and establish their worth as antibacterial agents be precise and subjected to rigorous testing. The most important studies regarding the validation of the antimicrobial activity of medicinal plants, the underlying mechanisms of action, the mechanisms of bacterial resistance, the plant-derived chemical compounds that may be responsible for such activity, the challenges and future perspectives of medicinal plant antimicrobials have therefore been analysed in order to gain a more comprehensive understanding of the potential use of medicinal plant extracts as alternatives to conventional treatments. In this review, we have examined the antimicrobial and phytochemistry, as well as its different pharmacological and therapeutic research of Ferula spp. and the bioactive content as well as the mechanistic basis for antibacterial actions is also discussed (illustrated in Fig. 1).
Microbial growth inhibition using Hing (Created with BioRender.com and Mega Creator).
2 Search Plan
The following search phrases were used to look through the literature in a planned way: biological activity; antioxidant and calming properties; bioavailability; data on F asafoetida the information about asafoetida came from several different places, such as books and articles in the arena, online databases (like Web of Science, Medline/Pubmed, Scifinder, Scopus, Embase, and Google Scholar), and interviews with experts. Approximately, 318 nos. of published papers were screened for this purpose. All information regarding plants was gathered utilizing published data from multiple sources such as Science Direct, PubMed, Google Scholar, and books with various keywords like Ferulaspp., drug resistance, antimicrobial activity, toxicity profile and bioactive composition. The botanical names were verified through an online website (https://www.plantsoftheworldonline.org/).
3 Antimicrobial potential of different Ferula spp.:
There is a growing demand not only for the discovery of new therapeutic agents but also for the modification of currently available antimicrobials. Particularly F. asafoetida and F. gummosa, members of the genus Ferula have a long history of application in alternative and traditional forms of treatment. This is particularly relevant to the former case. They are frequently utilised in treating infectious diseases and ailments, including, but not limited to, the common cold, skin infections, intestinal parasites, and diarrhea, amongst others. During this research, we want to determine if the metabolites made from Ferula spp. can be used to make a lot of compounds with antibacterial properties. It also emphasises information gaps that merit additional exploration and draws attention to such gaps. The authors of this paper discuss the relationship between the structure and antibacterial activity of compounds derived from Ferula spp. to point the way for future research and to provide a direction for such research. The terms “Ferula” and “antimicrobial,” “antileishmanial,” “antifungal,” “antiprotozoal,” “antimalarial and ”antiviral“ were searched through every relevant database in great detail. Electronic searches were conducted to gather information. In addition to local books on traditional medicine, Scopus, Pubmed, Web of Science, and Science Direct were used (see Fig. 2).
Multifaced role of Ferula spp. in treating infectious organisms.
Investigators have discovered several metabolites derived from Ferula spp. exhibit various biological features, the most notable of which include antibacterial properties. In recent years, this genus has also been responsible for discovering several promising antiviral sesquiterpene coumarins, some of which have shown promise in the fight against dangerous viral illnesses such as AIDS and influenza H1N1. In addition, antimycobacterial metabolites that are incredibly efficient, such as ferulenol, have been isolated from species of the genus Ferula.
In order to get a complete understanding of the potential antiviral properties of the drimane-type sesquitepene coumarins initiate in the genus Ferula, further research is required. The antiviral effects of sesquiterpene coumarins and Ferula spp is also established. It will be the subject of much more research in the not-too-distant future.
4 Ferula ammoniacum (D.Don) Spalik, M.Panahi, Piwczynski & Puchalka
The methanolic extract of Dorema ammoniacum(synonym) seeds when examined against several Gram positive, Gram negative, and yeast strains exhibited good activity against all tested strains with the MIC values ranged between < 0.3–10 mg/mL. In addition, the extract exhibited potent activity against Gram positive bacterial strains (Enterococcus faecalis, Mycobacterium smegmatis, Staphylococcus aureus 8146 (methicillin-kanamycin resistance), S. aureus 8147, S. epidermidis 5001, S. epidermidis 10282, S. lugdunensis T26A3, S. warneri T12A12, Corynebacterium striatum T25-17) and Gram-negative bacterial strains (Stenotrophomonas maltophilia) as compared to yeast strains (Abedini et al., 2014).
