Translate this page into:
Antimicrobial and synergistic properties of green tea catechins against microbial pathogens
⁎Corresponding author. tdawoud@ksu.edu.sa (Turki M. Dawoud)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Bacterial diseases caused by pathogenic bacteria are one of the health problems in recent times. The continuous use of antibiotics and increased hospitalization increased drug resistance. The increased drug resistance has led to the development of novel lead molecules. Hence, alternate medicine or secondary metabolites from plant sources is a possible therapeutic option to treat bacterial infection alone or with existing drugs. The present study aimed to evaluate the antimicrobial property of green tea extract, catechin, benzoyl peroxide and a combination of antibiotics against acne-causing Staphylococcus epidermidis, Staphylococcus aureus, and Propionibacterium acnes isolated from the skin surface of the clinical subjects. The aqueous and solvent extract of green tea showed antibacterial activity against the screened bacteria and it was further improved by combing with existing drugs. The Minimum Inhibitory Concentration (MIC) was performed using the broth dilution method and showed the least MIC value (<2.5 µg/mL). The tea extract exhibited a catechin compound and was determined using High Performance Liquid Chromatography. The mechanism of action of catechin on bacterial pathogens was determined. The catechin treated bacterial cells exhibited morphological changes, structural changes in the cell walls, separation of cells and membrane rupture. The effect of catechin in combination with antibiotics on both Gram-positive and Gram-negative bacteria was antagonistic and synergistic activity. Therefore crude green tea extract and catechin can reduce bacterial infection in acne.
Keywords
Green tea
Phenols
Catechin
Antimicrobial
Synergy
1 Introduction
Tea is one of the most widely used beverages across the world and has several biological activities such as anti-obesity, hypoglycaemic, antioxidant, anti-carcinogenic, anti-arteriosclerotic, and antiviral activities (Turkmen et al., 2007). The tea leaf extract exhibited antimicrobial potential against various bacteria and may be useful to develop as a potential antimicrobial agent. More than 300 types of tea were produced across the world. Based on the process, teas can be categorized into green tea (non-fermented tea), oolong tea (semi-fermented tea), and dark tea (post-fermented tea) (Sharma et al., 2012). Green tea is the non-fermented product of Camellia sinensis leaves, and it has been considered a traditional drink in several Asian countries. Green tea has various health benefits and it is widely used in preventive medicine. Green tea has various bioactive properties, against cardiovascular diseases, diabetes, cancer, oral health, obesity, cognitive function and bone health. Green tea was effective to treat various bacterial infections (Singh Arora et al., 2009).
Green tea is rich in polyphenols also termed catechins. In green tea, catechins such as (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), (−)-epicatechin (EC), and (−)-epigallocatechin-3-gallate (EGCG) were determined (Dulloo et al., 1999). Catechins such as EGC, ECG and EGCG are the major antimicrobial compounds against various pathogenic organisms. In green tea EGCG and EGC are predominant and these two compounds are excreted in bile. Moreover, EGC is finally excreted in the urine, revealing that green tea shows antibacterial properties against urinary tract bacterial pathogens (Fukushima et al., 2009).
In green tea, polyphenols such as EGC, ECG, EC, and EGCG were reported. The green tea extract exhibited antibacterial activities. The green tea extract showed antibacterial activity against Bacillus cereus, Escherichia coli, Campylobacter jejuni, Helicobacter pylori, Clostridium perfringens, Staphylococcus aureus, Legionella pneumophila, Pseudomonas aeruginosa, Shigella flexneri, and Vibrio cholerae (Marchese et al., 2014). In tea, the bioactive catechins are active against various infectious bacterial pathogens. In green tea, catechin showed activity against penicillinase-producing Staphylococcus aureus, and methicillin-resistant S. aureus by inhibiting the production of β-lactams. The amount of catechin in green tea is significantly influenced by the harvesting season and the degree of the fermentation process. The available organic acids in the tea extract showed activity against various bacteria and fungi. The essential oil extracted from the tea leaves showed antibacterial and antifungal activities. Catechins isolated from the green tea showed activity against Gram-negative and Gram-positive bacteria using several molecular mechanisms, including, the inhibition of cell membrane synthesis, cell wall synthesis, nucleic acid synthesis and protein synthesis, or the inhibition of various metabolic pathways, such as extracellular matrix virulence factors, toxins, iron chelation, and oxidative stress. Green tea was effective against Vibrio cholera and inhibited the biosynthesis of cholera toxins and bacterial growth (Kajiya et al., 2004).
