Translate this page into:
Antimicrobial and cytotoxic potential of an endophytic fungus Alternaria tenuissima AUMC14342 isolated from Artemisia judaica L. growing in Saudi Arabia
⁎Corresponding author. aalmosa@ksu.edu.sa (Amal A. Al Mousa),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, 19 endophytic fungal isolates associated with Artemisia judaica L. were isolated from Al-Shihiyah, Haʾil, Saudi Arabia. Those isolates represented five genera, including: Alternaria sp., (predominantly), Aspergillus sp., Chaetomium sp., Cladosporium sp., and Fusarium sp. All isolates (6 species) were grown on a solid rice medium and their ethyl acetate crude extracts, beside A. judaica L. extract, were tested for their antimicrobial activity. One endophytic fungal isolate demonstrated the highest activity and was chosen for further investigation. Based on its phenotypic, ITS ribosomal gene sequences, and phylogenetic characterization, this isolate was identified as Alternaria tenuissima AUMC14342 with accession number (MT468650). Ethyl acetate (EtOAc) extract from the isolated strain, AUMC14342, was fractioned using VLC. The active fraction of the EtOAc exhibited good antimicrobial effect against P. aeruginosa, S. aureus, F. solani, and A. niger at conc. 30 µ/mL using disc diffusion assay. A moderate cytotoxic effect was reported against a Hepatocellular carcinoma cell line (HepG2), with the lowest inhibitory concentration (IC50) of 0.52 µM. The active fraction was characterized by the presence of different chemically terpene, ester, volatile, and other organic compounds, determined by GC–MS. Conclusively, the endophytic fungus, A. tenuissima, derived from A .judaica L., is an ideal candidate for the potential industrial production of antimicrobial agents with a variety of applications.
Keywords
Endophytic fungi
Artemisia judaica
Alternaria tenuissima
Antimicrobial
Cytotoxicity
GC–MS
- EtOAc
-
Ethyl acetate
- PCR
-
polymerase chain reaction
- GC–MS
-
gas chromatography-mass spectrometry
- DMSO
-
dimethyl sulfoxide
- TLC
-
thin-layer chromatography
- VLC
-
vacuum liquid chromatography
- HepG2
-
Hepatocellular carcinoma cell line
- IC50
-
inhibitory concentration
- ITS
-
internal transcribed spacer
- PDA
-
potatoes dextrose agar medium
- MHB
-
Mueller Hinton broth
- MHA
-
Mueller Hinton agar
Abbreviations
1 Introduction
The genus Artemisia L. belonging to family Asteraceae, includes a variable number of species, with over 500 species found throughout the northern hemisphere of the world (Hayat et al., 2009). Artemisia is a good source of various secondary metabolites that has a high value in medicine and bio-pesticides (Cosoveanu and Cabrera, 2018). A. judaica L. is a perennial, fragrant little shrub that grows broadly in the southern desert of Algeria, the Sinai desert of Egypt, and in the Middle East across Jordan and Saudi Arabia (Wyk and Wink, 2004; Abu-Darwish et al., 2016). Many plants have the potential to produce bioactive compounds that help in preventing or relieving illnesses and providing a special environment for endophytic fungi (Rebecca et al., 2011). Endophytes are prevalent livening microorganisms in the internal of plant tissues without affecting obvious signs of infection. Most of these endophytic fungi that have a mutualistic association with their host, protecting them from microbial pathogen (Strobel and Daisy, 2003; Kharwar et al., 2008). The majority of endophytes are well known to have biosynthetic abilities more prominent than their host plant, because of their long co-evolution combined with genetic recombination (Fernandes et al., 2009).
Endophytic fungi are pervasive to plants and are mainly members of Ascomycota or their mitosporic fungi; some are members of Zygomycota, Oomycota, and Basidiomycota (Zheng and Jiang, 1995). Various endophytic fungi isolated from different plant groups have been identified using polyphasic approaches, including phenotypic and genotypic methods (Govindarajulu et al., 2013). About 80% endophytic fungi are responsible for production of bioactive products that have antimicrobial, and herbicidal effects (Schulz et al., 2002). Numerous antimycotic products have been created using endophytic fungi, belonging to different structural groups in particular alkaloids, phenols, flavonoids, terpenoids, and peptides (Kumar et al., 2008; Fernandes et al., 2009).
The genus Alternaria Nees (Dematiaceae), in the fungi Imperfecti, is extensive if the species found worldwide are counted, in association with an enormous assortment of substrates comprising plants, animals, soil, agricultural products, and the atmosphere (Lou et al., 2013; Eramet al., 2018). They live as plant pathogens, saprophytes, weak facultative parasites, and endophytes (Thomma, 2003). Simmons (2007) summarized the Alternaria taxonomy based on morphological characteristics, particularly 275 fungal species were identified as Alternaria spp. Metabolites from Alternaria showed a variety of bioactivities, including antimicrobial, phytotoxic, and cytotoxic activities (Bräse et al., 2009; Tsuge et al., 2013). A. tenuissima, as an endophytic fungus isolated from Tylophora indica, was found through genotypic and phenotypic examination to be more active against S. sclerotiorum and F. oxysporum, using the dual culture technique (Kumar et al., 2011). The objective of the current investigation has been to isolate endophytic fungi from A. judaica collected in Saudi Arabia, and further screen them for their antibacterial, antimycotic, and cytotoxic activity. In addition, this study has aimed to explore the bioactive metabolites profile using GC–MS.
2 Materials and methods
2.1 Collection of plant samples
Artemisia judaica L. was randomly selected from Al-Shihiyah, (27.8942°N, 42.6832°E), Haʾil, Saudi Arabia, during October–December 2018. Healthy plant parts were collected for further isolation of endophytic fungi; plant samples were transported to the lab under aseptic conditions.
2.2 Isolation of fungal endophytes
The collected plant materials were surface sterilized for the isolation processes described by Kumar et al. (2011) with some modifications. The isolation of endophyic fungi was carried out on Petri dishes that contained a potatoes dextrose agar medium (PDA). All plates were incubated at 28 ± 2 °C for up to two weeks. The fungal mycelium that were growing from the plant fragments were then isolated, purified, and maintained on PDA slants at 4 °C, for further investigations. Endophytic fungal mycelia were grown in PDA media, in order to assess their characterization and morphological identification.
