Translate this page into:
Antimicrobial and anticancer activities of Periplaneta americana tissue lysate: An in vitro study
⁎Corresponding author. aaalkhalaf@pnu.edu.sa (Areej A. Al-Khalaf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Cancer has emerged as a life-threatening disease over the past few decades, accounting for a higher rate of mortality. The advancement in cancer therapy has improved the quality of life of patients, however, the chemotherapy/ radiotherapy resistance conferred by the cancer cells emerged as a new threat. It demands the need for novel anticancer molecules from natural products. Animal product-based anticancer therapeutics are emerging as novel drug candidates and they are also highly nutritional.
Methods
In this study, the cyclohexane extract of the American cockroach, Periplaneta americana L., has been evaluated for its inhibitory activities against fungal and bacterial human pathogens; Candida albicans and MRSA and its effect as a cancer therapeutic agent.
Results
Results showed that inhibition zones were (8, 9 mm) with minimum inhibitory concentration (MIC) of 62.5 µg/ml and 0.9 µg/ml, for both fungal and bacterial cultures, respectively. The ultrastructural changes of Candida albicans and MRSA, after treatment, were observed using a transmission electron microscope (TEM). Cockroach extracted lysate had no cytotoxic effect on normal MRC-5 human-lung cell line with CC50 value = 118 ± 3.4 µg/ml. However, the antitumor activity of Cockroach lysate showed an inhibitory effect against MCF-7 (breast human carcinoma) cell line with IC50 values = 30.2 ± 1.62 µg/ml. Chemical analysis of cockroach extract using the HPLC technique coupled with an ESI mass spectrometer proved that this extract contained high biologically active compounds.
Conclusion
Overall, the study proves the potential of P. americana lysate as a potential therapeutic agent against microbial infections and cancer cells.
Keywords
Periplaneta americana
Lysate fractionation
Biological activity
Antitumor activity
Antibacterial activity
1 Introduction
Cancer incidence and mortality show a rapid increase worldwide, making cancer the major cause of death in most regions, it has become one of the leading causes of death in many geographic areas such as Europe, China, and the United States (Mathur et al., 2020; Torre et al., 2016). Management of cancers is done with the help of a combination of radiotherapy, chemotherapy, and modern immunotherapy. However, these methods could not effectively change the causal interaction of individual factors related to the pathological process (Bower, 2014). In addition, the increased chemotherapy/ radiotherapy resistance conferred by the neoplastic cells often raises concern about the current cancer-treatment regimen (Narayanankutty, 2020; Zhang et al., 2016). Drug resistance is also a complex process involving multiple factors such as the glutathione system of the body; this enables the cancer cells to escape from the therapeutic regimen and thereby reducing the treatment efficacy and increasing the recurrence of the tumor. Thus, it has become essential to have novel strategies and improvised therapeutic compounds to manage the multi-drug resistant cancer cells (Zhao et al., 2017).
Phytochemicals have been widely utilized as the anticancer agents and primary target in drug discovery; however, these compounds are facing serious flaws in terms of bioavailability and half-life in the body. As an alternative, natural secretions from insects and animals have demonstrated potential as alternative medicine therapies (Alves and Alves, 2011; Ratcliffe et al., 2011). Ethanolic extract of the Pieridae family of butterflies led to more than 70% inhibition of tumor growth in albino rats bearing Walker 256 carcinomas (Wang et al., 2011). Similar reports of arthropod extracts as anticancer agents emerged regarding the Asian Rhinocerous beetle, Allomyrina dichotomus (Dossey, 2010). Five new phenolic compounds were extracted from the medicinal insect Blapsrynchopetera Fairmaire, the cytotoxicity of these five compounds was tested against ten common human tumor cell lines and the results showed that these compounds exhibited antitumor activities against tumor cell lines (Xiao et al., 2017). P. americana extract showed significant anti-tumor activity that might be related to enhancing immune function in vivo (Zhao et al., 2017). Periplaneta americana L., constituents also have favorable tissue-repairing, antibacterial, antitumor, and immune-enhancing activities. This insect had been used in traditional Chinese medicine (Yang and Lee, 2015; Zhao et al., 2017). In addition, Periplaneta americana L. was shown to protect the liver, promote blood vessel growth, aid in tissue repair, improve microcirculation, and enhance immunity (Tingshun et al., 2012). This study aimed at exploring Periplaneta americana L. full-body extracts for their antimicrobial and anticancer activities against human Breast cancer cell lines.
2 Materials and methods
2.1 Collection and identification of the American cockroaches
Adult cockroaches (n = 50) were collected from sewage pipes, (Giza), Egypt, using bait trapping and active collection methods. The collected samples were classified and identified as Periplaneta americana L. using a dichotomous key.
2.2 Organ lysate of cockroach
Collected cockroaches were immobilized after exposure to 4 °C for 15 min., Legs and wings were removed. Extraction was carried out by homogenizing the cockroaches using a tissue grinder (mortar) with two types of solvents (ethanol 95%, and cyclohexane), (10 gm of cockroaches: 100 ml. solvent). The samples were collected and filtered. Finally, lysates were stored at −20 °C for further studies.
