Translate this page into:
Antimicrobial activity of Litchi chinensis and Nephelium lappaceum aqueous seed extracts against some pathogenic bacterial strains
*Corresponding author. Tel.: +966 14785968x1204 ramesa.aftab@gmail.com (Ramesa Shafi Bhat) rbhat@ksu.edu.sa (Ramesa Shafi Bhat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Available online 6 June 2013

Abstract
Seeds aqueous extracts from Litchi chinensis and Nephelium lappaceum were investigated for antibacterial activity by disc diffusion method and protein profile. Both seed aqueous extracts show moderate inhibition against pathogenic bacteria, both gram positive including Staphylococcus aureus, Streptococcus pyogenes, and Bacilllus subtillis and gram negative bacteria including Escherichia coli and Pseudomonas aeruginosa. Overall analysis of the antibacterial activity of tested samples revealed that the highest inhibitory activity was produced by Litchi chinensis (15 ± 0.55 mm) against S. pyogenes. Tris glycine SDS PAGE revealed major protein band approximately 15.5 kDa and 22-kDa. Protein contents of Seeds of Litchi chinensis and Nephelium lappaceum were approximately 7.5 and 13.5 mg/g, respectively.
Keywords
Litchi chinensis
Nephelium lappaceum
Peptides
Seeds
Antibacterial
1 Introduction
Plants defend themselves against microbial pathogens by various defence responses like production of antimicrobial peptides, secondary metabolites, lytic enzymes, membrane-interacting proteins and reinforcement of cell walls (Scheel, 1998; Karin et al., 2000; Freeman and Beattie, 2008; Walter, 2012). Most antimicrobial proteins that have been identified belong to a group of small molecular mass antimicrobial peptides (Garćıa-Olmedo et al., 1998, 2001). Antimicrobial proteins and peptides in plants have most commonly been discovered in seeds, where they accumulate to high levels and may also function as storage proteins (Candido et al., 2011). Homologues of the seed proteins have often been found subsequently at much lower concentrations in vegetative and floral tissues (Terras et al., 1995; Candido et al., 2011). These antimicrobial agents are very similar to those of human antimicrobial peptides in structure and function and are produced as a part of their defence systems (Thevissen et al., 2007). Most of these are small Cystein-rich basic peptides which have been also classified as Cationic Antimicrobial Peptides (CAMPs) in all living organisms (Peschel and Sahl, 2006). These antimicrobial peptides are now known as good antifungal substances and occasionally have antibacterial activities in vitro (Portieles et al., 2006; Thevissen et al., 2007).
The plants chosen for the present study are L. chinensis (litchi) and N. lappaceum (Rambutan), subtropical and tropical fruit of the Sapindaceae family (Marisa, 2006). L. chinensis is a subtropical species originating from South China, while N. lappaceum is a tropical species common to Southeast Asia and but nowadays cultivation has been extended to China, India, Thailand, Taiwan, Malaysia, and Australia (Davidson et al., 2006; Jalikop, 2012). Traditionally the L. chinensis is used to relieve coughing, cure stomach ulcers diabetes, obesity and also has analgesic action (Obrosova et al., 2010; Morton, 1987). It is also used to kill intestinal worms. Recent medical reports have shown that L. chinensis fruit and seeds impede the growth of cancerous cells (Xiao et al., 2004). Its seeds also show antihyperglycemic, antihyperlipidemic, antiplatelet, and antiviral activities (Chen et al., 2007). The fruit is a rich source of flavonoids which are very effective against breast cancer (Xu et al., 2011). Rambutan has been used as traditional medicine from centuries especially as a remedy for diabetes, high blood pressure (Kaushik et al., 2010; Peter, 2010). Although antibacterial activity of various extracts of L. chinensis and N. lappaceum have been reported (Thitilertdecha et al., 2008, 2010; Wisitsak et al., 2012) but antibacterial activity of their aqueous seed extract (which has high concentration of protein) has been found lacking in literature. Therefore the present study was undertaken to compare the protein profile and antibacterial activity of aqueous seed extract of L. chinensis and N. lappaceum against human pathogens which included S. aureus, S. pyogenes, B. subtillis, E. coli and P. aeruginosa.