2.3 Fermentation and extraction of fungal metabolites
Fermentation of pure fungal isolates was carried out in a solid rice medium (100 g rice and 110 mL H2O in 1 L Erlenmeyer flasks), autoclaved for 20 min at 121 °C. After cooling down, 2 mL spore suspension (scraped from a freshly cultivated culture) were inoculated into flasks, and incubated at 28 °C under static conditions for 30 days. After that, 500 mL of EtOAc was added to the fermented rice medium, and EtOAc extract was filtered and dried up with a vacuum rotary evaporator (BÜCHI R-114, Switzerland) at 40 °C until dry (Pretsch et al., 2014).
2.4 Preparation of plant extract
Dried A. judaica was ground into a coarse powder, and 100 g was soaked in EtOAc at room temperature for 48 h, then the filtered solution was concentrated by rotary evaporator to a small volume at 45 °C, in order to yield the crude extract for further antimicrobial activities (Pandey, 2007).
2.5 Antimicrobial activity using fungal and plant crude extracts
2.5.1 Test microorganisms
In our study microorganisms were obtained from the Bacteriology and Mycology Labs of the Botany and Microbiology Department, Faculty of Science at Al-Azhar University (Assiut), Egypt. The bacterial strains obtained for testing were Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC6538P, Escherichia coli ATCC 8739, Klebsiella pneumonia ATCC43816, and Pseudomonas aeruginosa ATCC9027, and pathogenic fungi Aspergillus flavus (Hassane et al., 2018), A. niger ATCC 16404, Alternaria alternata MLBM09, A. solani MLBM 23, Fusarium oxysporum MLBM212, and F. solani MLBM227). The bacteria were grown in Mueller Hinton broth (MHB) at 37 °C for 24 h, and the fungi were grown in PDA medium, incubated for 5 days at 28 °C.
2.5.2 Antimicrobial activity of extracts
The antimicrobial assay of the fungal and plant crude extracts underwent preliminary evaluation using the well diffusion method (Jahangirian et al., 2013), on MHA and PDA medium. Wells (diameter ∼ 6 mm) made in the inoculated plates were filled with 50 µL of a 10% (w/v) dissolved extract of dimethyl sulfoxide (DMSO). All plates were incubated for 24 h at 37 °C for bacteria and 3–5 days at 28 °C for fungi. The diameter of zone of inhibition around the well was measured in millimeters (mm). Chloramphenicol (1 mg/mL) was used as a positive control for the bacteria, and nystatin (100000 Iu/mL) for fungi, while DMSO was used as a negative control. All tests were performed in triplicate.
2.6 Identification of fungal culture
The A. tenuissima fungal isolate was identified based on colonial morphological features, microscopic observation, and well-established molecular biological protocols for the amplification and sequencing of DNA in the Internal Transcribed Spacer (ITS) region of the rRNA gene. DNA was extracted using a Patho-gene-spin DNA/RNA extraction kit, provided by the Intron Biotechnology Company (Seongnam, South Korea). The fungal DNA was then sent to SolGent Co. Ltd (Daejeon, South Korea) for polymerase chain reaction (PCR) and rRNA gene sequencing. The 18S rRNA encoding gene was amplified by PCR from purified genomic DNA using the fungal-specific primers, ITS1 (5′ - TCC GTA GGT GAA CCT GCG G − 3′), and ITS4 (5′- TCC TCC GCT TAT TGA TAT GC −3′). The purified PCR product (amplicon) was sequenced with the same primers, with the incorporation of ddNTPs, in the reaction mixture (White et al., 1990). The fungal ITS sequence fragment that was obtained was then aligned with closely-related species sequences, obtained from the NCBI nucleotide database using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The phylogenetic analyses of sequences were constructed using MegAlign (DNA Star) software, version 5.05.
2.7 Fractionation of the most active extract
Fungal and plant crude extracts were obtained through extraction by (VLC) vacuum liquid chromatography (10 × 15 cm) on a sintered funnel filled with silica gel (60–200 mesh size, Fisher, UK). Column chromatography (50 × 3 cm) was eluted with various solvent systems—methanol:water (80:20, v/v), n-hexane 100%, ethyl acetate 100%, n-hexane:ethylacetate (50:50, v/v), dichloromethane 100%, dichloromethane:methanol (50:50, v/v), and butanol—and successive fractions were collected. (TLC) Thin layer chromatography of the fractions was carried out on sheets (Silica gel 60 F254, Merck, Germany). Fractions that had the same spots on the TLC with similar Rf values were pooled, then antimicrobial activity was checked, and the most active fraction was used for further analysis.
2.8 Antimicrobial activity of AUMC14342 fractions
The antimicrobial activities of different fungal fractions were carried by agar disc diffusion method (Parekh et al., 2005). Pre-sterilized filter paper discs (Whatman no. 3, with 5 mm in diameter) were soaked in all fractions at a 30 µg/mL concentration, along with positive and negative controls, and dried in a laminar flow biological safety cabinet. The soaked discs were placed on the surface of the inoculated solidified plates, at equal distances, and then plates were incubated at 37 °C for 24 h for bacteria, and at 30 °C for 72 h for fungi. The plates were observed for the presence of inhibition of microbial growth and measured in mm.
2.9 Cytotoxicity activity
A hepatocellular carcinoma cell line (HepG2) was obtained from Nawah Scientific Inc. (Al-Mokattam, Cairo, Egypt). The cells were maintained in DMEM media, supplemented with 100 mg/mL streptomycin, 100 units/mL penicillin, and 10% heat-inactivated fetal bovine serum, in a humidified, 5% (v/v) CO2 atmosphere at 37 °C. The percentage of cell viability was calculated with the following:
Cell viability was assessed by WST-1 assay using Abcam® kit ab155902 WST-1 Cell Proliferation Reagent, as previously reported by Alaufi et al. (2017).
2.10 Gas chromatography-mass spectroscopy (GC–MS) analysis
The active fraction of the A. tenuissima strain that was obtained, along with plant ethyl acetate extract, were individually dissolved in 100% methanol and dehydrated with anhydrous Na2SO4, then filtered through a syringe filter (0.45 μm pore size) prior to injection. A Trace GC Ultra-ISQ mass spectrometer (Thermo Scientific, Austin, TX, USA), was used for the chromatographic analysis, and the compounds were separated according to Salem et al. (2016). The extract components were determined and identified using the mass spectra and retention time database that is found in the Wiley 09 and NIST 11 library.
2.11 Statistical analysis:
All the experiments were conducted in triplicate and analysis of variance was made using SPSS, program version 16. The mean of the data was calculated by analysis of variance (ANOVA), and statistical analysis was observed (at P < 0.05) to establish significant differences.