2.3 Antimicrobial assay
The antimicrobial activity of the whole lysate was measured against human pathogenic yeast and bacteria; (Candida albicans (RCMB005003(1) ATCC® 10231™) and MRSA (clinical isolate), obtained from the Regional Center for Mycology and Biotechnology Antimicrobial Unit Test Organism, Al-Azhar University, Nasr City, Egypt. respectively and expressed as the diameter of inhibition zones using agar well diffusion method according to the methods of (Narayanankutty et al., 2021b). Briefly, about 20 ml of the medium (using nutrient agar media for testing bacteria and malt extract agar media for testing yeast) was poured into sterile plates (9 cm) and allowed to solidify. 5 mm diameter holes were cut in the agar using a sterile cork borer. Plates were inoculated with 0.5 ml of fixed inoculum of (MRSA and Candida albicans). Plates were dried for 30 min. Holes were filled with 100 μl of the prepared lysate dissolved in DMSO. Negative control wells were loaded with DMSO; as it was used as negative control; plates were left in a cooled refrigerator at 4 °C for one hour for diffusion; then the plates were incubated for 24 h at 37 °C for testing human pathogenic bacteria and 28 °C for testing human pathogenic yeast. At the end of the incubation period, the inhibition zones were measured at three points along the diameter of the plate and the mean was calculated (Narayanankutty et al., 2021b).
2.4 Determination of the MIC
The minimum inhibitory concentrations (MIC) of cockroach extract. After testing against Candida albicans and MRSA were measured using the dilution method (Narayanankutty et al., 2021a,b) using two-fold serial dilution starting with 1000 μg/ml. After incubation, the inhibition zones were observed on all of the plates, and the diameter of these zones was measured in millimeters. MIC was determined as the lowest concentration of the lysate giving visible inhibition.
2.5 Evaluation of the cytotoxic effects of insect lysate against human cancer cells
2.5.1 Cell lines and culture medium
Mammalian breast cancer cell line MCF-7 (passage number 25) was obtained from the American Type Culture Collection, (ATCC, Rockville, MD). Chemicals used were Dimethyl sulfoxide (DMSO), MTT, and trypan blue dye purchased from Sigma (St. Louis, Mo., USA). Fetal Bovine serum, DMEM, RPMI-1640, HEPES buffer solution, L-glutamine, gentamycin, and 0.25% Trypsin-EDTA which were purchased from Lonza (Belgium). The cells were grown on RPMI-1640 medium supplemented with 10% inactivated fetal calf serum and 50 µg/ml gentamycin. The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 and were sub-cultured two to three times a week.
2.5.2 Cytotoxic assay
The tumor cell lines were suspended in the medium at a concentration 5x104 cell/well in Corning® 96-well tissue culture plates and then incubated for 24 h. The cockroach lysate was then added to 96-well plates (three replicates) to achieve twelve concentrations. Six vehicle controls with media or 0.5% DMSO were prepared for every 96 well plates. After incubating for 24 h, the numbers of viable cells were determined including the untreated controls. All well plates were then incubated at 37 °C and 5% CO2 for 4 h. Eighty-five µl aliquot of the media was removed from the wells, and 50 µl of DMSO was added to each well and mixed thoroughly with the pipette, and incubated at 37 °C for 10 min. Then, the optical density of aliquots was measured at 590 nm using the microplate reader (SunRise, TECAN, Inc, USA) to determine the number of viable cells and the percentage of viability was calculated as [(ODt/Odc)]x100% where Odt is the mean optical density of wells treated with the tested sample and Odc is the mean optical density of untreated cells by using standard MTT assay (Koottasseri et al., 2021; Roy et al., 2016).
The media was removed from the well plate and replaced with 100 µl of fresh culture RPMI 1640 medium without phenol red then 10 µl of the 12 mM MTT stock solution (5 mg of MTT in 1 ml of PBS) was added to each well.
The relation between the survived cells and drug concentration is plotted to get the survival curve of each tumor cell line after treatment with the lysate. IC50 required to cause toxic effects against 50% of intact cells, was estimated from graphic plots of the dose–response curve for each concentration using Graph pad Prism software (San Diego, CA. USA).
2.5.3 Microscopic observation of the tumor cells treated with cockroach lysate
After the end of the treatment, the plates were inverted to remove the medium, the wells were washed three times with 100 μl of phosphate-buffered saline (pH 7.2) and then the cells were fixed to the plate with 10% formalin for 15 min. at room temperature. The fixed cells were stained with crystal violet for 20 min. The cellular morphology was observed using an inverted microscope (CKX41; Olympus, Japan) equipped with a digital microscopy camera to capture the images representing the morphological changes compared to control cells.
