Translate this page into:
Antimicrobial activity and stability evaluation of soap from caprine milk, yogurt, and kefir
⁎Corresponding author at: Biology Department, Brawijaya University, Jl. Veteran No 1, Malang 65145, East Java, Indonesia. fatchiya@ub.ac.id (Fatchiyah Fatchiyah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Caprine milk is a nutraceutical source with various benefits for human health. The content of bioactive peptides in caprine milk has biological functions as an antioxidant, anti-inflammatory, and antimicrobial. Based on the benefits of caprine and its dairy products, we utilized them as the main ingredient for soap formulation. Stability evaluation is important to determine the shelf life and safety of the soap. This study aimed to examine the effect of different temperatures and storage time on the physical characteristic, pH level, heavy metal contents, and the antimicrobial activity of the soaps. We showed that the color and aroma of the soaps were relatively stable with a minor change in their hardness levels. The level of pH for all samples was around 7,00–8,70, which could be safely used on human skin. The level of heavy metal including Cd, Pb, and Hg were below the maximum standard allowed for the soap products. A little increase in the number of bacteria colonies were found when the soaps were stored at the longer durations. Based on the physical stability, pH level, heavy metal contents, and antimicrobial activity, these soaps might be safe for application on human skin.
Keywords
Antimicrobial activity
Caprine milk
Dairy
Heavy metal
Soap
Stability test
1 Introduction
Caprine milk is a nutraceutical source with a higher nutrition than cow’s milk. Caprine milk has a higher calcium, potassium, phosphorus, vitamins, and less lactose. Furthermore, caprine milk contains six of ten essential amino acids with higher levels than cow’s milk (Kumar et al., 2016). The bioactive peptide in caprine has many benefits for human health such as playing an important role in the digestive system, pathogenesis, and physiology. In the previous reports, we found that alpha-S2-casein (CSN1S2) in caprine milk has eight peptide residues that have various functions such as antioxidant, anti-inflammatory, and antimicrobial (Chotimah et al, 2015; Bia et al, 2015; Triprisila et al, 2016). To increase its nutritional value, caprine milk can be processed by several methods such as pasteurization and fermentation. Pasteurization can prevent damage of the milk from the microbial and enzymes, extend shelf life, and maintain the aroma, color, and flavor same as the fresh milk condition (Sulmiyati et al, 2016).
Bioactive peptides from caprine milk yogurt have greater antioxidants activity than yogurt made from cow’s milk. During fermentation process, antioxidant is produced by the activation process of lactic acid bacteria (LAB). In addition, the LAB activity can stimulate the immune system, control the balance of intestinal flora, and function as a natural anti-aging agent. Several studies showed that the casein of caprine milk yogurt can prevent the increase of Malondialdehyde levels (MDA) (Sada et al, 2020; Papaioannou et al., 2021). Kefir is a dairy product fermented by lactic acid bacteria, acetic acid bacteria, and yeast. Peptides and other bioactive compounds produced during kefir fermentation and has several functions such as an anticarcinogenic, increased insulin sensitivity, provide therapeutic effect on osteoporosis, and antihypertensive effect (Sulmiyati et al, 2019; Sharifi et al, 2017; Alihosseini et al, 2017; Tu et al, 2015; O’Brien et al, 2015). Interestingly, the lactic acid found in the dairy product can be beneficial of acne-prone skin and eczema (Vaughn and Sivarmani, 2015). Based on their biological functions, caprine milk and its dairy products can be used as a natural ingredient to increase the value of the cosmetic products.
Soap is one of the skincare products produced by saponification reaction. Saponification is the reaction between fatty acid and base compounds (NaOH and KOH). The oil on the soap formula will be heated with the alkali (Febriani et al, 2020; Kirkbride et al, 2021). In this research, we formulated a soaps product using caprine milk, yogurt, and kefir as the main ingredients. The stability and the safety of the new cosmetic products needs to be examined before its commercialization and distribution. The physical stability of the cosmetic product can measure based on its product appearance, pH level, viscosity, and microbial activity. The quality and safety standard of the cosmetic products in Indonesia were determined and supervised by the BPOM (National Agency of Drug and Food Control). This study aimed to predict the effect of different temperatures and storage times on the stability, pH level, heavy metal contents, and antimicrobial activity of solid soap from caprine milk, yogurt, and kefir. The soaps were stored under different conditions and temperatures for 12 months. Based on our observation, we found that there is no significant change in the physical appearance of the soaps. The pH level and heavy metal contents of the soap were on the range that could be safely used in human skin.