3 Results
3.1 Isolation of endophytic fungi
In this study, 19 fungal isolates were obtained from the 150 fragments analyzed (75 leaves and 75 stems fragments), which were obtained from healthy leaves and stem parts of A. judaica L. Samples of the leaves and stem parts differed in their endophytic fungal colonization, with 15 isolates (78.9%) obtained from leaves, and 4 isolates (21.1%) obtained from stem parts. The isolates belonged to Ascomycota and Hyphomycetes. Alternaria sp. isolated from the leaves was found to be predominant, with 63.1% (Alternaria alternata (Fries) Keissler (26.3%), and A.tenuissima (Kunze ex Pers.) Wiltshire (36.8%); followed by Aspergillus niger Van Tieghem from the leaves, with 15.7%; and from stem, Chaetomium sp. Kunze ex Fresen., with 5.2%; Cladosporium cladosporioides (Fresen.) G. A. de vries, with 5.2%; and Fusarium oxysporum Schlechtendal, with 10.5%.
3.2 Preliminary antimicrobial screening of isolated fungi and host plant extracts
The EtOAc crude extracts of the isolated fungi (6 species), obtained from the fermented medium and A. Judaica extract, were evaluated for antimicrobial activities by agar well diffusion method. The data obtained (Supplementary material Table S1, and Fig. 1) showed that the antibacterial activities of the fungal and plant extracts presented different degrees of inhibition, and a significant difference was observed as compared to controls. The results recorded high antibacterial activity when compared with the bacterial strains that were tested (B. subtilis, S. aureus, E. coli, K. pneumonia, and P. aeruginosa), with inhibition zones that ranged between 14.66 ± 1.45 to 38.66 ± 0.88 mm. Two isolates, A. tenuissima and Aspergillus niger crude extracts, exhibited no significant antibacterial activity when compared with all the bacteria that were tested, as compared to chloramphenicol. Meanwhile, the A. judaica plant crude extract showed varied significance with an inhibition zone that ranged between 20.33 ± 0.33 to 26.33 ± 0.88 mm. Other crude extracts of endophytic fungi showed significant inhibition when compared with the control. A. alternataand C. cladosporioides did not affect E. coli, while Chaetomium sp. showed no activity on K. pneumonia.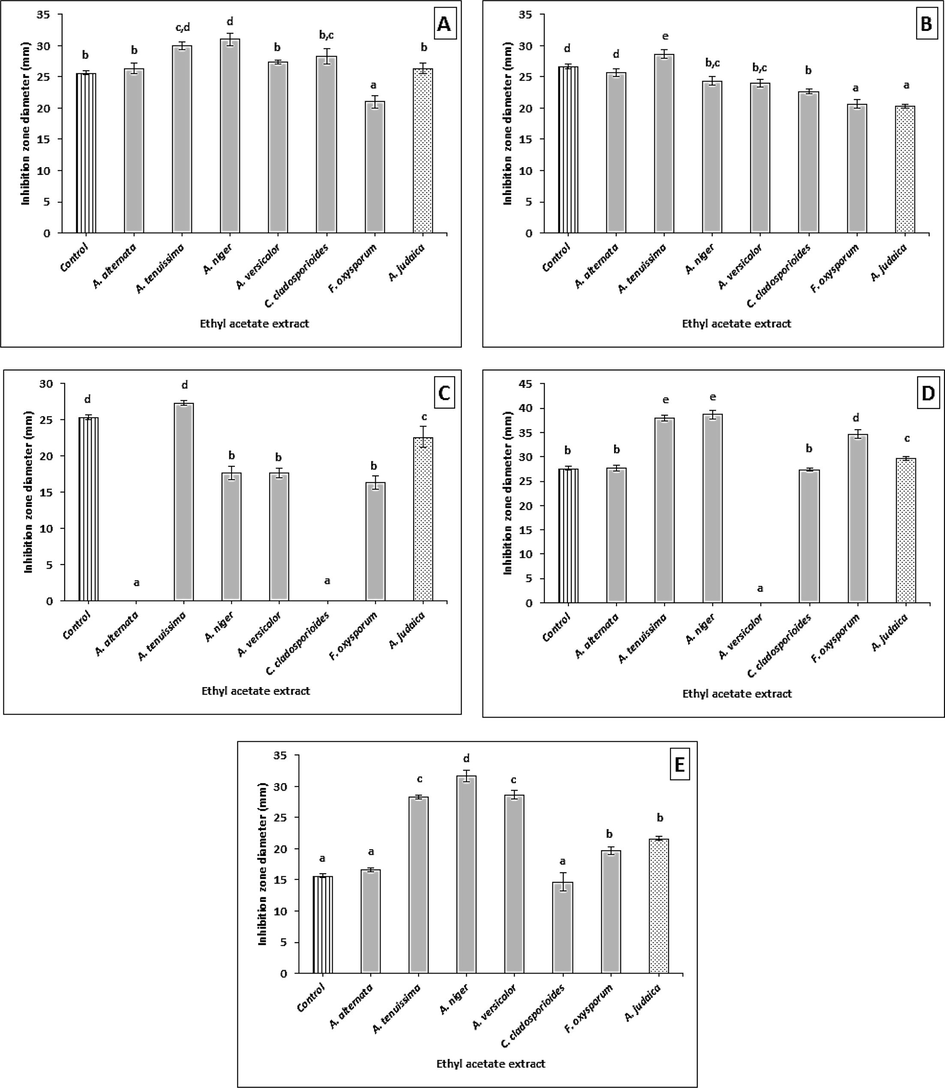
Antibacterial activity of the crude extracts against (A) B. subtilis, (B) S. aureus, (C) E. coli, (D) K. pneumoni, and (E) P. aeruginosa. Error bars represent standard error of the means (n = 3).