Tea is consumed in several forms. In Japan, “Tencha”, a thick green tea, is used and has several antioxidant compounds. Tencha has antibacterial activity against bacteria, including Bacillus, Clostridium, and Salmonella. Acne vulgaris is one of the infectious diseases prevalent in humans (Beylot et al., 2014). The development of red skin, blackhead and whitehead, and large papule are the major symptoms. These symptoms were associated with inflammation and in some cases were non-inflammatory.In some cases, patients with acne often have bacteria, including Propionibacterium acnes, Propionibacterium granulosum, Staphylococcus aureus, and Staphylococcus epidermidis on their skin (Bojar and Holland, 2004). Acne vulgaris may cause scarring and more than 650 million people are affected globally. The use of retinoids, benzoyl peroxide, keratolytic soaps and antiseborrheic treatments was suggested. Using complementary and alternative medicine, including, medicinal plants is useful to treat acne infections (Jin and Lee, 2018). The major objective of this study is to develop an alternative medicine to treat acne in humans.
2 Materials and methods
2.1 Experimental design
This study was performed from September to November 2022 at the Department of Microbiology, King Saud University (KSU), Riyadh, Saudi Arabia. A total of 30 samples were collected from females and males by swabbing face acne, the age range was between 15 and 30 years. A standard questionnaire includes the following components: the severity of the acne, experience with antibiotics to reduce acne, and regular skin care. The collection of the sample and the preparation of working procedures were performed for one month.
2.2 Isolation of aerobic and anaerobic bacteria
The sterilized cotton swabs moistened in sterile distilled water were used to collect the sample from the skin surfaces of 35 volunteers. It was inoculated immediately on Nutrient Agar (Himedia, Mumbai, India) plates and incubated at 37 °C for 18–24 h. To determine anaerobic bacteria samples from the skin of 35 volunteers, the samples were inoculated in Brain Heart Infusion (BHI) Broth medium. Three drops of 20 % glycerol were added to provide anaerobic conditions. The test tubes were placed in an anaerobic jar to provide an oxygen-free environment. After that, it was further incubated at 37 °C for 72 h (Zhang et al., 2020).
2.3 Culture and characterization
The aerobic bacteria were subcultured using nutrient agar plates. The selected bacterial strains were further cultured on blood agar plates, and haemolytic properties were determined. The anaerobic bacteria were cultured on brain heart infusion agar plates and maintained in anaerobic conditions (Wu et al., 2020).
2.4 Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) Mass spectrometry analysis
MALDI-TOF Mass spectrometry analysis was performed, and the bacterial strains were identified. The bacterial strains were cultured in a blood agar medium, and the three active bacteria were identified.
2.5 Green tea extract preparation
Green tea was prepared from the leaves using aqueous extraction and solvent extraction methods. Briefly, 5 g of dry ground green tea leaves were soaked in 50 ml of water for four hours, and then 50 ml of ethanol was added. It was heated at 80 °C for 20 min and filtered using Whatman filter paper. The liquid–liquid extraction method was followed using ethyl acetate. The filtrate was further concentrated by a rotary evaporator (Buchi R-215 Rotavapor, Switzerland) under reduced pressure at 55 °C for 90 min.
2.6 High-Performance liquid Chromatography (HPLC) analysis
High-Performance Liquid Chromatography (Agilent liquid chromatographic system, USA) was used to analyze the extracted compounds from the green tea. The HPLC system was equipped with a column SB-C18 (1.8 μm, 4.6 × 150 mm), and the program was controlled by software (G 4226A). The amount of catechins in green tea was estimated. The mobile phase used for the separation of Catechins consisted of acetonitrile and methanol (65:35). The flow rate of the mobile phase was 1 mL/min (35.31 bar), and the injection volume was 1 µL with a 5 min run. The chromatogram was acquired at wavelengths of 274 nm. The Catechins in the samples were identified by using standard Catechins (1 µg/mL).
2.7 Antimicrobial susceptibility analysis
Antibacterial susceptibility test was performed using antibiotics, benzoyl peroxide, catechins and decaffeinated green tea extract. The strains, S. epidermidis and S. aureus were isolated and characterized from the skin samples. The strain, Propionibacterium acnes was isolated from King Khalid University Hospital. To analyze the antibiotic susceptibility of S. epidermidis and S. aureus, these two were cultured in Mueller Hinton Agar, whereas, P. acnes were cultured in brain heart infusion agar medium. Antibiotics (Tetracycline − 30 µg, Erythromycin – 15 µg, and Clindamycin 20 µg), Benzoyl Peroxide BPO (5 %-10 %), and tea extract (aqueous and organic solvent extract) were prepared. Catechins were prepared at 30 % (0.3 g/mL), and 40 % (0.4 g/mL). A well diffusion method was used for the antimicrobial susceptibility test. The MHA plates were incubated for 24 h at 37 °C, and 72 h at 37 °C in 5 % CO2 for BHI agar plates (Malar et al., 2020).