On the other hand, dichloromethane and methanolic (1:1, v/v) extract of D. ammoniacum oleo gum resin (1000 and 500 μg/mL) successfully inhibited Bordetella bronchiseptica, Bacillus cereus var. mycoides, B. pumilus, B. subtilis, Saccharomyces cerevisiae, Candida albicans, Staphylococcus aureus, S. epidermidis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Streptococcus faecalis, and Aspergillus niger when evaluatedusing agar dilution method (Kumar et al., 2006).
5 Ferula assa-foetida L.
Asafoetida (Ferula asafoetida) is a well-knownspecies of Ferula which is cultivated at large scale mainly in Iran. It is a 2-m tall medicinal plant with 5–8 cm diameter small, hollow, succulent stems at the base of the plant. It is acknowledged by a communal name calledHing or Devils dung (depicted in Fig. 3).
Extraction of Hing or Devils dung from Asafoetida (originally clicked from Ladakh, and Himachal Pradesh).
In natural form it has very pungent fragrance. The antibacterial properties of the essential oils extracted from the oleo-gum resin of F. assafoetida (collected on June 15, 30, and July 15, 2011)when investigated against Candida albicans, Salmonella typhi, Escherichia coli, Bacillus subtilis, and Aspergillus niger, exhibited MIC values ranged between 0.015 and 0.111 mg/mL (Kavoosi et al., 2013). Likewise, hydro-alcoholic extract of F. assafoetida shoot when investigated for anti-bacterial potential against Listeria monocytogenes, its serotypes 4a and 4b using disc diffusion method and macro dilution method was found active against L. monocytogenes with MIC and MBC 7.25 and 12.50 μg/mL, respectively. Moreover, the extract (30 μg/mL) showed inhibition zone diameter of 16.35 and 15.87 mm, against L. monocytogenes 4a and 4b, respectively as compared with standard ampicillin (24.31 and 20.18 mm)(Akhlaghi et al., 2018). The purified protein fractions U7 (5 g) and U8 (10 g) from F. asafoetida root exudate were tested employing the agar-well diffusion assay to combat Staphylococcus aureus and Pseudomonas aeruginosa. Chloramphenicol (Inhibition zone 28 mm at 5 μg against P. aeruginosa) was used as positive control. U7 and U8 fractions showed activity against P. aeruginosa with inhibition zone diameters of 10 and 18 mm. Whereas, both fractions were found inactive against S. aureus (Chandran et al., 2017).
6 Ferula aucheri (Boiss.) Piwczynski, Spalik, M. panahi & Puchalka
A member of the Apiaceae family is ferula from the subtribe of Ferulinae. From the old-time numerous species of these genera have been the origins of oleo gum resins utilized as traditional medicine including ammoniacum, sagapenum, asafoetida and galbanum (Duraipandiyan et al., 2006). This medicinal plant is well known for the presence of chemical constituents such as volatile aromatic acids and sesquiterpene. The inhibitory activity of essential oil from Dorema aucheri (synonym) was examined against Pseudomonas aeruginosa and Chromobacterium violaceum. The essential oil exhibited activity at 25 μg/mL and reduced the violacein production by C. violaceum. Pyoverdine and elastase synthesis were also reduced with essential oil, although pyocyanin and biofilm generation were unaffected.These findings suggested that essential oil could be used as quorum sensing and virulence inhibitors (Sepahi et al., 2015).