2.6 Transmission electron microscopy preparation
Cells; Candida albicans, MRSA, and MCF-7 (human carcinoma) were centrifuged at 4000 rpm for 10 min from 24 hrs old cultures grown on nutrient broth media and washed with distilled water; then residual cells were fixed in 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.0) for 2 hrs at room temperature, rinsed in the same buffer, and postfixed in 1% osmium tetroxide for 2 h at room temperature. Samples were dehydrated in an ethanol series ranging from 10% to 90% for 15 min in each alcohol dilution and finally with absolute ethanol for 30 min. Through a graded sequence of epoxy resin and acetone infiltrations, samples were eventually penetrated in pure resin. On copper grids, ultrathin sections were collected. The sections were then dyed twice with uranyl acetate and lead citrate, at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Egypt. Stained slides were examined at 80 kV using a JEOL-JEM 1010 transmission electron microscope (Amin, 2016; Amin et al., 2020).
2.7 Liquid Chromatography-Mass Spectrometry (LC-MS), separation and analysis
HPLC analysis coupled with an ESI mass spectrometer as a detector allows the simultaneous isolation of the compounds of cockroach lysate with the determination of the molecular weight of the isolated peaks.
Cyclohexane extracted lysates of the American cockroaches were subjected to LC-ESI-MS analysis (Center of Drug Discovery and Development Research, Faculty of Pharmacy, Ain Shams University) for the identification of homologous compounds through Agilent Mass Hunter software, while keeping in view compensation needed for charges in positive ESI MS as well as electron fragmentations, to ensure searches for the correct parent mass, to obtain the chromatograms and the prospective mass spectra of every separated fraction of the mixture of compounds.
2.8 Identification of the separated compounds
Cyclohexane-extracted lysates of cockroaches were subjected to LC-ESI-MS analysis as described above, to obtain the chromatograms and the prospective mass spectra of every separated fraction of the mixture of compounds. The MS spectra for the compounds present in cyclohexane extracted lysates were run against the NIST Mass Spectral Search Program-2009 version 2.0f for the identification of homologous compounds through Agilent Mass Hunter software, while keeping in view compensation needed for charges in positive ESI MS as well as electron fragmentations, to ensure searches for the correct parent mass.
3 Results
3.1 Antibacterial potential of cockroach extracts
The cyclohexane and ethanol extracts of the whole cockroach are found to have significant antibacterial properties. Among these, the cyclohexane extract had significantly higher potential against S. mutans, E. cloacae, and MRSA. Ethanol extract showed antibacterial activity against MRSA alone. None of these extracts were effective against S. enterica (Table 1). *Calculated mean is for triplicate measurements ± SD.
Extract
Streptococcus mutans
Enterobacter cloacae
MRSA
Salmonella enterica
Cyclohexane extract
15 ± 0.4 mm
10 ± 0.2 mm
28 ± 0.3 mm
−ve
Ethanol extract
−ve
−ve
20 ± 0.1 mm
−ve
3.2 Antifungal activity
The antifungal activity of the cockroach extracts was analyzed against Aspergillus niger, A. fumigatus, A. flavus, C. albicans, and P. italicum. Cyclohexane extract was most effective one with highest activity against C. albicans; it was also effective against all other fungi, except for A. niger. On contrary, the ethanol extract was effective against A. niger and A. fumigatus (Table 2). In all experiments, diameters were calculated by mm, without subtracting the diameter of the well-diameter (5 mm), the calculated mean is for triplicate measurements ± SD.
Extract
Aspergillus niger
Aspergillus fumigatus
Aspergillus flavus
Candida albicans
Penicillium italicum
Cyclohexane extract
−ve
18 ± 0.2 mm
13 ± 0.4 mm
27 ± 0.2 mm
15 ± 0.3 mm
Ethyl alcohol 95% extract
11 ± 0.2 mm
15 ± 0.1 mm
−ve
−ve
−ve
3.3 Determination of MIC against microbial strains
The MIC values of cockroach lysate were estimated against C. albicans and MRSA strains. The activity was higher against MRSA compared to the C. albicans (Table 3; Fig. 3).
MIC (µg/ml) inhibition zones (mm)
Test organisms
No.
Conc. (µg/ml)
Candida albicans
MRSA
1
1000
27
28
2
500
23
26
3
250
17
26
4
125
12
23
5
62.5
9
18
6
31.25
00
15
7
15.6
00
14
8
7.8
–
11
9
3.9
–
10
10
1.9
–
9
11
0.9
–
8
12
0.45
–
00
13
0.22
–
00
3.4 Cytotoxic activity on breast cancer cells
As shown in Fig. 4, there observed a dose-dependent cytotoxic activity for the P. Americana extract with an IC50 value of 30.2 ± 1.62 µg/ml. On contrary, the toxicity against normal human lung fibroblast cells was significantly lower (IC50 value = 118 ± 3.4 µg/ml).