The antifungal activities of these extracts were also evaluated against toxigenic and plant pathogenic fungi (A. flavus, A. niger, A. alternata, A. solani, F. oxysporum, and F. solani); inhibition zones ranged from 15.00 ± 0.57 to 33.00 ± 2.51 mm. The results demonstrated that the A. tenuissima, A. niger, and A. judaica plant crude extracts exhibited a significant antimycotic activity, when compared with nystatin. On the other hand, C. cladosporioides showed no antifungal activity when compared against all of the fungi that were tested. Meanwhile, the A. alternata and F. oxysporum crude extracts only showed inhibition against pathogenic A. alternataand A. solani (Supplementary material Table S2, and Fig. 2). The results above have demonstrated that bacteria were more susceptible to ethyl acetate extracts than fungi. The A. tenuissima crude extract presented high broad-spectrum activity among other extracts; therefore, it was selected for further study.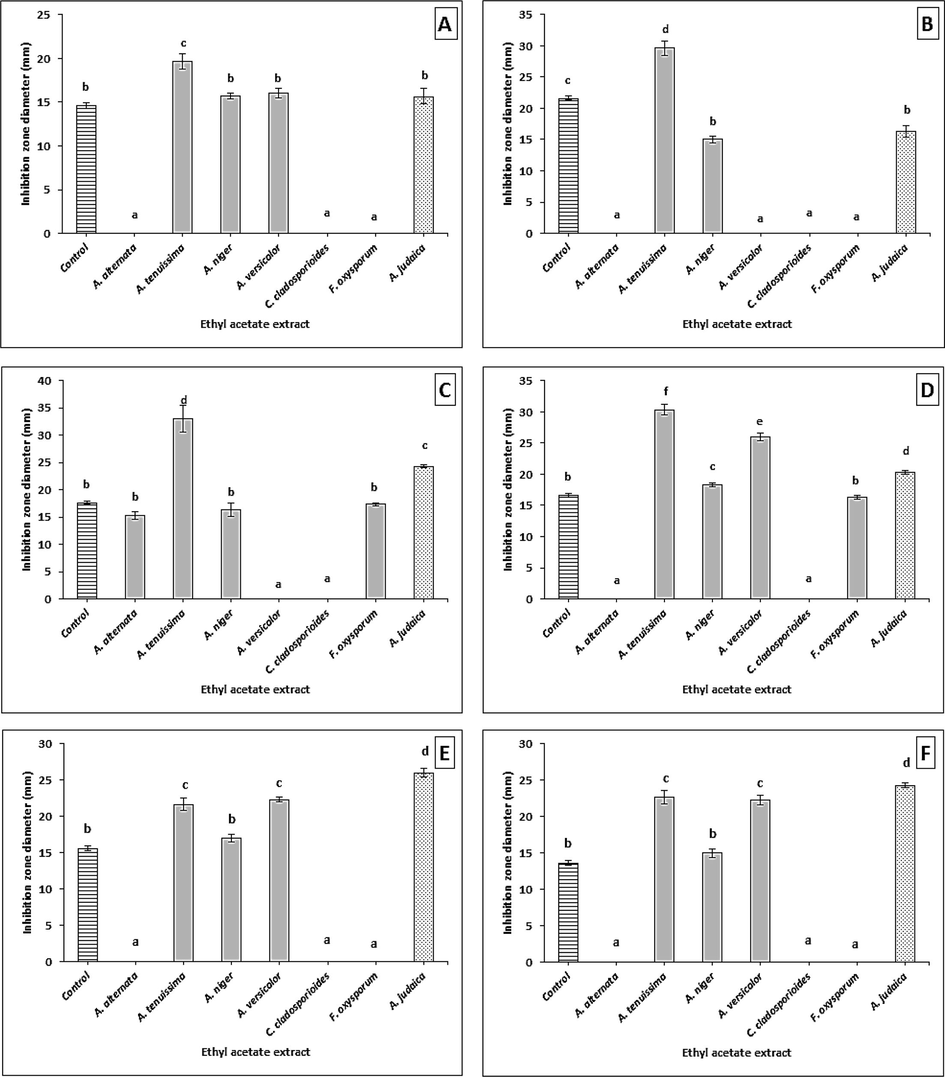
Antifungal activity of crude extracts against (A) A. flavus, (B) A. niger, (C) A. alternata, (D) A. solani, (E) F. oxysporum, and (F) F. solani. Error bars represent standard error of the means (n = 3).
3.3 18S rDNA sequencing and phylogenetic analysis of the most active culture
The molecular identity and phylogenetic characterization of the promising isolate, AUMC14342, were determined using 18S rDNA gene sequencing. The partial 18S rDNA gene sequence was detected using gel electrophoresis as a clear band at a region of ∼500 bps. Sequence homology analysis of the AUMC14342, with previously published reference sequences found using the BLASTn tool on the GenBank Database, indicated that the AUMC14342 isolate belongs to the genus Alternaria, with the greatest homology (100%) to A. tenuissima published sequences (Fig. 3). Therefore, the isolated strain was designated as A. tenuissima AUMC14342 with the registered accession number MT468650.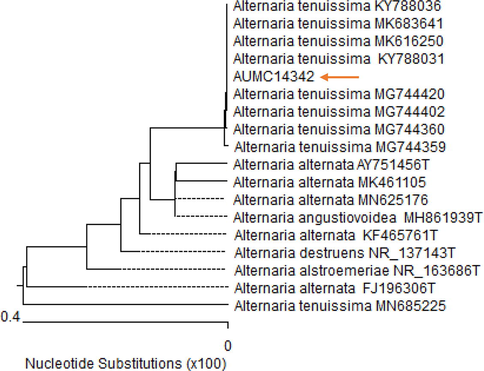
The neighbour-joining (NJ) phylogenetic tree based on ITS gene sequences of AUMC14342, with closely related strains accessed from the GenBank, (arrowed) showed a 100% identity with several strains of A. tenuissima.