2 Methods
2.1 Formulation of solid soap
Three different soaps were made based on their main ingredients that are caprine milk, kefir, and yogurt. The main ingredient is obtained from UPTD (Unit Pelaksana Teknis Daerah) in Malang. The pasteurized caprine milk, yogurt, or kefir was mixed with the other materials as formulated on Patent No. IDP000079150. The soap mixture was stirred until it was thick, then poured into the mold. The mixture was incubated to solidify for 24 h at room temperature. The caprine milk soap was treated at 4 °C (M4C), 25 °C (M25C), 25 °C under light exposure (M25CLE), and 33 °C (M33C). The kefir soap was also treated at 4 °C (K4C), 25 °C (K25C), 25 °C under light exposure (K25CLE), and 33 °C (K33C). Similarly, the yogurt soap was treated at 4 °C (Y4C), 25 °C (Y25C), 25 °C under light exposure (Y25CLE), and 33 °C (Y33C). The observation was performed at the 0, 1, 3, 6, 9, and 12 months of incubation.
2.2 Organoleptic and pH test
The organoleptic parameters of the soap are the aroma, color, and hardness level. The scoring for the organoleptic test can be seen in Table 1 (Rahayu et al, 2021). The pH test was carried out by dissolving 1 g of the soap in 10 mL of distilled water. pH paper was used to measure the alkalinity of the soap (Febriani et al, 2020). Twenty panelists participated in the organoleptic test of the soaps with the age range is 20–40 years old, and the gender percentage is 20% of men and 80% of women. The panelist is always the same for examining the soap organoleptic for 12 months.
Organoleptic parameters
Scale
1
2
3
4
5
Color
Black
Brown
Yellow
Slightly yellow
White
Aroma
No fragrant
Slightly fragrant
Strong fragrant
Hardness Levels
Slightly soft
Slightly hard
Hard
2.3 Heavy metals level
The level of heavy metals including Cadmium (Cd), Lead (Pb), and Mercury (Hg) were measured using the Atom Absorption Spectrophotometer (AAS). To determine the level of lead (Pb), 1 g sample were diluted with 65% HNO3 and 30% H2O and then heated at 100 °C. After cooled down, the ash of the mixture was filtered and analyzed by AAS. The level of cadmium (Cd) was measured by dissolving 1 g sample in HNO3: perchloric acid (3:1) solution. The mixtures were heated on a hotplate for 2–3 h. After that, the mixtures were dissolved with distilled water and then filtered using a filter paper. The filtrate was analyzed using AAS. The Hg levels were determined by dissolving 1 g sample in aqua regia solution (65% HNO3 and 35% HCl) and the boiled. After that, the solutions were subjected for AAS analysis (Chauhan et al, 2010; Jelic et al, 2017; Endah and Surantaatmadja, 2019).
2.4 Total Plate Count test (TPC)
Each solid soap sample is 5 g was dissolved into 45 mL of distilled water and diluted into the serial dilution method in a 10 mL test tube. Then, 1 mL of each dilution was taken and poured into Nutrient Agar (NA) media. The media was incubated at 37 °C at the incubator for 24 h. The colonies were counted by the Total Plate Count (TPC) method (Triprisila et al, 2016).
2.5 Antimicrobial activity test
The bacteria used in antimicrobial activity test is S. aureus, P. aeruginosa, and C. albicans. The bacteria culture was obtained from the Medical Faculty, Brawijaya University Type Culture Collection. The diffusion method analysis was carried out based on Tripisila et. al (2016) with some modifications. The microbial were grown in Luria Berthani (LB) agar at 37 °C for 24 h. A well of 8 mm was made in the agar and 200 µL of each soap solution was added to the well. The plate was incubated at 37 °C for 24 h. The diameters of the clear zone were measured.