2 Materials and methods
2.1 Medicinal plants
Commercially available fresh fruit samples of L. chinensis and N. lappaceum were collected from Riyadh. The seeds were separated out from the fruits by hands, cleaned with distilled water and shade dried for 2 weeks.
2.2 Microorganisms for antibacterial assay
Reference bacterial strains which included S. aureus, S. pyogenes, B. subtillis, E. coli and P. aeruginosa (clinical isolates) were provided by the Department of Botany and microbiology, King Saud University. The strains were maintained on agar slant at 4 °C and sub cultured at 37 °C for 24 h on nutrient agar (Sigma–Aldrich, Germany) prior to any screening.
2.3 Preparation of seed extract
One gram of dry powder of samples was dissolved in 3 ml of 0.01 M HCl containing 0.15 M NaCl. (Ratio of sample/extract solution of 1:3 w/v). The residue was then removed by filtering through cheese cloth; the filtrate was then centrifuged at 8,100g, for 5 min. This seed extract was subjected to antibacterial activity experiments and protein determination on the same day (Franco et al., 2006).
2.4 Determination of protein content and protein pattern analysis
Protein content was determined as described by Bradford (1976) using a Bio-Rad protein assay reagent (Bio-Rad, USA) and using bovine serum albumin (BSA) as a protein standard. Absorbance was measured at 595 nm after the mixture was allowed to stand for 5 min at room temperature. Protein profile analysis of seed and fruit extract was performed by a 12% separating Tris–tricine sodium dodecyl sulphate polyacrylamide gel electrophoresis (Tris–glycine SDS–PAGE) and stained with coomassie brilliant blue R250.
2.5 Media preparation and antibacterial activity
The antibacterial assay was performed using the paper disc diffusion method (Bhat et al., 2012). Using a sterile cotton swab, the nutrient broth (Sigma–Aldrich, Germany) cultures were swabbed on the surface of sterile nutrient agar (Sigma–Aldrich, Germany) plates and allowed to dry for 5 min. Sterile filter paper discs (6 mm in diameter) impregnated with different test extracts (100 μl/disc) were then placed on the surface of inoculated agar plates. Kanamycin (30 μg/disc) was used as positive control. The plates were then incubated at 37 °C for 24 h after which microbial growth was determined by measuring the diameter of the inhibition zone (mm) using a transparent scale. Each extract was analysed in triplicate, the mean values are presented.
2.6 Minimum inhibitory concentration
The Minimum Inhibitory Concentration (MIC) was determined by using the serial dilution technique (Anu Kiruthika et al., 2011). In this technique copious of test tubes were used and each test tube was filled with 1 ml of sterile nutrient broth media and graded doses of sample solution were added. Then these test tubes were inoculated with the selected organisms (inoculums contains 1 × 106 cells/ml) followed by incubation at 37 °C for 24 h to allow the growth of the bacteria. The test tubes which showed minimum concentration as well as clear content were selected. This lowest or minimum concentration was considered as the Minimum Inhibitory Concentration (MIC).
2.7 Statistical analysis
The results were analysed by using standard deviation (SD) statistical methods.
3 Results and discussion
3.1 Protein content of crude extract from L. chinensis and N. lappaceum seed
Ten grams of dry Seeds of L. chinensis and N. lappaceum were extracted with 30 ml of extraction solution. Protein contents of Seeds of L. chinensis and N. lappaceum were approximately 7.5 and 13.5 mg/g, respectively.