7 Ferula communis L.
Ferula communis is a well-known herbal plant belongs to family Apiaceae and it mostly grows in temperate regions.This plant is used in many countries to treat different diseases specially for stomach related diseases, arthritis, headache. Ferulenol and its acetate isolated from F. communis were found active against Mycobacterium intracellulare, M. smegmatis, M. xenopei and M. cheloneia withthe MIC value ranged between 1.25 and 5 μg/mL.Moreover, at 12.5 g/mL, ferulenol acetate was found to be ineffective against M. tuberculosis H37Rv (Mossa et al., 2004). The diverseamalgams like ferulenol, E-ω-benzoyloxyferulenol, E-ω-hydroxyferulenol and E-ω-acetoxyferulenol from F. communis roots were found active against Mycobacterium fortuitum, M. phlei, M. aurum and M. Smegmatis with the MIC values between 0.5 and 64 μg/mL. Among them, ferulenol showed pronounced effect against tested mycobacterial strains (MIC 0.5–2 μg/mL). When compared to the reference standards, ethambutol (0.5–8 g/mL) and isoniazid (0.5–4 g/mL), ferulenol exhibited excellent activity (Appendino et al., 2004) (see Fig. 4).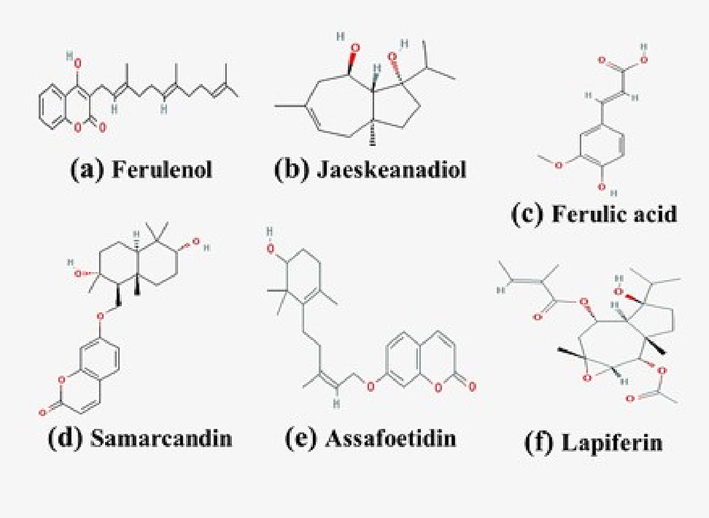
Chemical structures of some compounds from theFerula spp.(a)Ferulenol; (b)Jaeskeanadiol; (c) Ferulic acid; (d) Samarcandin; (e) Assafoetidin; F: Lapiferin().
Source: https://pubchem.ncbi.nlm.nih.gov/
8 Ferula foetida (Bunge) regel
Foetithiophenes A-F, thiophene derivatives isolated from methanolic extract of F. foetida roots showed moderate activity against Candida albicans and Bacillus cereus with MIC values ranged between 50 and 400 μg/mL. Interestingly, foetithiophene F was the most effective against B. cereus, with a MIC of 50 μg/mL (Chitsazian-Yazdi et al., 2015).
9 Mechanistic basis of antimicrobial action
The presence of phytoconstituents is thought to be responsible for the Ferula's antimicrobial action.Ferula ammoniacum contains coumarins, and salicylic acid (Dymock, 1995; Khare and Katiyar, 2012). While, its gum resin contains ammoresinol, anthraquinones, coumarins, doremone A, dshamirone, flavonoids, steroids and triterpenes (Motevalian et al., 2017;Adhami et al., 2013). Subsequently, Ferula assa-foetida contains assafoetidin, conferol, gummosin, neveskone, polyanthinin and samarcandin (Zhou et al., 2011a; Zhou et al., 2011b). While its roots contain asaresinotannol, azulene, bassorine, badrakemin and ferulic acid (Duke and Ayensu, 1985). On the other hand, Ferula aucheri contains alkaloids, coumarins, flavonoids, saponins and terpenes (Ahangarpour et al., 2014; Etebari et al., 2016). Ferula communis contains alkaloids, diterpenes, glycosides, flavonoids and terpenoids (Gamal and Atraiki, 2015). Its roots contain erutinin, ferulenol derivatives, ferutidin, jaeskeanadiol and lapiferin (Appendino et al., 2004; Poli et al., 2005). Ferula foetida contains cadinene, farnesiferols, ferulic acid, sesquiterpene coumarins and rutadisulfide A (Zhou et al., 2011b; Khare and Katiyar, 2012). While, its roots contain foetithiophenes A-F and thiophene derivatives (Chitsazian-Yazdi et al., 2015). Fig. 5 shows how the chemical structures of certain compounds are represented. Balkrishna et al. outlined the processes of antibiotic resistance, including efflux pump hyperactivity, biofilm development, enzyme-induced degradation, and drug transformation (2021). They discovered that the bioactive components of plants have an antibacterial effect, and that this effect can be mediated by inhibiting processes like the creation of biofilms, the production of efflux pumps, and the synthesis of proteins and DNA (Fig. 5).