3 Results
3.1 Physical characteristics of the solid soap
To examine the stability of the soap made from caprine milk, yogurt, or kefir, we performed organoleptic analysis of the soaps under different temperature storage (4 °C, 25 °C, 25 °C LE, and 33 °C) at 0, 1, 3, 6, 9, and 12 months. Twenty panelists measured the hardness level, color, and aroma of each soap, and the results were shown in Fig. 1. The aroma of the soaps was scored into 1 (no fragrant), 2 (slightly fragrant), and 3 (strong fragrant) (Table 1). The soap made of caprine milk, yogurt, or kefir has a strong fragrance. We found that there is no change in the aroma of the soap stored at different temperatures for 12-month period of incubation. The color change of the soap was categorized into 1 (black), 2 (brown), 3 (yellow), 4 (slightly yellow), and 5 (white) (Table 1). We observed that there is no significant change in the color of the soaps, and it was stable at score of 5 (Fig. 1). Caprine milk soap has a decreased score at 6 until 12 months because some of the panelists have the rate of soap color is yellow. The mean of this score is 4,67 and was still categorized as white color. The stability of the sample's color with different temperatures can indicate that the sample is safe to use.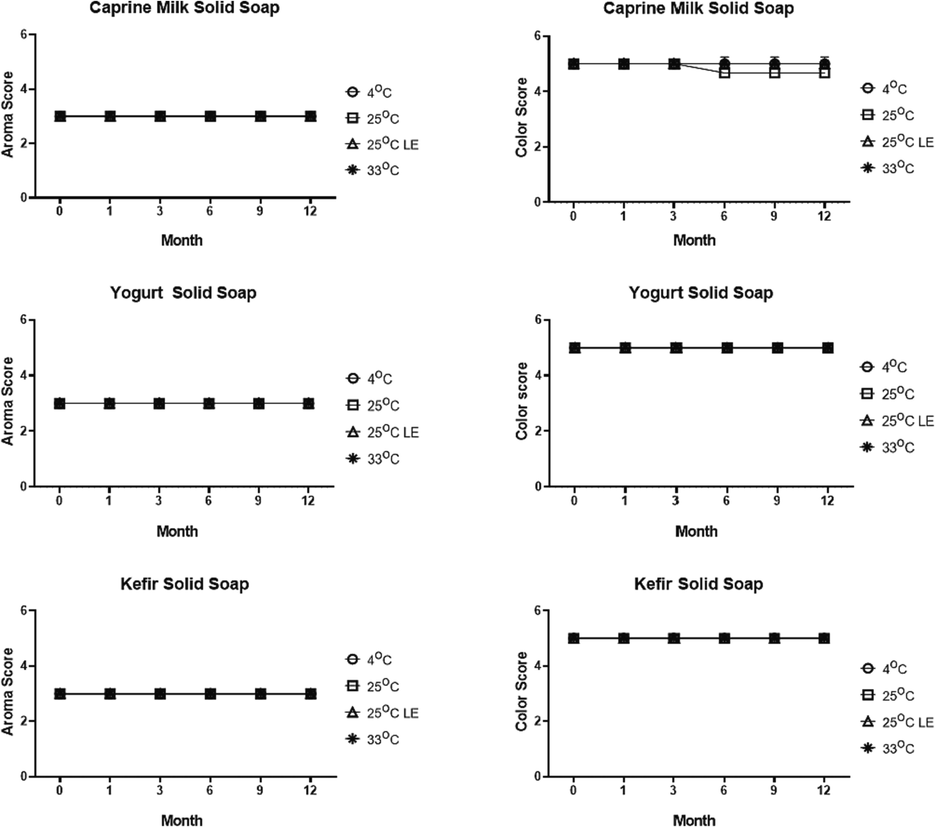
Aroma and color parameter of organoleptic test of each solid soap treatment in different temperature (in 4 °C, 25 °C, 25 °C light exposure, and 33 °C) and storage time. The two-way ANOVA test were obtained by Graphpad Prism 8.0.2 (p = 0.05).