3.5 Pathological changes after treatment of MCF-7 cells with cockroach extract
Morphological alteration of MCF-7 cells line upon exposure using Periplaneta americana L. extract was observed under an inverted microscope. The crystal-stained cells indicated the number of dead cells which increased correspondingly with the concentration of the lysate. The presence of apoptotic bodies and extensive vacuolation in the cell cytoplasm indicated an autophagy-like mechanism of cell death which was seen in the treated cells. Cell elongation shrinkage and signs of detachment from the surface of the wells denoting cell death (Fig. 5)
3.6 Transmission electron microscopy analysis
The integrated MCF-7cells in control group are interconnected with many plasma cell membrane extensions with many microvilli, as demonstrated by electron microscopy (Fig. 6-A). The separation of apoptotic bodies from apoptotic cells, the disrupted cell membrane, and the contracted nucleus were all signs of apoptosis in the treated group. Finally, all of the injured cells were lysed (Fig. 6-B).
3.7 Ultrastructural changes in MRSA and Candida albicans
In Candida albicans, the untreated cells (control) represented a typical morphology of rounded cells with an intact regular cell wall (CW), cell membrane (CM), mitochondria (M), nucleus (N), and vacuole (V) with a uniform central cytoplasmic density (Fig. 7A), while, the affected cells lost their integrity, aggregation of the cytoplasmic contents and large detachment of cell membrane from cell wall that had increasing thickness and irregularity were observed (Fig. 7B). Untreated control of Staphylococcus aureus (MRSA) cells showed uniform cytoplasmic density, a spherical shape, and a rigid surface with intact cell membranes (Fig. 7C), however, cells treated with pure compound showed detachment from the cell wall, translucent cytoplasm, shrunken, misshapen cells and completely deformation of the cells (Fig. 7D).
3.8 Fractionation and identification of compounds using LC-MS
Body extract of the American cockroaches was prepared using cyclohexane and subjected to LC-MS, for qualitative analyses. thirty compounds were separated based on the m/z ratio and retention time in the column. The identified compounds are listed in Table 4.
No.
Compounds
Formula
1
1-(S,S) -1,1-Bis(ethoxycarbonyl)-,2-bis-ptolylsulfinyl-1- ethanol
C22H26O7S2
2
1-Allyl(2-nitro-1-phenylethyl)sulfane
C11H13NO2S
3
2–2-Ethyl-3-tertbutoxyaminoquinaz olin-4(3H)-one
C14H19N3O2
4
7-(Isopropoxy)-2,2,5-trimethylchromene
C15H20O2
5
5-Ethyl-6,7-dihydr 5Hcyclopentapyrazine
C9H12N2
6
Di-isopropyl 2-phenyl ethenyl phosphonate
C14H21O3P
7
(E)-3-Benzylidene-5 methyldihydrofuran-2(3H)-one
C12H12O2
8
2,5-Dihydroxy-4′-methoxy-flavanone
C16H14O5
9
4,4-Dimethyl-3-oxacholest-5-en-7-one
C28H46O2
10
5-Methyl-2-(thiophen-2-yl) pyridine
C10H9NS
11
(2S,5R, Rs)-2-Allyl-5-methyl-2-(ptolylsulfinylmethyl) tetrahydropyran
C17H22O2S
12
(E)-3-Benzylidene-5 methyldihydrofuran-2(3H)-one
C16H16N2S
13
2-Hydroxy-2-methylhexanoic acid
C7H14O3
14
2,7,12,17-Tetraethyl-3,8,13,18-tetramethyl-21H,23H-porphine copper
C32H36Nu
15
2-Methyl-6-(3,7,12-trihydroxy-10,13-dimethylhexadecahydro1Hcyclopenta[a]phenanthren-17-yl) heptanoic acid
C27H46O
16
2-Ethyl-3-tertbutoxyaminoquinazol in-4(3H)-one
C14H19N3O2
17
Dimethyl alpha-(N acetylamino)benzyl phosphonate
C11H16NO4P
18
2-(Allylthio)-1-nitro-2-phenylethane $$ [1-(allylsulfanyl)-2-nitroethyl] benzene
C11H13NO2S
19
Methyl 5-formyl-4-methoxycarbonylmethyl-2-methylpyrrole-3-propionate
C13H17NO5
20
1-Oxa-2-oxo-5,5,8atrimethyl-8-phenylbicyclo [3.5.0(5a,8a)] dec-5(4a)-ene
C18H22O2
21
(E)-6,8-Dimethyl-1-phenyl-3-styryl-1H-[1,2,4] triazolo [3,4-f] purine-5,7(6H,8H) dione
C22H18N6O2
22
2-(3,6-Dihydropyridin-2-yl)-5,6,7,8-tetrahydro Spiro [benzo [d][1,3]thiazine 4,1′-cyclohexane]