Antibiotic resistance machineries and defensive role of plant bioactive compounds against bacterial strains. Reproduced from Balkrishna et al. (2021) under the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
10 Toxicity of Ferula species
As mentioned in the introduction section, the plants of this genus have traditionally been used as medicine as well as for flavouring, thus the toxicity of this significant plant should be considered. The toxicity profile of various species of the genus is shown below.
-
Ferula assafoetida L.
As aqueous extract of F. assafoetida oleo-gum-resin (25, 50, and 100 mg/kg) was examined for acute toxicity in mice, there were no evidence of paralysis, weight loss, tremor, or autonomic behavioural abnormalities contrasted with the control group. During the 10-day observation period, there was no mortality in the treated animals (Bagheri et al., 2014). Further, an aqueous suspension of F. assafoetida oleo gum resin (25, 50, 100, and 200 mg/kg) when tested for acute toxicity in rats had no harmful effects in the short or long term (Bagheri et al., 2015). Additionally, no fatality or evidence of toxicity was seen even at maximal dose of aqueous extract of F. asafoetida gum(2000 mg/kg, b.w.) (Vijayalakshmi et al., 2012). Kellerin, isolated from F. assafoetida gum-resin (collected from root) was tested for cytotoxic activity against vero cells using XTT assay. Kellerin showed no influence on vero cell viability and had no cytotoxic effect up to 10 g/mL (Ghannadi et al., 2014). F. assa-foetida oleo-gum-resin exhibited cytotoxic effect against Brine shrimp (Artemia salina) with LC50 value of 28 μg/mL (Bagheri et al., 2010).
-
Ferula communis L.
Ferulenol, a compound derived from F. communis, was tested in Albino mice for acute toxicity. The hypoprothrombinemia with internal and external haemorrhages was observed after 3 days after ferulenol administration with acute LD502100 and 319 mg/kg, b.w., p.o and i.p., respectively (Fraigui et al., 2002).
-
Ferula aucheri (Boiss.) Piwczynski, Spalik, M. panahi & Puchalka
The comet assay was used to test the genotoxic activity of hydro-alcoholic and aqueous extracts of Dorema aucheri aerial parts against human hepatoma cells (HepG2). When compared to the control group, hydro-alcoholic extract at 500 and 1000 μg/mL and aqueous extract (at 500 μg/mL) displayed genotoxic effects on HepG2 cells evident from the increased tail length, percent DNA in tail, and tail moment considerably (Etebari et al., 2016). On the other hand, ethanolic extract of D. aucheri leaves (0.4, 0.8, 1.6 and 3.2 mL/kg) was investigated for hepatotoxicity in Albino mice. According to pathologic and biochemical study, the injection of the extracts resulted in necrosis, inflammation of the liver tissue, cell growth, and cholestasis. In addition, when compared to the non-injected control group, there were considerable increases in the release of bilirubin and liver enzymes. The severity of liver damage varied according on the dose. The ethanolic extract of D. aucheri leaves was found to have possible hepatotoxic properties, which could be linked to the high prevalence of cancer in particular Iranian locations (Mostafavi et al., 2013).