3.4 Fractionation of the AUMC14342 crude extract and its antimicrobial activity
The EtOAc crude extract (3.4 g) of AUMC14342 was separated by silica gel for VLC, followed by partial purification via TLC plates, affording 7 different fractions (Fr.1-Fr.7). All of these fractions were evaluated for antimicrobial activities using the disc diffusion method (30 µg/mL). Results found that the fraction no. 3 was the most potent; it showed significantly higher activity than even the positive control used in this experiment, against different pathogenic bacteria that were tested with inhibition zones from 13.00 ± 0.57 to 21.33 ± 0.88 mm. Most of the bacteria that were tested were sensitive to the applied fractions with different degrees, and a few strains were resistant to certain fractions (Supplementary material Table S2, and Fig. 4). In addition, the antifungal activity of A. tenuissima fractions showed that fr. 3 was also the most promising active fraction, with zones of inhibition that ranged from 7.00 ± 0.57 to 16.00 ± 0.57 mm. Our data demonstrated that fungi were more resistant to most of the tested fractions, showing significant sensitivity in some fractions in comparison to control (Supplementary material Table S2, and Fig. 5). Fraction no. 3 was selected as the most potent antimicrobial extract, for further study to determine its chemical components using GC/MS analysis as well as the crude extract of the A. Judaica plant.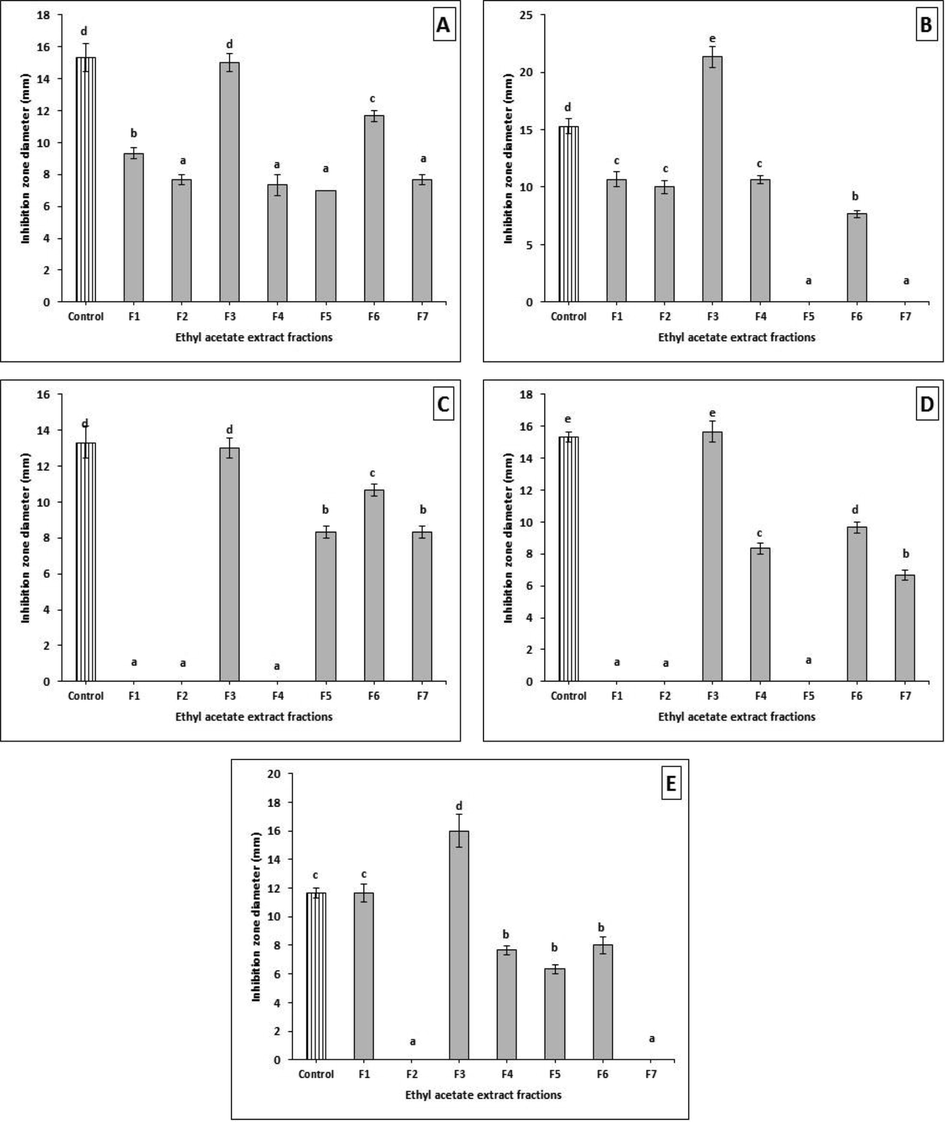
Antibacterial activity of A. tenuissima crude extract fractions against (A) B. subtilis, (B) S. aureus, (C) E. coli, (D) K. pneumonia, and (E) P. aeruginosa. Error bars represent standard error of the means (n = 3).
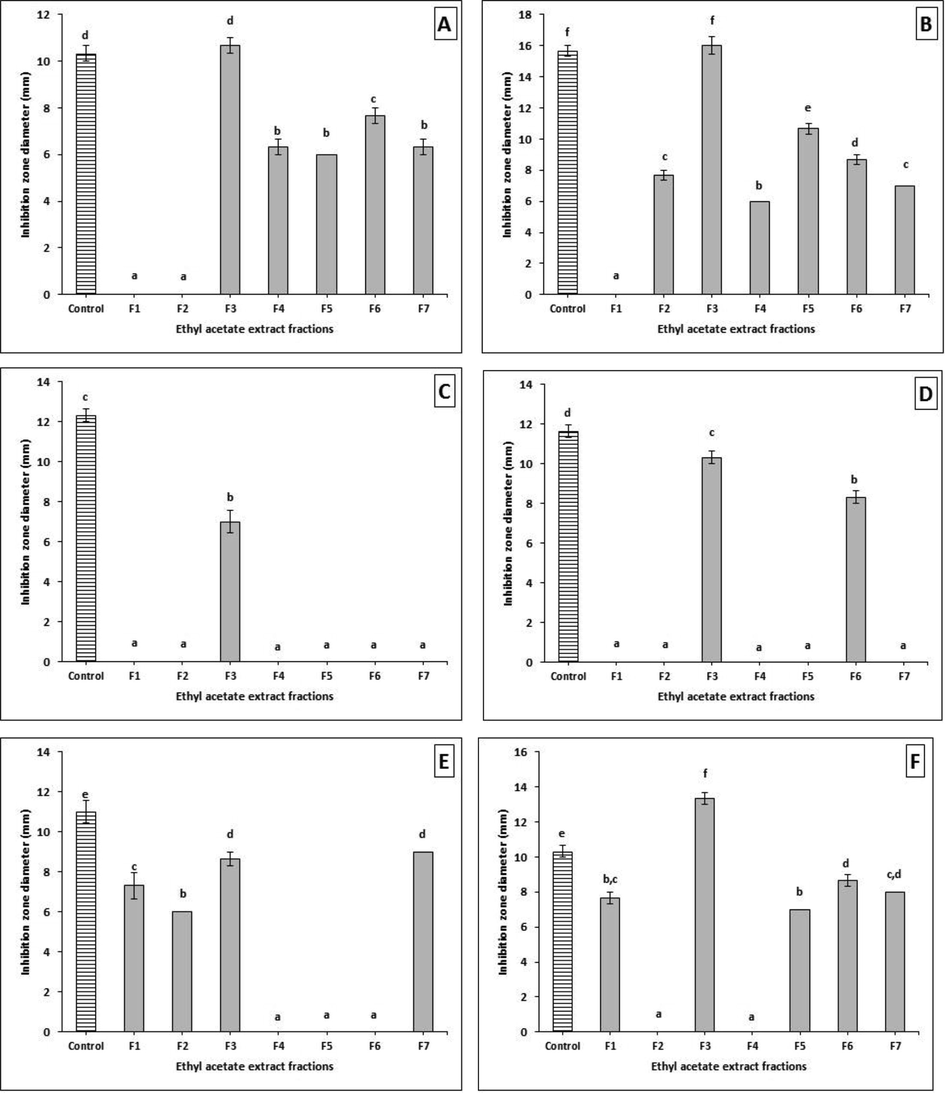
Antifungal activity of A. tenuissima crude extract fractions against (A) A. flavus, (B) A. niger, (C) A. alternata, (D) A. solani, (E) F. oxysporum, and (F) F. solani. Error bars represent standard error of the means (n = 3).