C18H22N2S
23
2-Methoxy-2-methyl octanoic acid
C10H20O3
24
4,4 - (Iminomethylene)bis (N, N-dimethylaniline)
C17H21N3
25
4-Phenyl-3-(phenylamino)-1H-1,2,4-triazole-5(4H)-thione
C14H12N4S
26
Thiomorpholine
C4H9NS
27
6a-Hydroxy-4-methoxy-6a,6b,7,8,9,10,10a,10b-octahydro-6Hbenzo[3,4]cyclobuta[1,2-c] chromen-6-one
C16H18O4
28
3-(2′-Phenylethyl)-2,3-dihydro-6-phenyl-2,4-dioxo-4H-1,3-thiazine
C18H15NO2S
29
3,9-Dimethoxy-12,13-dihydro-5Hindolo [2,3-a] pyrrolo [3,4-c]carbazole-6-benzyl
C29H21N3O4
30
2-Isopropoxy-5-phenyl-1,3,4-oxadiazole
C11H12N2O2
4 Discussion
Natural products, especially plant extract has been the major source of bioactive compounds with pharmacological and biological potentials (Shweta and Arunaksharan, 2017; Vinayak et al., 2019). Apart from these, the utility of animal-derived compounds is also emerging in the past few decades (Abd El-Sattar et al., 2021). Periplaneta americana has been extensively studied for its uncommon ability to resist environmental threats, which has contributed to its evolutionary presence for more than 300 million years. Insect extracts are traditionally used in many diseases’ treatments, including the treatment of arthritis and diabetics (Srivastava et al., 2009). Insect larval extracts have been also used in folklore medicine in different parts of the world (Hosni et al., 2022; Talbot et al., 2006). Insects can rapidly clear microbial infections by producing varieties of immune-induced molecules including antibacterial and/or antifungal types. With emerging of antimicrobial-resistant pathogens and the passage of the resistance via resistant genes (R-factor) to susceptible microbial individuals, commonly simple infections may become serious leading to death (Nicasio et al., 2008; Nordmann et al., 2007). Antibiotic-resistant microbes continue to elevate at an alarming rate as a health problem facing humanity (Devasahayam et al., 2010; Salzet, 2001). Cockroach extract proved its inhibition activity against Gram-positive and Gram-negative resistant pathogenic bacteria (Table 1, Fig. 1)Such insect-antimicrobial activity was previously documented by Gordya et al. (2017). Different insect peptides described as defensin, diptericins, cecropins, and proline proved their activity against bacterial infection (Hedayati et al., 2007). Our results proved the antifungal activity of cockroach extract against different pathogenic fungi and yeast (Fig. 2) as Aspergillus flavus which produces aflatoxin B1, the most toxic and potent hepatocarcinogenic natural compound ever characterize (Walsh et al., 2008), A. fumigatus which incriminated as the causative pathogen of chronic pulmonary aspergillosis, A. niger which produce ochratoxins and the pathogenic Candida albicans initiate a wide range of diseases such as chronic disseminated candidiasis, endocarditis, vaginitis, meningitis, and endophthalmitis- and Penicillium italicum -attributed to the pathogenesis of pneumonia, hypersensitivity, allergic alveoli, skin sensitivity and emphysema; these pathogenic fungi resist wide range of anti-fungal drugs. The before mentioned results are in agreement with Chernysh et al. (2002), who proved the fungicidal activity of grasshopper gut bacteria against the yeast-like fungus Candida. Treatment of human breast cancer cell line with cockroach lysate recording obvious cell inhibition at IC50 concentrations = 30.2 ± 1.62 µg/ml res which are less than that needed to produce cytotoxicity for normal cells (CC50 = 118 ± 3.4 µg/ml). The safety of this lysate and its cheap supply recommend it for therapeutic manufacturing. The lysate can inhibit the growth of tumor cells which lowered the cell adhesion capacity and cell viability in a concentration-dependent manner. Calliphora maggots extract at 0.28 mg/ml could inhibit human cancer cells as