11 Anti-microbial studies activities Ferula spp. & its crucial phyto-constituents
Several factors influence the antimicrobial activity of spices. These include the type of species involved, the spices' composition and concentration, the frequency with which they occur, the substrate's composition, the processing conditions, and the storage environment. Infections brought on by moulds and germs can be remedied with the help of the spice and herb, Asafoetida. Testing the antimicrobial effects of Asafoetida crude extracts was done using a variety of fungal and bacterial strains. Both alcoholic and water extracts of asafoetida were found to be highly efficient against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Aspergillus niger, and Bacillus subtilis using the agar disc diffusion method.Both alcoholic and water extracts of asafoetida were found to be highly efficient against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Aspergillus niger, and Bacillus subtilis using the agar disc diffusion method. The crude extract showed a wide range of antibacterial properties by stopping the relevant fungus and bacteria from growing. Using the agar disc diffusion method to test for antibacterial activity, the size of the inhibitory zone for Asafoetida extracts was between 4 and 16 mm (Shrivastava et al., 2015). The extracted essential oils OGR1, OGR2, and OGR3 had distinct antioxidant, ROS, RNS, H2O2, and TBARS scavenging chemical compositions. OGR1 essential oil contains bicyclic sesquiterpenes [10-epi—eudesmol] and acyclic sulphur-containing compounds [(E)-1-propenyl sec-butyl disulphide and (Z)-1-propenyl sec-butyl disulphide]. These compounds had excellent radical-scavenging action but limited antifungal and antibacterial activity. OGR2 essential oil contains bicyclic monoterpenes [beta-pinene and alpha-pinene], (E)-1-propenyl sec-butyl disulphide], [(Z)-1-propenyl sec-butyl disulphide and acyclic sulphur-containing compounds. This oil has mild radical-scavenging, antibacterial and antifungal properties, OGR3′s essential oil includes heterocyclic disulphide (1,2-dithiolane) and bicyclic monoterpenes (beta-pinene and alpha-pinene) and. It had the lowest radical-scavenging activity and the highest antibacterial and antifungal activity. To improve the oxidative stability of fatty foods during storage, asafoetida could be used as a safe and reliable source of natural antioxidants in the food business. Asafoetida essential oil, on the other hand, can be utilised as a medical antimicrobial (Shrivastava et al., 2015; Kavoosi et al., 2013). F. asafoetida comes in two varieties: Pathani and Irani volatile oils were hydrodistilated and tested for antibacterial activity against various food-borne germs and fungi. Pathani had more robust antibacterial properties than E. coli and B. subtilis. While Penicillium chrysogenum and Aspergillus ochraceus were prevented from growing by Irani's volatile oilby 70% and 75%, respectively, Pathani's volatile oil inhibited growth by just 50% and 45%. Pathani oil is a powerful antibacterial agent, and irani oil is a fungicide. Findings of Bhatnager used two different varieties of F.asafoetida the antibacterial activity of Asafoetida, including red gum and white gum, was tested against five different bacterial strains (Bhatnager et al., 2015). In contrast to Shigella flexneri, the highest levels of antibacterial activity were found in S. aureus as red, and white in hexane extracts. Given that the antibacterial activity of extracts from red and white forms was equal, both forms were most likely chemically identical. Given that it had an inhibitory effect on every bacterial species that was tested, F. asafoetida possesses extensive antibacterial activity. As a result, bioactive substances derived from this plant may one day be used to develop antibacterial medications to treat a number of bacterial illnesses related to the digestive system. Studies of Patil explored the antibacterial and antifungal properties of aqueous extracts ofAsafoetida, ethyl acetate, chloroform, methanol, ethanol (Patil et al., 2015). Antibacterial activity was tested on B. subtilis. Antifungal properties of Candida albicans were investigated. Escherichiacoli, Bacillus subtilis, Klebsiella pneumoniae, Staphylococcus aureus the antifungal effectiveness against A. aureus remained scrutinized. Ethyl acetate, ethanol, and methanol extract have influential antibacterial effects due to anassorted diversity of phytoconstituents. Furthermore, this extract has the potential to yield novel antibiotic molecules. Further studies by Mostafa on antifungal impact of Asafoetida seed essential oil on plant disease fungi such as Verticillium spp, A. niger, Bipolaris sorokiniana and Fusarium graminearum.. Moreover, Fusarium solani using an in vitro approach and a completely randomised design (Mostafa et al., 2013) were evaluated. Associated to controls, Asafoetida seed vital oil stalwartly reduced the growth of all examined fungus species. B. The growth was inhibited by asafoetida seed essential oil, while the effect on other species varied depending on the amount. Deeb's study further investigated Asafoetida's capability to combat several Blastocystis species.. in-vitro growth. Asafoetida extracts in powder and oil form were incubated with Blastocystis spp. subtype three isolates and compared to the antiprotozoal medication metronidazole as a control (Deeb et al., 2012). The concentration, form, and length of incubation with asafoetida extracts all significantly affected the inhibitory activity, reducing the counts and viability of all examined isolates of Blastocystis spp. subtype 3. The lowest concentrations of asafoetida powder and oil inhibited Blastocystis growth, and the most extensive percentage suppression of development was 16 and 40 mg/mL, respectively. For the treatment of Blastocystis spp. infection, asafoetida may be a potent natural substitute to phytomedicine.