2.8 Scanning electron microscopy (SEM) analysis
The bacterial isolates (Staphylococcus epidermidis, Staphylococcus aureus, and Propionibacterium acnes) were subjected to SEM analysis. The bacterial cells were pre-treated with catechin, and membrane integrity was studied. To the control, catechin was not added. The cultured bacterial strains were fixed in 2.5 % of glutaraldehyde and 1 % of osmium tetroxide. The dehydration step was performed with ethanol. It was covered with a platinum lid and its morphology was analyzed.
3 Results
3.1 3.1.Survey, analysis and causes of acne among individuals
The severity of the acne that appeared on volunteers' faces ranges from mild (60 %) to moderate (40 %). The age group of volunteers was categorized into three groups (15–20, 20–25, and 25–30 years). The first group represents 17 % of volunteers, followed by the second (23 %), and the final group (60 %). The use of antibiotics reduced acne among individuals. Among the population, only 53 % used antibiotics. Of the volunteers (%) who assume that there is an association between face masks and acne, 57 % agreed that face masks could be a causative agent for increasing acne in the face. The percentage of the aged 15–20 was 17 %, while the percentage of the aged 20–25 was 60 %, and the percentage of the aged 25–30 was 23 %. The questionnaire also showed the percentage of volunteers who take care of their skin, which was 57 % of the sample study.
3.2 Morphology, microscopy observation and characterization of skin bacteria
The isolated bacteria were observed under a microscope. The isolates were cocci, and bacilli. The screened bacteria were hemolytic, and certain strains were non-hemolytic type on blood agar plates. Staphylococcus strains were gamma hemolytic, whereas, P. acne exhibited beta hemolysis activity. The selected bacterial strains exhibited catalase activity. S. epidermis, and S. aureus strains (S1 and S2) and P. acne were identified in this study based on biochemical tests, antibiotic sensitivity, and MALDI-TOF Mass spectrum analysis.
3.3 Antibiotic susceptibility of the bacterial strains
S. epidermidis S1 was resistant to Benzylpenicillin and Erythromycin. It was sensitive to tested antibiotics such as Amoxicillin/clavulanic acid, Cloxacillin, Oxacillin, Cefazolin, Cefradine, Gentamycin, Tobramycin, Levofloxacin, Moxifloxacin, Trimethoprim, Mupirocin, Clindamycin, Vancomycin, Tetracycline, Tigecycline, and Fosfomycin. Moreover, it exhibited intermediate susceptibility to Rifampicin and Linezolid.S. aureus S2 was resistant to Benzylpenicillin, and showed intermediate susceptibility to Levofloxacin and Linezolid. However, it showed sensitivity against other tested antibacterial agents. The MIC value was less against Oxacillin, Gentamycin, Tigecycline, Clindamycin, and Erythromycin. The isolated P. acues exhibited sensitivity to antibiotics such as Penicillin, Erythromycin, Clindamycin, Lymecycline, Tetracycline, Doxycycline, Minocycline, and Nadiflaxacin.