3.5 WST-1 cytotoxicity activity
The results obtained from WST-1 cytotoxicity assay were rated based on cell viability compared with a control (diluted DMSO). The different degrees of cell viability were categorized as strongly cytotoxic (>30%), moderately cytotoxic (30–59%), slightly cytotoxic (60–90%), and non-cytotoxic (>90% cell viability). Cytotoxicity results showed moderate cytotoxic activity of the A. tenuissima ethyl acetate active fraction (no. 3) against the HepG2 Hepatocellular carcinoma cell line. The lowest IC50 0.52 µM of the A. tenuissima fractions was recorded at a viability of 43.33%, compared with the negative control which did not exhibit any activity.
3.6 GC–MS based identification of bioactive compounds
The chemical composition of the ethyl acetate extract of A. judaica leaves was analyzed using GC–MS. Eighteen chemical compounds were identified, supported by a NIST database comparison in which specific peaks bore a resemblance with the nearest compound. The most abundant constituents were; camphene, artemisia ketone, camphor, (+)-dihydrocarvone, farnesene epoxide, α-bisabolol, humulol, nerolidol-epoxyacetate, and limonene dioxide (Table 1, and Fig. 6). Furthermore, GC–MS analysis of the most potent ethyl acetate fraction of A. tenuissima (no. 3) identified seventeen chemical components. The dominant compounds were hexadecanoic acid, methyl ester; 1-hexadecanol; 9-octadecenoic acid (Z)-, methyl ester; 1-hexadecanol, 2-methyl-; methyl octadeca-9,12-dienoate; and 1,2-benzenedicarboxylic acid. The peak area of each compound was directly proportional to its quantity in the extract.
a
GC–MS analysis of ethyl acetate extract of A. Judaica
No.
Compounds
Chemical formula
Molecular weight
RT (min.)
Match Factor
Area (%)
1
Camphene
C10H16
136
5.08
969
2.20
2
Artemisia ketone
C10H16O
152
7.80
937
37.03
3
Camphor
C10H16O
152
9.97
964
16.62
4
(+)-dihydrocarvone
C10H18O
154
14.87
782
1.31
5
Farnesene epoxide
C15H24O
220
21.10
786
1.83
6
α-bisabolol
C15H26O
222
23.74
953
3.72
7
Humulol
C15H26O
222
25.69
743
8.53
8
Nerolidol-epoxyacetate
C17H28O4
296
27.70
792
7.79
9
Limonene dioxide
C10H16O2
168
28.89
758
3.13
b
GC–MS analysis of ethyl acetate fraction (no. 3) of A. tenuissima
1
1-Hexadecanol
C16H34O
242
15.14
910
1.02
2
1-Eicosanol
C20H42O
298
18.30
891
0.92
3
Tert-Hexadecanethiol
C16H34S
258
19.57
790
0.65
4
Tetradecane, 2,6,10-trimethyl-
C17H36
240
20.99
812
0.84
5
cis-11-Eicosenoic acid
C20H38O2
310
21.18
764
0.63
6
Hexadecanoic acid, methyl ester
C17H34O2
270
21.50
941
21.15
7
1-Hexadecanol, 2-methyl-
C17H36O
256
22.33
746
1.08
8
Isochiapin B
C19H26O6
350
23.63
762
0.82
9
9-Octadecenoic acid (Z)-, methyl ester
C19H36O2
296
24.14
913
12.64
10
Methyl octadeca-9,12-dienoate
C19H34O2
294
24.37
918
20.95
11
Erucic acid
C22H42O2
338
25.31
816
0.92
12
1,2-Benzenedicarboxylic acid
C24H38O4
390
30.68
909
1.22
13
Squalene
C30H50
410
32.11
823
0.46
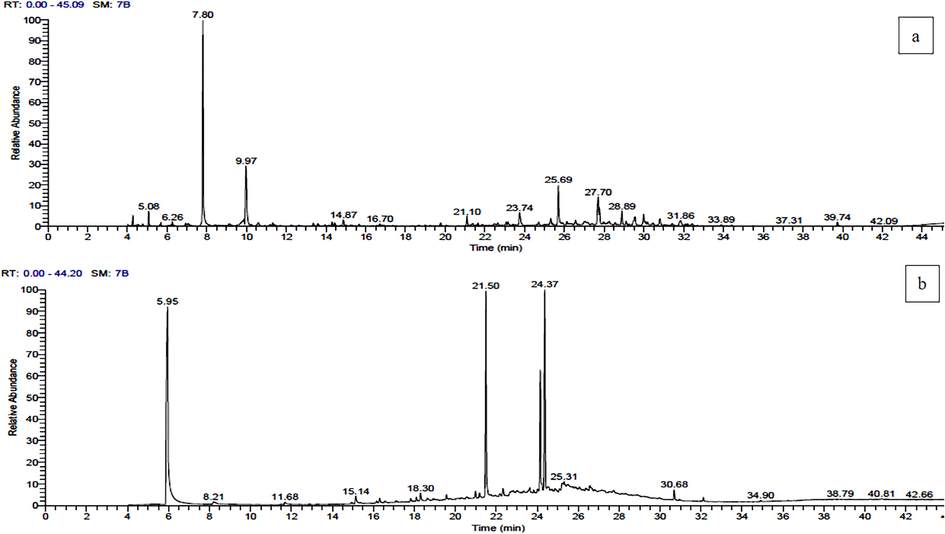
GC–MS chromatogram of (a) ethyl acetate extract of A. Judaica, and (b) ethyl acetate fraction (no. 3) of A. tenuissima.