The hardness level and pH were related to the water content in the soap sample (Fig. 2). At month 0, the mean score of all solid soaps were around 2,00 (moderately hard). From the first until the twelfth month, the hardness level of all samples was increased and had a score of 3 (hard soap). The increase of hardness can be affected by the temperature factors and storage time of the solid soap. The stability test of the cosmetic product including pH value has important. This is because the pH of the product can directly impact the health of the skin. The measurement of pH level is fluctuated in several months of incubation. The pH level of all samples was ranged from 7,00–7,50 at month 0. Subsequently, at month 12 the pH value of each soap ranged from 8,10–8,70.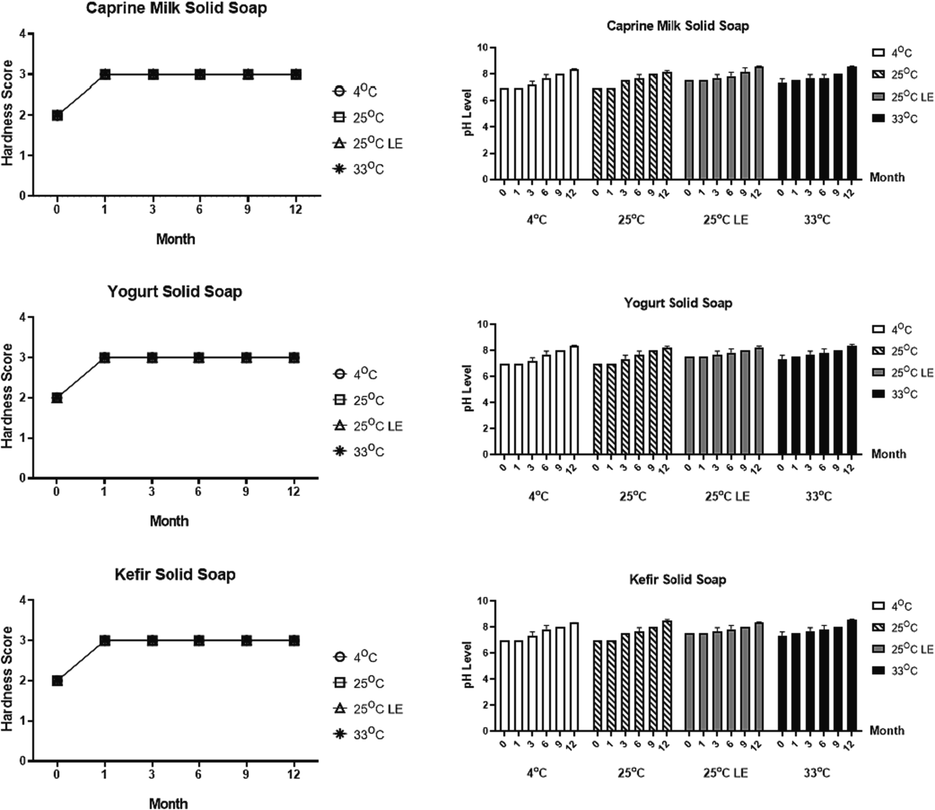
Hardness level and pH value of each solid soap treatment in different temperature (in 4 °C, 25 °C, 25 °C light exposure, and 33 °C) and storage time. The two-way ANOVA test were obtained by Graphpad Prism 8.0.2 (p = 0.05).
3.2 Heavy metal levels
The content of heavy metals in each solid soap sample was tested using the AAS. The level of Cadmium (Cd), Lead (Pb), and Mercury (Hg) were measured at 0, 1, 3, 6, 9, and 12 months (Tables 2–4). The results were then compared with the BPOM standard. The low level of cadmium was found in all sample and it was relatively stable for several months. All of the soap sample has Cd content around < 0,01 ± 0,00 mg/kg or not determined at 0–12 months, except for the kefir solid soap at 25 °C and at 4 °C is 0,25 ± 0,00 mg/kg and 0,94 ± 0,06 mg/kg in month 0. However, these results were still below the maximum standard for cosmetic product from BPOM RI (2019) which is 5 mg/kg. The levels of lead (Pb) in each soap sample with the different storage time showed the same value, <0,01 ± 0,00 mg/kg, except for the kefir solid soap at 25 °C (K25C) and light exposure (KLE) is 0,49 ± 0,00 mg/kg and 0,20 ± 0,00 mg/kg. The Hg levels of each soap sample were < 0,01 ± 0,00 mg/kg. This result indicates that the contamination of heavy metals in the solid soap is very low, and it can be categorized as safe and healthy for the application in human skin.