3.2 Antibacterial activity
In our study we used aqueous solution for extraction of seed material which contain higher amount of protein. Four of the five selected strains were found sensitive to the protein extracts of L. chinensis and N. lappaceum seeds. The seed extracts inhibited the growth of gram positive bacteria better than gram negative strains (Table 1). Among gram (+) bacteria, the strongest activity of L. chinensis was observed against S. pyogenes (15 ± 0.55) followed by B. subtilis and S. aureus and N. lappaceum seed extract shows highest activity against S. aureus. However, P. aeruginosa and E. coli exhibited moderate to weak activities, respectively for both the test extracts. Gram (−) bacteria are surrounded with the cell wall which restricts diffusion of hydrophobic compounds through its lipo polysaccharide covering. The absence of this barrier in gram (+) bacteria allows the direct contact of the essential oil’s hydrophobic constituents with the phospholipid bilayer of the cell membrane, causing either an increase of ion permeability and leakage of vital intracellular constituents, or impairment of the bacterial enzyme systems (Zhao et al., 2001).
Bacteria
Inhibition zone amm
L. chinensis
N. lappaceum
Kanamycin
S. pyogenes
15 ± 0.55
12 ± 0.10
28
B. subtilis
11 ± 0.00
12 ± 0.40
21
S. aureus
10 ± 0.25
13 ± 0.80
26.5
E. Coli
7.5 ± 0.30
6.5 ± 0.66
20
P. aeruginosa
9 ± 0.45
10 ± 0.55
25
3.3 Minimum inhibitory concentration
S. pyogenes was found most sensitive among all the strains. Therefore MIC was determined only against S. pyogenes. To determine MIC different concentrations of sample extracts were used against selected bacteria. Results are presented in Table 2.
Extracts
S. pyogenes
Con. (mg/ml)
0
5
10
15
20
Litchi chinensis L.
−
−
−
−
+
Nephelium lappaceum L.
−
−
−
+
+
3.4 Tris–glycine SDS–PAGE