3.4 Antibacterial activity of tea extract and synergistic properties
The aqueous and organic solvent green tea extract was tested for antibacterial activity against S. epidermidis. The green tea extract showed significant activity, against this bacterium. To determine synergistic activity, tetracycline, erythromycin, and clindamycin were used with extracts, and the results are depicted in Fig. 1a-d. The aqueous and solvent extracts combined with antibiotics improved antibacterial activity. (Fig. 2 and Fig. 3).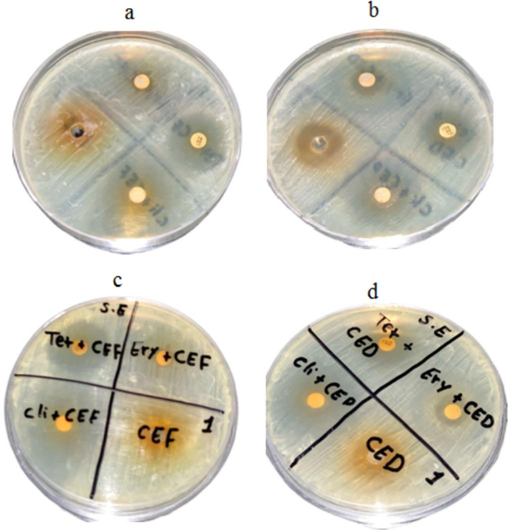
Antibacterial activity of green tea extract and synergistic activity against S. epidermidis. The aqueous green tea extract was loaded on MHA plates at various concentrations (a); solvent extract of green tea (b); synergistic activity of aqueous tea extract and antibiotics (c), and synergistic activity of solvent extract and antibiotics (d). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
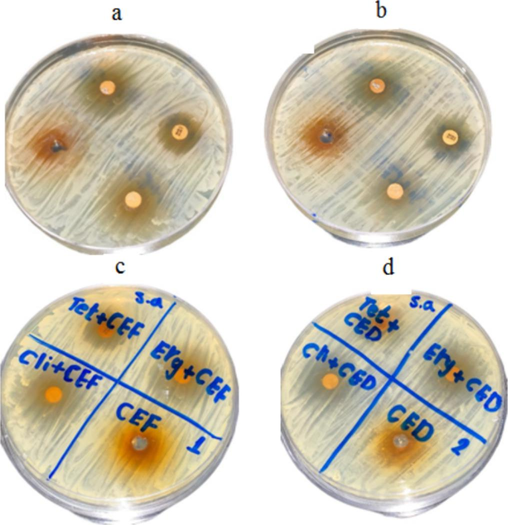
Antibacterial activity of green tea extract and synergistic activity against S. aureus. The aqueous green tea extract was loaded on MHA plates at various concentrations (a), and the solvent tea extract (b). The synergistic activity of aqueous tea extract and antibiotics (c) and synergistic activity of solvent extract and antibiotics (d). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
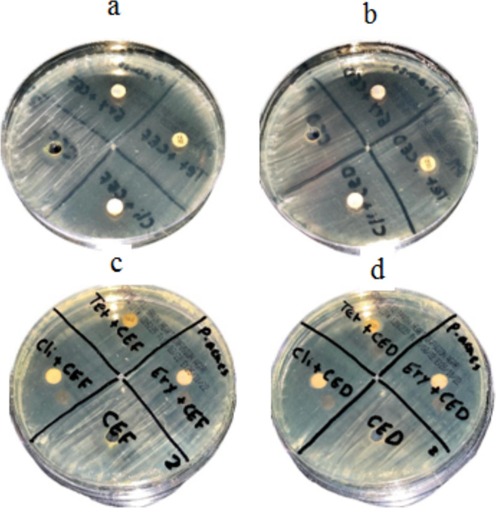
Antibacterial activity of green tea extract and synergistic activity against P. acnes. The aqueous green tea extract was loaded on MHA plates at various concentrations (a) and the solvent extract (b). The synergistic activity of aqueous tea extract and antibiotics (c) and synergistic activity of solvent extract and antibiotics (d). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5 Antibacterial activity of catechins and synergistic activity
Catechin powder was prepared at 30 % (0.3 g/mL), and 40 % (0.4 g/mL) concentrations. At 40 % concentration, antibacterial activity was higher than 30 % in S. epidermis, and the result is depicted in Fig. 4.To determine synergistic antibacterial activity, tetracycline, erythromycin, and clindamycin were mixed with catechins (Fig. 4). The antibacterial activity of Catechins was tested at two different concentrations and synergistic properties against S. aureus. Antibacterial activity was dose-dependent (Fig. 5). The inhibitory effect of catechins (30 % and 40 %) and synergistic activity against P. acnes on BHI agar is depicted in Fig. 6. The effect of Catechins and green tea extracts against S. epidermis, S. aureus, and P. acnes and synergistic activity are depicted in Fig. 7.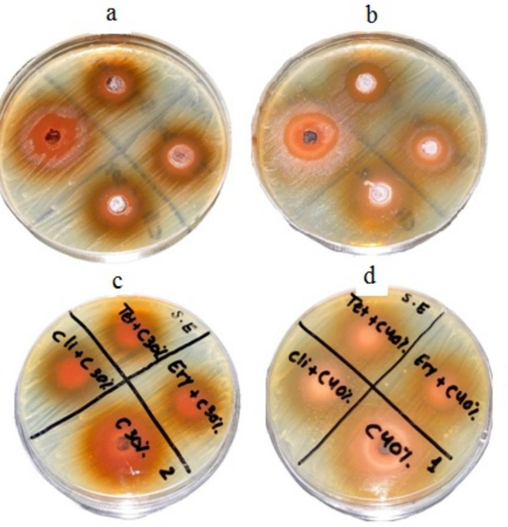
Antibacterial activity of Catechins at two different concentrations and synergistic properties against S. epidermis (a) 30% Catechins at various volumes (b) 40% Catechins at various volumes, (c) antibacterial activity of 30% Catechins and antibiotics, and (d) antibacterial activity of 40% Catechins and antibiotics.