mentioned by Oliver et al. (1994). The most available antitumor agents are derived from plants, microbes, and animal secondary metabolites (Anusmitha et al., 2021; Illam et al., 2017; Kim et al., 2022). Few studies are available concerning bioactive molecules derived from insects which are considered a cheap and abundant source of therapeutics; arthropod extracts as anticancer agents were evidenced regarding the Asian rhinocerous beetle, Allomyrina dichotomus, the Texas lubber grasshopper, Brachystola magna.2 and the North American yellow jacket wasp, Vespula pensylvanica (Wang et al., 2011). Results concerning tumor cell apoptosis (Fig. 6) after treatments with cockroach extract hypothesized that the lysate may inhibit the synthesis of DNA, RNA, and proteins, and block the energy metabolism of tumor cells. Previous studies proved the cytotoxicity of cockroach lysate against different human cancer lines (Hu et al., 2011). Apoptosis might also be one of the mechanisms of the inhibitory activity of Periplaneta americana extract against cancer cells. Apoptosis, to as a process of programmed cell death, has a vital role in cancer development and therapies (Narayanankutty, 2019, 2020; Roy et al., 2018). Multiple genes are involved in apoptosis in cancer cells, as the gene coding proteins, pro-apoptotic and anti-apoptotic types. Periplaneta americana extract is suggested to inhibit the proliferation of human hepatoma cells by inducing apoptosis and reducing the mitochondrial membrane potential, up-regulating apoptotic protein expression. In addition, a previous study revealed that Periplaneta americana extract induced apoptosis in human hepatocellular carcinoma cells via the mitochondrial pathway (Zhao et al., 2017). Thirty compounds were separated from cockroach lysate (Table 4) through fractionation using liquid chromatography-mass spectrometry. It is expected that the compounds can act together to produce their biological activities; previous studies in plant extract have reported such cumulative and additive effects (Parathodi Illam et al., 2019). These compounds possess characteristics in their structure like the arrangement of specific functional groups and the presence of active components, which make them biologically significant for their potential therapeutic value against various infectious and non-infectious diseases.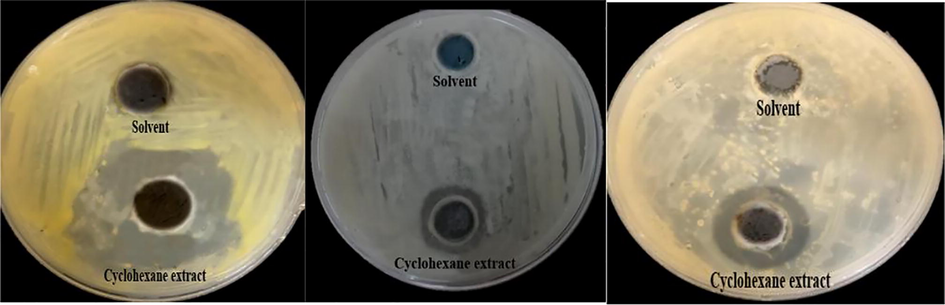
The inhibitory effect of cyclohexane extract against Streptococcus mutans, Enterobacter cloacae and Methicillin-Resistant Staphylococcus aureus respectively.

The inhibitory effect of cyclohexane extract against Aspergillus fumigatus, Aspergillus flavus, Candida albicans and Penicillium italicum respectively.

The minimum inhibitory concentrations (MIC) of pure compound extracted from Periplaneta americana L. against Candida albicans; (A): 1;1000 μg/ml, 2; 500 μg/ml, 3; 250 μg/ml, 4; 125 μg/ml, 5; 62.5 μg/ml and methicillin resistant Staphylococcus aureus (MRSA); (B): 1; 1000 μg/ml, 2; 500 μg/ml, 3; 250 μg/ml, 4; 125 μg/ml, 5; 62.5 μg/ml, 6; 31.25 μg/ml, 7; 15.6 μg/ml, 8; 7.8 μg/ml, 9; 3.9 μg/ml, 10; 1.9 μg/ml and 11; 0.9 μg/ml.

In vitro antitumor activity of different concentrations of cockroach extract against MCF-7 tumor cell lines and MRC-5 cells (normal fibroblast).

Morphological characteristics of human breast MCF-7cells treated with and without the lysate observed under an inverted microscope after 24hr. The tumor cells stained with crystal violet stain. (A); Control human breast MCF-7 carcinoma cells, (B); treated with pure compound.
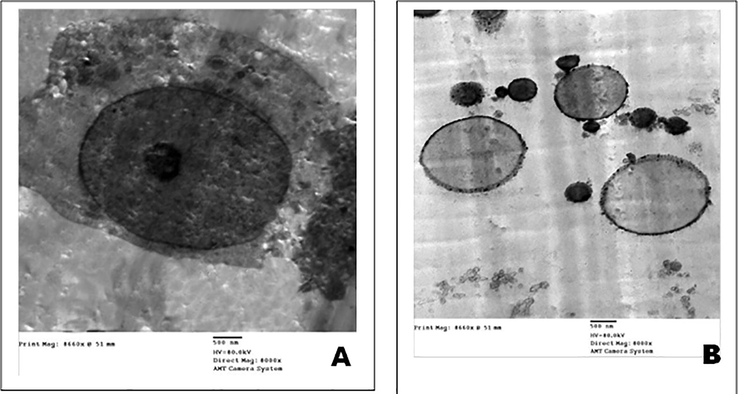
TEM micrographs of the ultrastructural characteristics of MCF-7cells treated with and without cockroach extract. (A) Control MCF-7 cells. (B) MCF-7 cells treated with cockroach extract. (Scale bar = 500 nm).