12 Future prospects
Although there is a lot to be gained by reviewing medicinal plants, only a fraction of their properties has been explored, making them a largely untapped source of bioactive compounds. Since many medicinal plants have not been examined in depth, there are vast natural resources that have not been used to search for new chemicals that alter resistance and could one day be employed as effective therapeutic agents. Nevertheless, there is still a great deal of natural molecular combinations that are intricate enough to necessitate ongoing chemistry study. Further investigation into the potential of utilising this resource would be beneficial for all parties involved. A relevant correlation between in vitro efficacy statistics and the therapeutic use of such medications may be established in the future through in-vivo research on animal infection models. In the event the study proves fruitful, this may be the case. In vitro testing is when a chemical is put through its paces in a test tube or petri dish to see how well it performs. To acquire more about the pharmacokinetics and pharmacodynamics of medications, as well as the structure–activity correlations associated with these substances, more in-depth studies of the change of drug chemical structures are needed. Antibiotics and substances may have synergistic effects, although more study is needed to determine this. So, scientists can look beyond the antibiotic effects of these drugs and uncover other targets. Interactions between medicinal plant extract and antimicrobial drugs may involve synergism or antagonism. Before these products may be recognised as biological agents, further research, especially in vivo and toxicity assessments, are necessary.
13 Conclusion
In inference, plants of the genus Ferula were recognized as efficient antimicrobials against several different infections. The majority of the research was done in vitro with crude extracts except ferulenol from Ferula communis and Foetithiophenes A-F from Ferula foetida. Remarkably, ferulenol was found to be more effective than ethambutol and isoniazid. On the basis of scientific data, Ferulaassa-foetida was found to be safeas no toxic phytochemicals have not yet reported till now. On the other hand, giving someone ferulenol is associated with hypoprothrombinaemia and hemorrhage. The researchers have also revealed that Ferula aucheri has genotoxic and hepatotoxic effects. As a result, the major concern is raised by toxicity studies. The findings of this review will assist as an advantageous etiquette for researchers of herbal drug engineering and in treating infectious diseases. By virtue of phytochemical and biological activity, asafoetida can be utilised as a variety of medicines, according to the existing information in the scientific literature. It is also often used as a flavouring spice in foods worldwide.
They have been used for many years to treat various diseases. Recent studies of the pharmacological and biological effects of Asafoetida have revealed a variety of effects, including calming, neuroprotective, memory-enhancing, digestive enzyme, anti-oxidant, anti-spasmodic, hypotensive, hepatoprotective, anti-microbial, anti-carcinogenic, anti-cancer, anti-cytotoxic, anti-obesity, anti-helminthic, and antagonistic. Although asafoetida has several potential medical applications (some of which are depicted in Fig. 6), the spice still needs additional study.
Multi-faced action of Asafoetida spp.(Created with BioRender.com & Adobe Illustrator).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial activity of selected Iranian medicinal plants against a broad spectrum of pathogenic and drug multiresistant micro-organisms. Lett. Appl. Microbiol.. 2014;59(4):412-421.