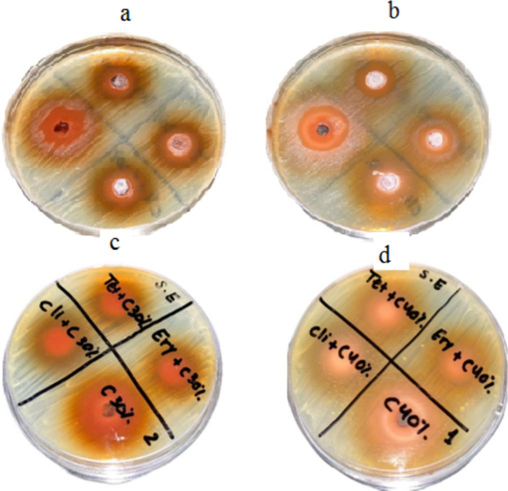
Antibacterial activity of Catechins at two different concentrations and synergistic properties against S. aureus (a) 30% Catechins, (b) 40% Catechins, (c) synergistic antibacterial activity of 30% Catechins and antibiotics, and (d) synergistic activity of 40% Catechins and antibiotics.
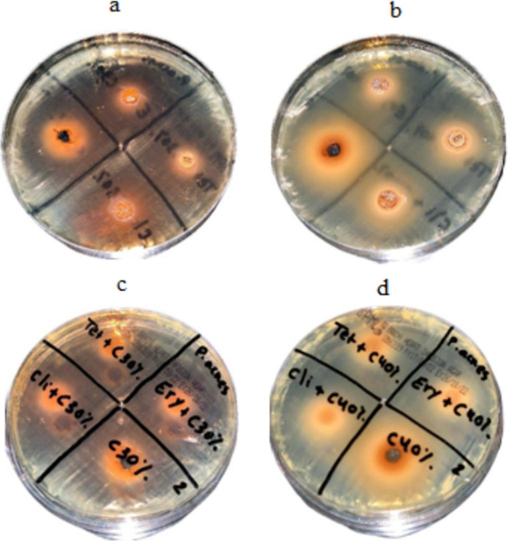
Antibacterial activity of Catechins at two different concentrations and synergistic properties against P. acnes (a) 30% Catechins (b) 40% Catechins, (c) synergistic antibacterial activity of 30% Catechins and antibiotics, and (d) synergistic activity of 40% Catechins and antibiotics.
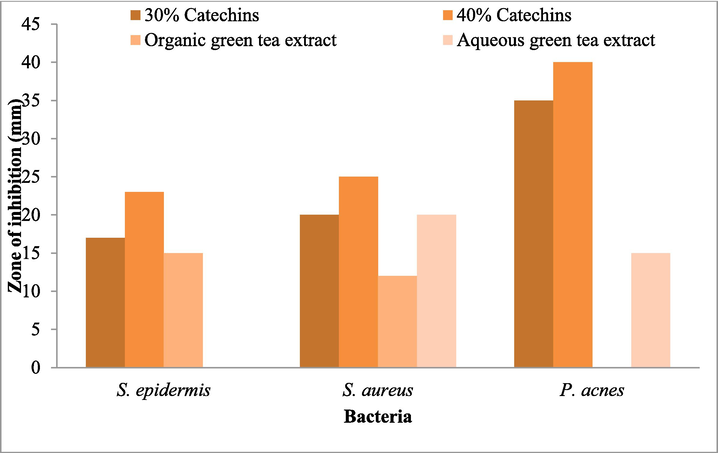
Effect of Catechins and green tea extracts against S. epidermis, S. aureus, and P. acnes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6 High-performance liquid chromatography analysis
In this study, a standard (Catechin) and tea extract (sample) were run in HPLC, and the results are depicted in Fig. 8. In this figure, a major absorbance peak was observed at 274 nm at 1.226 min (Fig. 8a) in the standard. In the sample, a major peak was observed at 1.226 min (Fig. 8a) which confirmed the presence of Catechin (Fig. 8b).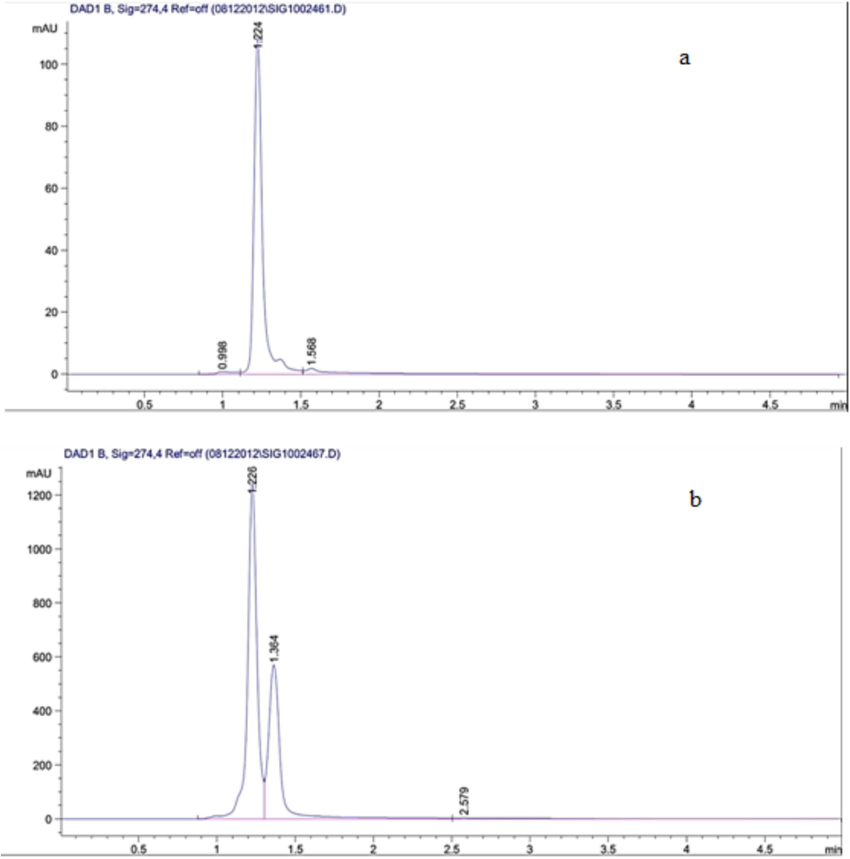
HPLC chromatogram of the standard of catechins at 274 nm (a) and Catechins compound in the green tea extract at 274 nm shows a major peak at 1.226 min (b).
3.7 Morphological analysis of bacteria using scanning electron microscopy
The effect of catechins on cell morphology was studied using SEM at 5000X and 15000X. S. epidermis isolated from face acne was cultured in BHI broth medium and showed grape-like clusters (control). Catechins extract induced structural changes, including, a deformed or shrunken appearance, and grouping of bacterial cells (Fig. 9 a –c). The isolated S. aureus exhibited a spherical shape, and the cells were arranged in grape-like clusters. The