Soap Treatment
Heavy Metals Level (mg/kg)
Month 0
Month 1
Month 3
Month 6
Month 9
Month 12
M4C
0,55 ± 0,00
0,91 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M25C
<0,01 ± 0,00
0,41 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y4C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y25C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K4C
0,25 ± 0,00
0,32 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K25C
0,94 ± 0,06
1,13 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Soap Treatment
Heavy Metals Level (mg/kg)
Month 0
Month 1
Month 3
Month 6
Month 9
Month 12
M4C
0,55 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M25C
<0,01 ± 0,00
0,25 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y4C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y25C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K4C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K25C
0,49 ± 0,00
0,34 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Soap Treatment
Heavy Metals Level (mg/kg)
Month 0
Month 1
Month 3
Month 6
Month 9
Month 12
M4C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M25C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
M33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y4C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y25C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
Y33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K4C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K25C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K25LE
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
K33C
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
<0,01 ± 0,00
3.3 Number of colonies of the solid soap samples
The calculation of the colony will be compared with the standard from BPOM for the contamination of microbial in the cosmetic product. Based on the analysis, there are different numbers of colonies in each soap sample at several months (Table 5). All the soap samples had some colonies under 3,0 × 102 CFU/mL from month 0 until 6. However, in the 9–12 months, there was an increasing number of colonies in all the samples. The highest of the colonies was in the kefir solid soap at 25 °C (K25C) is 5,6 × 102 CFU/mL at 12 months. Based on the BPOM regulations, the number of colonies that are counted in all samples is still within the standard and classified as healthy soap.
Treatment
Number of Colony (CFU/mL)
Month 0
Month 1
Month 3
Month 6
Month 9
Month 12
M4C
0,4 × 102
0,8 × 102
1,3 × 102
1,5 × 102
2,6 × 102
3,2 × 102
M25C
0,5 × 102
0,8 × 102
1,2 × 102
1,2 × 102
2,7 × 102
4,5 × 102
M25LE
0,7 × 102
1,0 × 102
1,0 × 102
1,4 × 102
3,0 × 102
4,0 × 102
M33C
0,7 × 102
1,5 × 102
1,5 × 102
2,0 × 102
4,0 × 102
4,3 × 102
Y4C
0,4 × 102
0,8 × 102
1,3 × 102
2,0 × 102
2,7 × 102
3,4 × 102
Y25C
0,6 × 102
1,1 × 102
1,3 × 102
1,6 × 102
2,5 × 102
3,8 × 102
Y25LE
0,5 × 102
0,7 × 102
1,2 × 102
1,5 × 102
2,4 × 102
3,2 × 102
Y33C
0,6 × 102
0,8 × 102
1,6 × 102
2,0 × 102
2,6 × 102
3,9 × 102
K4C
0,2 × 102
0,7 × 102
1,6 × 102
1,9 × 102
2,5 × 102
2,7 × 102
K25C
0,5 × 102
1,2 × 102
1,7 × 102
2,0 × 102
3,7 × 102
5,6 × 102
K25LE
0,3 × 102
0,7 × 102
1,2 × 102
2,2 × 102
3,0 × 102
3,9 × 102
K33C
0,4 × 102
1,1 × 102
1,8 × 102
2,7 × 102
4,0 × 102
4,8 × 102
3.4 Antimicrobial activity