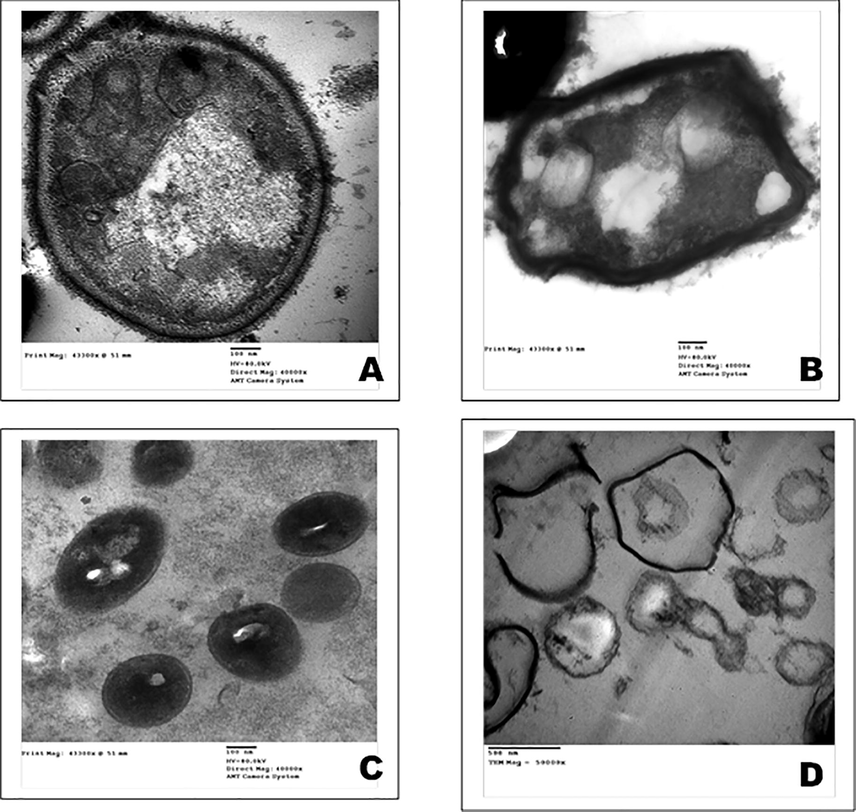
TEM micrographs of Candida albicans; A: control and B: treated with organ lysate C: control of Staphylococcus aureus (MRSA); and E: treated with organ lysate.
5 Conclusion
Traditionally the phytocompounds were utilized as the source of drug candidates against infectious diseases and cancers; however, the present study identifies the potential of insect-based drug discovery against these diseases. The cyclohexane extract of the cockroach has shown promising antibacterial and antifungal activities against drug-resistant pathogens; besides, the anticancer properties of the same are also found to be promising. In conclusion, these findings suggest that compounds present in cockroach body extract are of potential therapeutic value. Further identification, characterization, and functional studies in vitro and in vivo using individual compounds can act as a potential alternative in developing novel therapeutics against various pathogens.
Acknowledgement
The authors acknowledge Princess Nourah bint Abdulrahman University Researchers supporting Project number (PNURSP2022R37), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design, synthesis, molecular docking and in silico ADMET profile of pyrano [2, 3-d] pyrimidine derivatives as antimicrobial and anticancer agents. Bioorganic Chemistry. 2021;115
- [CrossRef] [Google Scholar]
- The faunal drugstore: Animal-based remedies used in traditional medicines in Latin America. J. Ethnobiol. Ethnomed.. 2011;7(1):9.
- [CrossRef] [Google Scholar]
- Isolation and characterization of antiprotozoal and antimicrobial metabolite from Penicillium roqueforti. Afr. J. Mycol. & Biotech.. 2016;21(3):13-26.
- [Google Scholar]
- Synthesis, characterization, and biological investigation of new mixed‐ligand complexes. Appl Organomet Chem.. 2020;34(8)
- [CrossRef] [Google Scholar]
- Phytochemical analysis, antioxidant, anti-inflammatory, anti-genotoxic and anticancer activities of different ocimum plant extracts prepared by ultrasound-assisted method. Physiol. Mol. Plant Pathol. 2021:101746.
- [CrossRef] [Google Scholar]
- Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol.. 2014;11(10):597-609.
- [Google Scholar]
- Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci.. 2002;99(20):12628-12632.
- [CrossRef] [Google Scholar]
- Newer antibacterial drugs for a new century. Expert. Opin. Investig. Drugs. 2010;19(2):215-234.
- [Google Scholar]
- Insects and their chemical weaponry: New potential for drug discovery. Nat. Prod. Rep.. 2010;27(12):1737-1757.
- [CrossRef] [Google Scholar]
- Natural antimicrobial peptide complexes in the fighting of antibiotic resistant biofilms: Calliphora vicina medicinal maggots. PLoS ONE. 2017;12(3):e0173559.
- [Google Scholar]
- Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153(Pt 6):1677-1692.
- [Google Scholar]
- Modeling the Potential Global Distribution of Honeybee Pest, Galleria mellonella under Changing Climate. Insects. 2022;13
- [CrossRef] [Google Scholar]
- Effect of Periplaneta americana extract on two human lung tumor cell lines. Chin. J. Pharm. Anal.. 2011;31(7):1245-1250.
- [Google Scholar]
- Epithelial mesenchymal transition in cancer progression: prev entive phytochemicals. Recent Pat. Anticancer Drug Discov.. 2017;12(3):234-246.
- [CrossRef] [Google Scholar]
- Azima tetracantha leaf methanol extract inhibits gastric cancer cell proliferation through induction of redox imbalance and cytochrome C release. Appl. Sci.. 2022;12(1):120.