catechins-treated S. aureus showed structural changes, cell lysis, membrane rupture, separation of cells, and structural changes in the cell wall (Fig. 9d-f). The isolated P. acneswere rod-shaped bacterium, and the morphology is depicted in Fig. 9g. The catechins-treated P. acnes showed deformed bacterial cells, and the morphology is illustrated in Fig. 9h.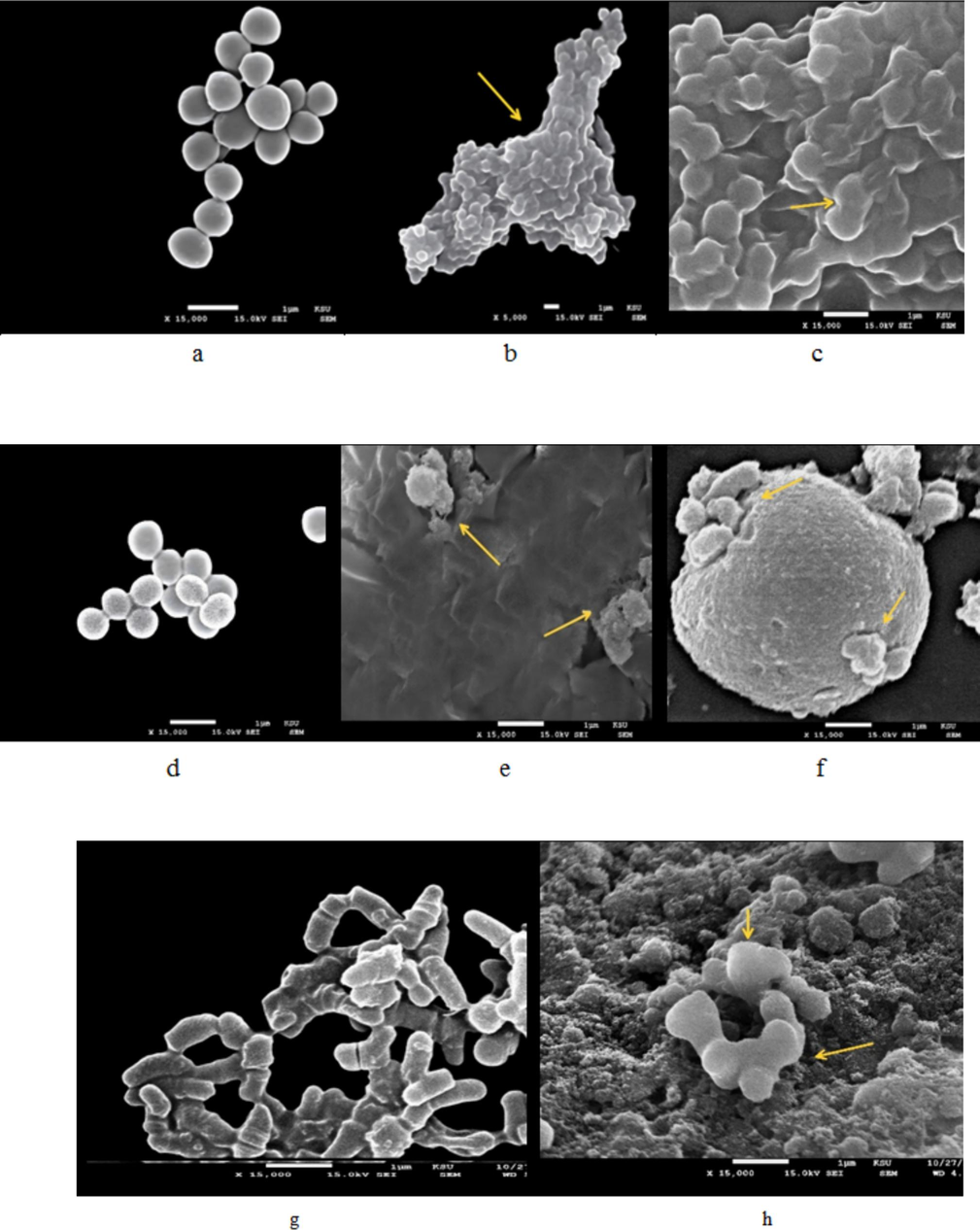
Scanning electron microscopy observations of Staphylococcus epidermis, Staphylococcus aureus, and Propionibacterium acnes. The bacteria were incubated individually with catechins and the morphology was tested at two different magnifications. In the control, catechins were not treated with bacterial cells.(a)S. epidermis – control; S. epidermis treated with catechinsand magnified at 5000X (b) and 15000X (c).S. aureus – control (d); S. aureus treated with catechins and magnified at 5000X (e) and 15000X (f). P. acnes – control (g) and P. acnes treated with catechins and magnified at 15000X (h).
4 Discussion
Thirty samples were collected from the volunteers suffering from acne vulgaris. The percentage of the severity of acne was estimated through the survey method (questionnaire analysis). The severity ranged between mild (40 %) and moderate (60 %). The bacteria that were isolated from face acne were S. aureus which was predominant in females, with a percentage of 60 %, and S. epidermidis, which exhibited more in males, with a percentage of 52 %. The age of volunteers ranges from 15 to 30 years. Also, clindamycin may enhance bacterial phagocytosis (Kehrenberg et al., 2005).
Erythromycin was effective against S. aureus and P. acnes. However, erythromycin did not affect the S. epidermidis growth. The green tea extracts, catechins and BPO were synergistically active against S. epidermidis (Bowman and Sadowski, 2022). This present result agrees with the previous report by Kim and Cha (2021). The EGCG and EC extracted