- [Google Scholar]
- Compounds from gum ammoniacum with acetylcholinesterase inhibitory activity. Sci. Pharm.. 2013;81(3):793-806.
- [Google Scholar]
- Antidiabetic and hypolipidemic effects of Dorema aucheri hydroalcoholic leave extract in streptozotocin-nicotinamide induced type 2 diabetes in male rats. Iran. J. Basic Med. Sci.. 2014;17(10):808-814.
- [Google Scholar]
- Data set on the antibacterial effects of the hydro-alcoholic extract of Ferula assafoetida plant on Listeria monocytogenes. Data Brief. 2018;20:667-671.
- [Google Scholar]
- Antimycobacterial coumarins from the sardinian giant fennel (Ferula c ommunis) J. Nat. Prod.. 2004;67(12):2108-2110.
- [Google Scholar]
- Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm. Biol.. 2010;48(3):242-246.
- [Google Scholar]
- Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. Research in Pharmaceutical Sciences. 2014;9(3):207-212.
- [Google Scholar]
- Effect of Ferula assa-foetida oleo gum resin on spermatic parameters and testicular histopathology in male wistar rats. Journal of Ayurveda and Integrative Medicine. 2015;6(3):175-180.
- [Google Scholar]
- Mechanistic insight into antimicrobial and antioxidant potential of Jasminum species: A herbal approach for disease management. Plants. 2021;10(6):1-25.
- [Google Scholar]
- Antibacterial activity of Ferula asafoetida: a comparison of red and white type. J Appl Biol Biotechnol.. 2015;3:18-21.
- [Google Scholar]
- A facile approach to the isolation of proteins in Ferula asafoetida and their enzyme stabilizing, anti-microbial and anti-oxidant activity. Int. J. Biol. Macromol.. 2017;102:1211-1219.
- [Google Scholar]
- Foetithiophenes CF, thiophene derivatives from the roots of Ferula foetida. Pharm. Biol.. 2015;53(5):710-714.
- [Google Scholar]
- Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med.. 2013;13(1):1-8.
- [Google Scholar]
- Duke, J. A., & Ayensu, E. S. 1985. Medicinal plants of China (Vol. 1). Michigan, China.
- Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med.. 2006;6(1):1-7.
- [Google Scholar]
- Pharmacographia indica. Vol Vol. 2. Dehradun, India: Bishen Singh Mahendrapal Singh Publishers; 1995.
- Inhibitory effect of Ferula asafoetida L. (Umbelliferae) on Blastocystis sp. subtype 3 growth in vitro. Parasitol Res.. 2012;111:1213-1221.
- [Google Scholar]
- Genotoxicity evaluation of hydroalcoholic and aqueous extracts of Dorema aucheri by the comet assay. Advanced Biomedical Research 2016:1-5.
- [Google Scholar]
- Acute toxicity of ferulenol, a 4-hydroxycoumarin isolated from Ferula communis L. Vet. Hum. Toxicol.. 2002;44(1):5-7.
- [Google Scholar]
- Phytochemical constituents of Ferula communis plant extracts and their antimicrobial and antioxidant activity. Lebda Medical Journal. 2015;1:6-9.
- [Google Scholar]
- Genomic profile of the plants with pharmaceutical value. 3 Biotech. 2014;4:563-578.
- [CrossRef] [Google Scholar]
- Anti-viral evaluation of sesquiterpene coumarins from Ferula assa-foetida against HSV-1. Iranian Journal of Pharmaceutical Research. 2014;13(2):523-530.
- [Google Scholar]
- Phytochemical screening and evaluation of the antimicrobial and antioxidant activities of Ferula caspica M. Bieb. extracts. Saudi Pharmaceutical Journal. 2019;27(4):525-531.
- [Google Scholar]
- Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: effect of collection time. Food Chem.. 2013;138(4):2180-2187.
- [Google Scholar]
- Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula asafoetida oleo-gum-resin: effect of collection time. Food Chem.. 2013;138:2180-2187.