Tris–glycine SDS–PAGE revealed the major protein bands at 15.5 kDa for L. chinensis and 22-kDa for N. lappaceum as shown in Fig. 1. Earlier antimicrobial peptide studies have revealed that extracts contain protein bands in the range from 6 to 20 kDa (Diz et al., 2006; Ribeiro et al., 2007; Berrocal-Lobo et al., 2002). Therefore, we deem from this SDS–PAGE result that those proteins or some of them probably are antimicrobial peptides.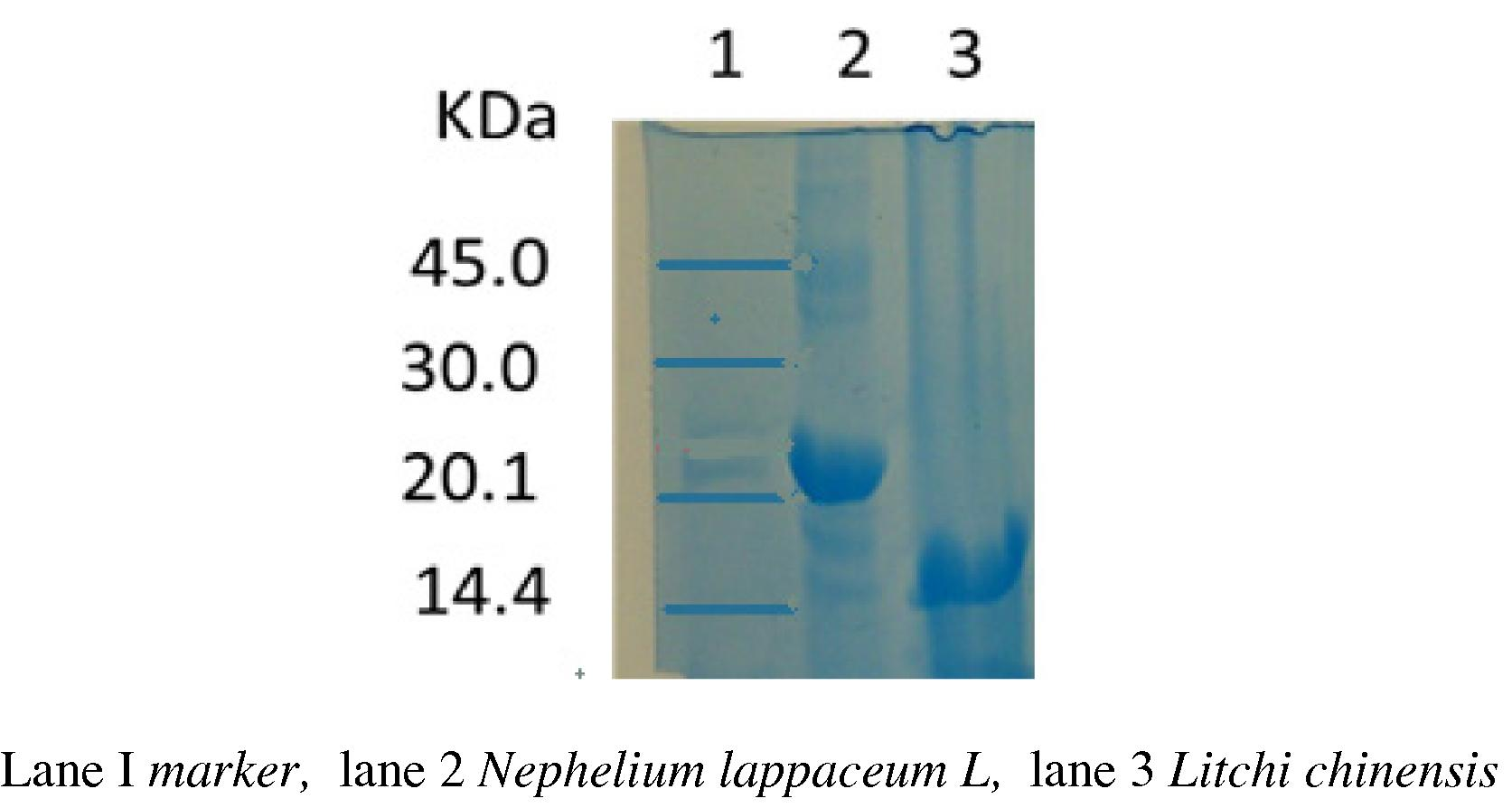
SDS–Polyacrylamide Gel Electrophoresis pattern of Nephelium lappaceum L., and Litchi chinensis crude extracts. About 55 μg of sample was electrophorised on 12% polyacrylamide gel in presence of 0.2% SDS. Electrophoresis buffer used was 0.01 M Tris–glycine containing 0.1% SDS, pH 8.4. Electrophoresis was performed at the current of 25 mA in the stacking gel region and at 30 mA as the sample entered separating gel.
4 Conclusion
The overall result of the present study concluded that aqueous seed extracts of L. chinensis L and N. lappaceum L. exhibit moderate antibacterial activity. The protein profile of the studied aqueous seed extracts showed N. Lappaceum is a rich source of protein as compared to L. chinensis. Furthermore, evaluation of active compounds responsible for the activity warns extensive investigation.
Acknowledgement
We extend our appreciation to Deanship of Scientific Research, King Saud University for funding the work through the research group project no RGP-VPP-063.
References
- Evaluation of the antimicrobial property of selected flower extracts when exposed in a hospital environment. Int. J. PharmTech Res.. 2011;2:769-774.
- [Google Scholar]
- Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol.. 2002;128:951-961.
- [Google Scholar]
- Antibacterial properties of different cultivars of Phoenix dactylifera L and their corresponding protein content. Ann. Biol. Res.. 2012;3(10):4751-4757.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Plant storage proteins with antimicrobial activity: novel insights into plant defense mechanisms. FASEB J.. 2011;25:3290-3305.