4 Discussion
Endophytic fungi are well known to be valuable sources of a wide range of bioactive metabolites, with antimicrobial, cytotoxic, anticancer, antioxidant, antimalarial, and antiviral activities (Aly et al., 2011). There is an urgent necessity to research new medications and pharmaceuticals because of the rise in drug-resistant pathogens and emergence of new infectious diseases (Espinel et al., 2001). In the present study, A. alternata, A. tenuissima, A. niger, Chaetomium sp., C. cladosporioides and F. oxysporum, were isolated from A. judaica. Qian et al. (2014) identified the A. alternata, A. tenuissima, C. globosum, C. cladosporioides, F. nematophilum, and P. radicum strains of fungal endophytes, deriving these from Artemisia argyi via ITS rDNA sequence data. While, Zhang et al. (2012) identified 11 endophytic strains isolated from the stems of Artemisia annua and shown to have four genera (Aspergillus, Fusarium, Cephalosporium, and Mucor). Cosoveanu et al. (2018) indicated A. alternata, A. flavus, and Cladosporium sp. endophytes, from Artemisia thuscula, in a study based on morphology and phylogenetic analysis.
Our results have shown that the A. tenuissima and A. niger ethyl acetate crude extracts exhibit a good antibacterial activity against the bacteria they are tested upon, while the A. judaica plant crude extract shows bioactivity of varied significance. In this respect, Artemisia thuscula fungal endophytes have exhibited antibacterial activity against E. coli, S. aureus (Zhang et al., 2012), and ethyl acetate extracts of A. argyi fungal endophytes at 200 µg/mL have demonstrated varied antibacterial activity against Clavibacter michiganensis and Pseudomonas solanacearum (Qian et al., 2014). Al-Wahaibi et al. (2020), meanwhile, proved the antibacterial susceptibility of B. subtilis, S. pneumonia, and E. coli to A. judaica essential oil, while Guetat et al. (2017) reported antibacterial activities of taxa belonging to the genus Artemisia against the common human pathogens E. coli, S. typhimurium, S. agalactiae, E. feacium, and S. aureus.
In the current study, the impacts of the ethyl acetate extracts that were tested upon pathogenic fungi showed that A. tenuissima and A. niger, as well as A. judaica, plant crude extracts all exhibited significant antimycotic activity. Qian et al. (2014) cleared that a 1 mg/mL EtOAc extract of fungal endophytes, from A. argyi, has an antifungal effect upon the phytopathogenic fungi F. graminearum, R. solani and P. capsici. A. judaica essential oil has also been proven to have an antifungal effectupon A. fumigatus, S. racemosum, G. candidum, and C. albicans (Al-Wahaibi et al., 2020). Moreover, Abu-Darwish et al. (2016) reported that the essential oil of A. judaica is active against A. flavusand A. fumigatus, as well as against A. niger. Badawy and Abdelgaleil (2014) noted that the oil of A. judaica caused the highest spore germination inhibition of F. oxysporum, and that the A. tenuissima ethyl acetate fraction recorded the most potent antibacterial and antimycotic efficiency among seven derived fractions of the crude extracts. This fraction also showed moderate cytotoxic activity against a HepG2. An endophytic fungus A. tenuissima fermentation broth has too exhibited moderate anticancer activity (Wu et al., 2014). However, Qian et al. (2014) reported no inhibition at 20 µg/mL of A. tenuissima ethyl acetate extract against human tumor cell lines [breast (MCF-7), colon (COLO205), and leukemia (HL-60)].
With regards to the GC–MS analysis of the A. judaica ethyl acetate extract in this study, camphene, artemisia ketone, camphor, (+)-dihydrocarvone, α-bisabolol, humulol, and limonene dioxide were identified. In similar results to ours, Sallam et al. (2011) isolated camphor and piperitone as major compounds in A. judaica from Egypt; and Abu-Darwish et al. (2016) detected camphene, camphor, and artemisia ketone in A. judaica from Jordan; while Al-Wahaibi et al. (2020) reported carvone, camphor, camphene, artemisia ketone, α-humulene, α-bisabolol, farnesol, and farnesyl acetate in essential oils of A. judaica from Saudi Arabia.
In this study, GC–MS analysis of the most potent ethyl acetate fraction of A. tenuissima identified seventeen chemical components, in which the dominant compounds were 9-octadecenoic acid (Z)-, methyl ester; hexadecanoic acid, methyl ester; and methyl octadeca-9,12-dienoate. Alternaria species have been recognized among the literature as a rich fungal origin of new pharmacologically active metabolites, such as pyrones, steroids, terpenoids, phenolics, quinones, and nitrogen-containing compounds, which present diverse biological activities and include among them phytotoxic, cytotoxic, and antimicrobial properties (Lou et al., 2013). Pyrone derivatives from the endophytic fungus A. tenuissima SP-07, were evaluated for their antibacterial activities against six bacteria (B. megaterium, B. subtilis, C. perfringens, E. coli, M. tetragenus, and MRSA) (Wang et al., 2014). Elgorban et al. (2019) used GC–MS to identify bis(2-ethylhexyl) phthalate; 1,2- dibutyl phthalate; phenol, 2,4-di-t-butyl-6-nitro; and 1-octadecene; 1-tetradecene;these demonstrate antimicrobial and anticancer activity in the ethyl acetate extract from the culture filtrate of Alternaria sp. (A8), from Salvadora persica from Saudi Arabia.
5 Conclusion
Endophytic fungi show high bioactive versatility, as well as their host A. judaica. Both extracts of A. tenuissima (AUMC14342) and A. judaica have been shown to contain a variety of bioactive compounds, including antibacterial and antifungal agents, in addition to bioactivity against the HepG2 Hepatocellular carcinoma cell line. This study has used GC–MS for the bioactivity-guided active fraction of the ethyl acetate organic solvent of the isolated strain and host plant extract, leading to the determination of various chemical compounds that possess broad bio-active properties. This study concludes that A. tenuissima can be considered to be an ideal candidate for use in the industrial production of antimicrobial agents, and in pharmaceutical development.
Acknowledgements
The authors would like to thank the Distinguished Scientist Fellowship Program through research supporting project at King Saud University, Riyadh, Saudi Arabia,for funding the project (No. RSP-2020/227).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J. Ethnopharmacol.. 2016;191:161-168.
- [CrossRef] [Google Scholar]
- Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci. Rep.. 2017;7:1-12.
- [CrossRef] [Google Scholar]
- Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arab. J. Chem.. 2020;13(1):2053-2065.
- [CrossRef] [Google Scholar]
- Fungal endophytes: unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol.. 2011;90(6):1829-1845.
- [CrossRef] [Google Scholar]
- Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crops Prod.. 2014;52:776-782.
- [CrossRef] [Google Scholar]
- Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev.. 2009;109(9):3903-3990.
- [CrossRef] [Google Scholar]
- Fungi as endophytes in Artemisia thuscula: Juxtaposed elements of diversity and phylogeny. J. Fungi.. 2018;4(1):17.