from the green tea leaves of C. sinensis showed bactericidal activity (Reygaert, 2018). In this study, catechins (30 %) exhibited a 17 mm zone of inhibition; and 40 % showed a 23 mm zone of inhibition against S. epidermidis. A catechins concentration of 30 % showed a 17 mm diameter zone of inhibition. The concentration of 40 % Catechins showed a 25 mm diameter zone of inhibition against S. aureus. The concentration of 40 % Catechins showed a 40 mm diameter against P. acnes. Catechins were highly effective against P. acnes, and this finding was agreed upon by Yoon et al. (2013). Catechins inhibited protein tyrosine phosphatase as well as enzymes involved in pathogenicity processes, and inhibited cysteine proteinases (Culp and Wright, 2017).
In this study, catechins affected bacterial cells and caused them to be deformed and shrunken. Catechins extract caused cell lysis, changes in bacterial cell morphology, and distorted bacterial cells. In P. acnes, bacterial cell numbers decreased considerably, and these findings revealed the significant effects of catechins on the structures of all three tested bacteria. This result was similar to previously published reports by Ma et al. (2019). In tea extract, the available EGC and EGCG have been demonstrated to be the most effective (Ma et al., 2019) antimicrobial compounds. Benzoyl peroxide has a broad-spectrum antibacterial compound (Tanghetti and Popp, 2009). In human skin, benzoyl peroxide has been found to reduce the metabolism of sebaceous gland cells. Because bacterial lipases are responsible for the formation of free fatty acids, there is a decrease in the serum of patients treated with benzoyl peroxide, mainly due to its antibacterial activity (Emmerich et al., 2021). According to a previous study, the benefit of combining benzoyl peroxide with antibiotics is to reduce the suppressive effect of bacteria against drug resistance (Boonchaya et al., 2022).