- [Google Scholar]
- Research progress in the chemical constituents and pharmacological effects of lychee seeds. Chin. J. Inf. TCM. 2007;14(5):97-98.
- [Google Scholar]
- The Oxford Companion to Food Oxford [Oxfordshire]. Oxford University Press; 2006.
- Antimicrobial peptides from chilli pepper seeds causes yeast plasma membrane permeabilization and inhibits the acidification of the medium by yeast cells. Biochim. Biophys. Acta. 2006;1760:1323-1332.
- [Google Scholar]
- Identification of a cowpea g-thionin with bacteria activity. FEBS J.. 2006;273:3489-3497.
- [Google Scholar]
- An overview of plant defenses against pathogens and herbivores. Plant Health Instructor 2008
- [CrossRef] [Google Scholar]
- Antibiotic activities of peptides, hydrogen peroxide and peroxynitrite in plant defense. FEBS Lett.. 2001;498:219-222.
- [Google Scholar]
- Jalikop, S.H., 2012. Rambutan. <http://www.fruitipedia.com/>.
- Specific binding sites for an antifungal plant defensin from Dahlia (Dahlia merckii) on fungal cells are required for antifungal activity. Mol. Plant-Microbe Interact.. 2000;13:54-61.
- [Google Scholar]
- Commonly consumed Indian plant food materials in the management of diabetes mellitus. Diabetes and metabolic syndrome. Clin. Res. Rev.. 2010;4(1):21-40.
- [Google Scholar]
- Ascorbic acid and mineral composition of longan (Dimocarpus longan), lychee (Litchi chinensis) and rambutan (Nephelium lappaceum) cultivars grown in Hawaii. J. Food Compos. Anal.. 2006;85(2):231-237.
- [Google Scholar]
- Lychee. In: Fruits of Warm Climates. Julia F. Miami, FL: Morton; 1987. p. :249-259.
- [Google Scholar]
- Diabetic cataracts: mechanisms and management. Diabetes Metab. Res. Rev.. 2010;26:172-180.
- [Google Scholar]
- The co-evolution of host cationic 5. Antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol.. 2006;4:529-536.
- [Google Scholar]
- Isolation and characterization of novel peptides from chilli pepper seeds: antimicrobial activities against pathogenic yeasts. Toxicon. 2007;50:600-611.
- [Google Scholar]
- Resistance response physiology and signal transduction. Curr. Opin. Plant Biol.. 1998;1:305-310.
- [Google Scholar]
- Small cysteine-rich antifungal proteins from radish: their role in host defence. Plant Cell. 1995;7:573-588.
- [Google Scholar]
- Therapeutic potential of antifungal plants and insect defensins. Drug Discov. Today. 2007;12:966-972.
- [Google Scholar]
- Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. Food Sci. Technol.. 2008;41:2029-2035.
- [Google Scholar]
- Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules. 2010;15(3):1453-1465.
- [Google Scholar]
- Plant defense mechanisms are activated during biotrophic and necrotrophic development of colletotricum graminicola in maize. Plant Physiol.. 2012;158(3):1342-1358.
- [Google Scholar]
- Wisitsak, P., Nimkamnerd, J., Thitipramote, N., Saewan, N., Chaiwut, P., Pintathong, P., 2012. Comparison the bioactive compounds and their activities between longan and litchi seeds extracts. 1st Mae Fah Luang University International Conference.
- Studies on the antitumor effect of lychee seeds in mice. J. Chin. Med. Mater.. 2004;27(7):517-518.
- [Google Scholar]
- Flavonoid glycosides from the seeds of Litchi chinensis. J. Agric. Food Chem.. 2011;59(4):1205-1209.
- [Google Scholar]
- Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillinresistant Staphylococcus aureus. Antimicrob. Agents Chemother.. 2001;45:1737-1742.
- [Google Scholar]