- [Google Scholar]
- Antioxidant, anti-inflammatory and anticancer activities of the methanolic extract of Thottea siliquosa: an in vitro and in silico study. Recent Pat. Anticancer Drug Discov.. 2021;16(3):436-444.
- [CrossRef] [Google Scholar]
- Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Global Oncol. (6):1063-1075.
- [Google Scholar]
- PI3K/ Akt/ mTOR pathway as a therapeutic target for colorectal cancer: a review of preclinical and clinical evidence. Curr Drug Targets. 2019;20(12):1217-1226.
- [CrossRef] [Google Scholar]
- Phytochemicals as PI3K/ Akt/ mTOR inhibitors and their role in breast cancer treatment. Recent Pat. Anticancer Drug Discov.. 2020;15(3):188-199.
- [CrossRef] [Google Scholar]
- Chemical Composition of Cinnamomum verum leaf and flower essential oils and analysis of their antibacterial, insecticidal, and larvicidal properties. Molecules. 2021;26(20)
- [CrossRef] [Google Scholar]
- Mango ginger (Curcuma amada Roxb.) rhizome essential oils as source of environmental friendly biocides: Comparison of the chemical composition, antibacterial, insecticidal and larvicidal properties of essential oils extracted by different methods. Environ. Res.. 2021;202:111718
- [CrossRef] [Google Scholar]
- The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy. 2008;28(2):235-249.
- [Google Scholar]
- Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr. Opin. Microbiol.. 2007;10(5):436-440.
- [Google Scholar]
- The discovery of marine natural products with therapeutic potential. Discov. Novel Nat. Prod. Therap. Potential. 1994;109–174
- [CrossRef] [Google Scholar]
- Natural combination of phenolic glycosides from fruits resists pro-oxidant insults to colon cells and enhances intrinsic antioxidant status in mice. Toxicol. Rep.. 2019;6:703-711.
- [CrossRef] [Google Scholar]
- Insect natural products and processes: New treatments for human disease. Insect Biochem. Mol. Biol.. 2011;41(10):747-769.
- [CrossRef] [Google Scholar]
- Garlic phytocompounds possess anticancer activity by specifically targeting breast cancer biomarkers - an in silico study. Asian Pac. J. Cancer Prev.. 2016;17(6):2883-2888.
- [Google Scholar]
- EGFR gene regulation in colorectal cancer cells by garlic phytocompounds with special emphasis on S-Allyl-L-Cysteine sulfoxide. Interdiscip. Sci.. 2018;10(4):686-693.
- [CrossRef] [Google Scholar]
- Vertebrate innate immunity resembles a mosaic of invertebrate immune responses. Trends Immunol.. 2001;22(6):285-288.
- [Google Scholar]
- Traditional fruits of kerala: bioactive compounds and their curative potential in chronic diseases. Curr. Nutr. Food Sci.. 2017;13(4):279-289.
- [CrossRef] [Google Scholar]
- Traditional insect bioprospecting–As human food and medicine. Indian J. Trad. Knowl. (IJTK). 2009;8(4):485-494.
- [Google Scholar]
- Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis.. 2006;42(5):657-668.
- [Google Scholar]
- Research advances in pharmacological action and clinical application of Periplaneta americana. Agric. Sci. Technol. Hunan. 2012;13:888-892.
- [Google Scholar]
- Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol. Biomarkers Prev.. 2016;25(1):16-27.
- [Google Scholar]
- Heat Shock Proteins (HSPs): A novel target for cancer metastasis prevention. Curr. Drug Targets. 2019;20:1-11.
- [CrossRef] [Google Scholar]
- Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis.. 2008;46(3):327-360.
- [CrossRef] [Google Scholar]
- Chemotherapeutic effects of bioassay-guided extracts of the American Cockroach, Periplaneta americana. Integr. Cancer Therap.. 2011;10(3):NP12-NP23.
- [CrossRef] [Google Scholar]
- Chemotherapeutic effects of bioassay-guided extracts of the American cockroach, Periplaneta americana. Integr. Cancer Ther.. 2011;10(3):NP12-NP23.
- [Google Scholar]
- Five new phenolic compounds with antioxidant activities from the medicinal insect Blaps rynchopetera. Molecules. 2017;22(8)
- [CrossRef] [Google Scholar]
- Antimicrobial activities of active component isolated from Lawsonia inermis leaves and structure-activity relationships of its analogues against food-borne bacteria. J Food Sci Technol. 2015;52(4):2446-2451.
- [Google Scholar]
- Traditional Chinese medicine targeting apoptotic mechanisms for esophageal cancer therapy. Acta Pharmacol. Sin.. 2016;37(3):295-302.
- [CrossRef] [Google Scholar]
- Zhao, Y., Yang, A., Tu, P., Hu, Z., 2017. Anti-tumor effects of the American cockroach, Periplaneta americana. Chin. Med. 12, 26–26. https://doi.org/10.1186/s13020-017-0149-6.