- [Google Scholar]
- Kew Science-Plants of the world online: Ferula. 2021. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30105171-2 (accessed on 10 November 2021).
- Khare C.P., Katiyar C.K., eds. the Modern Ayurveda: Milestones Beyond the Classical Age. CRC Press; 2012.
- Search for antibacterial and antifungal agents from selected Indian medicinal plants. J. Ethnopharmacol.. 2006;107(2):182-188.
- [Google Scholar]
- Evaluation of antimicrobial potential of cadmium sulphide nanoparticles against bacterial pathogens. International Journal of Pharmaceutical Sciences Review and Research. 2014;24(2):202-207.
- [Google Scholar]
- Evaluation of phytochemical, antioxidant, antibacterial and anti-cancerous activity of Ficus auriculata Lour. and Osyris wightiana Wall. ex Wight. Bulletin of Environment, Pharmacology and Life Sciences. 2018;7(8):64-70.
- [Google Scholar]
- Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacogn. Rev.. 2012;6(12):141-146.
- [Google Scholar]
- Antimycobacterial constituents from Juniperus procera, Ferula communis and Plumbago zeylanica and their in vitro synergistic activity with isonicotinic acid hydrazide. Phytother. Res.. 2004;18(11):934-937.
- [Google Scholar]
- Antifangal effects of asafoetida seed essential oil on in vitro growth of five species of plant pathogenic fungi. Int Res J Appl Basic Sci.. 2013;4:1159-1162.
- [Google Scholar]
- Hepatotoxicity of Dorema aucheri (Bilhar) in albino mice. Arch. Iran. Med.. 2013;16(9):530-532.
- [Google Scholar]
- Anticonvulsant activity of Dorema ammoniacum gum: evidence for the involvement of benzodiazepines and opioid receptors. Research in pharmaceutical sciences. 2017;12(1):53-59.
- [Google Scholar]
- Evaluation of antimicrobial activity of asafoetida. Int J Pharm Sci Res.. 2015;6:722-727.
- [Google Scholar]
- Paul, T., Debnath, S. (2018). Recent Researches on Molecular Breeding for Spice Crop Improvement. In: Sharangi, A. (Eds.), Indian Spices. Springer, Cham. Pp-317-339. https://doi.org/10.1007/978-3-319-75016-3_11.
- Antiproliferative effects of daucane esters from Ferula communis and F. arrigonii on human colon cancer cell lines. Phytother. Res.. 2005;19(2):152-157.
- [Google Scholar]
- Ferulone A and ferulone B: two new coumarin esters from Ferula orientalis L. roots. Nat. Prod. Res.. 2016;30(19):2183-2189.
- [Google Scholar]
- Inhibition of quorum sensing in Pseudomonas aeruginosa by two herbal essential oils from Apiaceae family. J. Microbiol.. 2015;53(2):176-180.
- [Google Scholar]
- Effect of solvents extraction on phytochemical profile and biological activities of two Ocimum species: A comparative study. J. Appl. Res. Med. Aromat. Plants. 2021;25:100348
- [Google Scholar]
- Antimicrobial activities of Asafoetida resin extracts (a potential Indian spice) J Pharm Res.. 2015;5:5022-5024.
- [Google Scholar]
- Metabolic profile, bioactivities, and variations in the chemical constituents of essential oils of the Ferula genus (Apiaceae) Front. Pharmacol.. 2021;11:1-28.
- [Google Scholar]
- Evaluation of the effect of Ferula asafoetida Linn. gum extract on learning and memory in Wistar rats. Indian Journal of Pharmacology. 2012;44(1):82-87.
- [Google Scholar]
- Encyclopedia of Traditional Chinese Medicines. Vol Vol. 2. New York, USA: Springer-Verlag, Berlin Heidelberg; 2011.
- Encyclopedia of Traditional Chinese Medicines. Vol Vol. 4. New York, USA: Springer-Verlag Berlin Heidelberg; 2011.
Further reading
- PubChem 2021. Chemical structures. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 November 2021).