- [CrossRef] [Google Scholar]
- Natural products of Alternaria sp., an endophytic fungus isolated from Salvadora persica from Saudi Arabia. Saudi J. Biol. Sci.. 2019;26(5):1068-1077.
- [CrossRef] [Google Scholar]
- Alternaria species: Endophytic fungi as alternative sources of bioactive compounds. Ital. J. Mycol.. 2018;47:40-54.
- [CrossRef] [Google Scholar]
- Global trends in resistance to antituberculosis drugs. N. Engl. J. Med.. 2001;344(17):1294-1303.
- [CrossRef] [Google Scholar]
- Biological activities of the fermentation extract of the endophytic fungus Alternaria alternate isolated from Coffea arabica L. Braz. J. Pharm. Sci.. 2009;45:677-685.
- [CrossRef] [Google Scholar]
- Endophytic Xylariaceae from the forests of Western Ghats, southern India: Distribution and biological activities. J. Mycol.. 2013;4:29-37.
- [Google Scholar]
- The genus Artemisia L. in the northern region of Saudi Arabia: Essential oil variability and antibacterial activities. Nat. Prod. Res.. 2017;31(5):598-603.
- [CrossRef] [Google Scholar]
- In vitro and in situ impact of safe synthetic and natural antioxidants on populations and aflatoxin B1 accumulation by Aspergillus flavus. Biotechnol. Sci. Res.. 2018;5(1):1-16.
- [Google Scholar]
- Ethnobotany of the genus Artemisia L. (Asteraceae) in Pakistan. Ethnobot. Res. Appl.. 2009;7:147-162.
- [Google Scholar]
- Jahangirian, H., Haron, M., Ismail, M.H.S., Rafiee-Moghaddam, R., Afsah-Hejri, L., Abdollahi, Y., Rezayi, M., Vafaei, N., 2013. Well diffusion method for evaluation of antibacterial activity of copper phenyl fatty hydroxamate synthesized from canola and palm kernel oils. Dig. J. Nanomater. Biostruct. 8(3), 1263–1270. http://www.chalcogen.ro/index.php/journals/digest-journal-of-nanomaterials-and-biostructures/8-djnb/32 volume-8-number-3-july-september-2013.
- Javanicin an antibacterial naphthaquinone from an endophytic fungus of Neem, Chloridiumsp. Curr. Microbiol.. 2008;58(3):233-238.
- [CrossRef] [Google Scholar]
- S. Kumar N. Kaushik R. Edrada-Ebel R. Ebel P. Proksch Endophytic fungi for pest and disease management A. Ciancio . andMukerji, K.G., Integrated management of diseases caused by fungi, phytoplasma and bacteria 2008 Springer Berlin 365 387 10.1007/978-1-4020-8571-0_17.
- Isolation, characterization and bioactivities of endophytic fungi of Tylophora indica. World J. Microbiol. Biotechnol.. 2011;27(3):571-577.
- [CrossRef] [Google Scholar]
- Metabolites from Alternaria fungi and their bioactivities. Molecules. 2013;18:5891-5935.
- [CrossRef] [Google Scholar]
- Anti-staphylococcal activity of a pan-tropical aggressive and obnoxious weed Parthenium histerophorus: An in vitro study. Natl. Acad. Sci. Lett.. 2007;30:383-386.
- [Google Scholar]
- Preliminary screening of some folklore medicinal plants from western India for potential antimicrobial activity. Indian J. Pharmacol.. 2005;37:408-409.
- [CrossRef] [Google Scholar]
- Antimicrobial and anti-inflammatory activities of endophytic fungi Talaromyces wortmannii extracts against acne-inducing bacteria. PLoS ONE. 2014;9(6):e97929.
- [CrossRef] [Google Scholar]
- Y. Qian J. Kang K. Geng L. Wang B. Lei Endophytic fungi from Artemisia argyi Levl. et Vant. and their bioactivity Chiang Mai J. Sci. 41 4 2014 910 921 https://scholar.google.com/scholar_lookup?journal=Chiang+Mai+J.+Sci.&title=Endophytic+fungi+from+Artemisia+argyi+Levl.+et+Vant.+and+their+bioactivity&author=Y.+Qian&author=J.+Kang&author=K.+Geng&author=L.+Wang&author=B.+Lei&volume=41&publication_year=2014&pages=910-921&.
- Isolation of Phomaspecies from Aloe vera: an endophyte and screening the fungus for taxol production. World J. Sci. Technol. Sustain. Dev.. 2011;1:23-31.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant and antibacterial activities of extracts from Schinusmolle L. wood branch growing in Egypt. J. Wood Sci.. 2016;62:548-561.
- [CrossRef] [Google Scholar]
- Effect of some essentials oil on in vitro methane emission. Arch. Anim. Nutr.. 2011;65:203-214.
- [CrossRef] [Google Scholar]
- Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res.. 2002;106(9):996-1004.
- [CrossRef] [Google Scholar]
- Alternaria: An identification manual, CBS Biodiversity Series 6. Utrecht, The Netherlands: CBS Fungal Biodiversity Centre; 2007. http://www.mycobank.org/BioloMICS.aspx?TableKey=14682616000000061&Rec=30658&Fields=All
- Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev.. 2003;67(4):491-502.
- [CrossRef] [Google Scholar]
- Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol.. 2003;4:225-236.
- [CrossRef] [Google Scholar]
- Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS Microbiol. Rev.. 2013;37(1):44-66.
- [CrossRef] [Google Scholar]
- Pyrone derivatives from the endophytic fungus Alternaria tenuissima SP-07 of Chinese herbal medicine Salvia przewalskii. Fitoterapia. 2014;99:184-190.
- [CrossRef] [Google Scholar]
- Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., eds. PCR Protocols: A guide to Methods and Applications. San Diego, U.S.A: Academic Press; 1990. p. :315-322.
- [CrossRef] [Google Scholar]
- A new compound from an endophytic fungus Alternaria tenuissima. J. Asian Nat. Prod. Res.. 2014;16(7):777-782.
- [CrossRef] [Google Scholar]
- Medicinal plants of the world. Briza publications: Pretoria, South Africa. 2004:54-56.
- [Google Scholar]
- Evaluation of anti-microbial activities of extracts of endophytic fungi from Artemisia annua. Bangladesh J. Pharmacol.. 2012;7:120-123.
- [CrossRef] [Google Scholar]
- Rhizomucor endophyticus sp. nov., an endophytic zygomycetes from higher plants. Mycotaxon. 1995;56:455-466.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101462.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