5 Conclusions
In this study, the antibacterial activity of green tea extract, catechin, and synergistic activity were determined. Green tea extract exhibited antibacterial activity against S. epidermidis, S. aureus, and P. acnes. The tea extract, catechin, tetracycline, erythromycin, and clindamycin exhibited maximum synergistic activity. The mechanism of action of catechin on bacterial cells was determined using scanning electron microscopy. The available phenolic compounds from the green tea extract may be useful to treat acne-mediated bacterial infections.
CRediT authorship contribution statement
Roua M. Alkufeidy: . Leen Ameer Altuwijri: . Noura S. Aldosari: . Nura Alsakabi: . Turki M. Dawoud: Conceptualization, Resources, Writing – review & editing.
Acknowledgement
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R197), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Propionibacterium acnes: an update on its role in the pathogenesis of acne. J. Europ. Acad. Dermatol. Venereol.. 2014;28(3):271-278.
- [CrossRef] [Google Scholar]
- Minimum contact time of 1.25%, 2.5%, 5%, and 10% benzoyl peroxide for a bactericidal effect against Cutibacterium acnes. Clini. Cosmet. Invest. Dermatol.. 2022;403–409
- [CrossRef] [Google Scholar]
- Antibiotic use and bacterial resistance in patients with acne vulgaris. J. Am. Acad. Phy. Assis.. 2022;35(8):34-39.
- [CrossRef] [Google Scholar]
- Bacterial proteases, untapped antimicrobial drug targets. J. Antibiotic.. 2017;70(4):366-377.
- [CrossRef] [Google Scholar]
- Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nut.. 1999;70(6):1040-1045.
- [CrossRef] [Google Scholar]
- Improving target assessment in biomedical research: the GOT-IT recommendations. Nat. Rev. Drug Discov.. 2021;20(1):64-81.
- [CrossRef] [Google Scholar]
- Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J. Agric. Food Chem.. 2009;57(4):1253-1259.
- [CrossRef] [Google Scholar]
- Kaempferia parviflora extract as a potential anti-acne agent with anti-inflammatory, sebostatic and anti-propionibacterium acnes activity. Int. J. Mol. Sci.. 2018;19(11):3457.
- [CrossRef] [Google Scholar]
- Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. J. Agric. Food Chem.. 2004;52(6):1514-1519.
- [CrossRef] [Google Scholar]
- A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol. Microbiol.. 2005;57(4):1064-1073.
- [CrossRef] [Google Scholar]
- Antibiotic resistome from the One-Health perspective: understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med.. 2021;53(3):301-309.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Cont.. 2019;106:106712
- [CrossRef] [Google Scholar]
- In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A. Juss and Melia azedarach Linn for anticancer activity. Saud. J. Biol. Sci.. 2020;27(2):682-688.
- [CrossRef] [Google Scholar]
- Influence of in vitro simulated gastroduodenal digestion on the antibacterial activity, metabolic profiling and polyphenols content of green tea (Camellia sinensis) Food Res. Int.. 2014;63:182-191.
- [CrossRef] [Google Scholar]
- An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol.. 2018;4(3):482.
- [CrossRef] [Google Scholar]
- Green tea extract: possible mechanism and antibacterial activity on skin pathogens. Food Chem.. 2012;135(2):672-675.
- [CrossRef] [Google Scholar]
- Antibacterial activity of tea and coffee: their extracts and preparations. Int. J. Food Prop.. 2009;12(2):286-294.
- [CrossRef] [Google Scholar]
- A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol. Clin.. 2009;27(1):17-24.
- [CrossRef] [Google Scholar]
- Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12(3):484-496.
- [CrossRef] [Google Scholar]
- Characterization of biofilm formed by multidrug resistant Pseudomonas aeruginosa DC-17 isolated from dental caries. Saud. J. Biol. Sci.. 2020;27(11):2955-2960.
- [CrossRef] [Google Scholar]
- Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J, Invest. Dermatol.. 2013;133(2):429-440.
- [CrossRef] [Google Scholar]
- Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saud. J. Biol. Sci.. 2020;27(12):3505-3513.
- [CrossRef] [Google Scholar]