The antimicrobial activity of soap samples can be determined with qualitative analysis using an agar diffusion method and measuring the diameter of the inhibition zone (Fig. 3). The storage time can increase the diameter of the inhibition zone in all the soap samples. In the S. aureus culture, all of the samples had an inhibitory diameter zone is 9,17 mm, except the caprine milk soap at 33 °C, the yogurt soap at 4 °C, and the kefir soap at 33 °C. The diameter at 6, 9, and 12 months have nearly stable in all temperatures which are 10,00–10,83 mm. The P. aeruginosa culture had a stable diameter of inhibition zone from 1 to 12 months is 10,00–10,83 mm for all soap treatments. The lowest diameter in the caprine milk soap is 8,00 mm at 0 months. Different results were seen in C. albicans culture than the others. The kefir soap had a more stable diameter of inhibition zone than caprine milk and yogurt soap at 1–12 months which is 10,00–10,50 mm. The highest diameter at 0 months was found on caprine milk soap at 25 °C, yogurt soap at 25 °C LE and 33 °C, and kefir soap at 25 °C. Yogurt soap at 25 °C LE had similar diameters at 0, 1, and 3 months.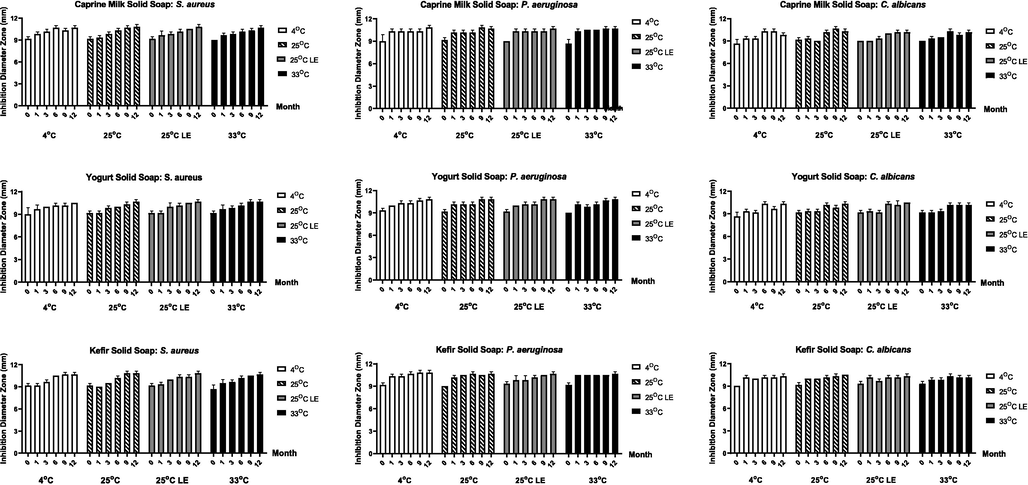
Inhibition diameter zone of each solid soap treatment in different temperature (in 4 °C, 25 °C, 25 °C light exposure, and 33 °C) and storage time. The two-way ANOVA test were obtained by Graphpad Prism 8.0.2 (p = 0.05).
4 Discussion
The panelist’s preference for the aroma of each soap sample is shown in Fig. 1. The stability of aroma in the different temperatures indicates that there was no change reaction in all samples. This can conclude that the soap samples are stable in terms of their aroma. Caprine milk, yogurt, and kefir contain volatile compounds such as octanoic acid (caprylic acid), decanoic acid (capric), nonanal aldehydes, and carboxylic acids, that play a role in influencing the aroma and quality of the product. The level of caprylic acid and capric acid is higher than the others and can be characterized by a sharp and persistent odor that is typical of caprine (Sant’Ana et al, 2017). Volatile compounds in yogurt are aldehydes, ketones, carboxylic acids, hydrocarbons, and terpenes. Aldehydes are a degradation product from milk fat or amino acid catabolism. Ketones are higher volatile compounds than others in yogurt (Papaioannou et al, 2021). The aroma of kefir is rather sour but not fresh. This aroma can produce from metabolite products such as lactic acid, carbon dioxide, ethanol, acetaldehyde, diacetyl, and acetone acid (Beshkova et al, 2003).
The ingredients in the soap formula can affect the color of the soap. Caprine milk is high in vitamins A and B but contains fewer carotenoids. There is the conversion of carotenoids to retinol that causes the color of caprine milk to become whiter. Therefore, the soap has white and clean (Kumar et al, 2016). Yogurt and kefir are fermented products and have the same color as the caprine milk which is white. The kefir grain that was added to the kefir mixture has a yellow color, but it does not change the color of the kefir. Kefir grain can change the taste and smell of the kefir (Sulmiyati et al, 2019; Bruzantin et al, 2016).
Moisture content is the parameter that is used to determine the shelf life of the product, especially soap. The water content of the soap product should not be more than 10–20% because it promotes the growth of microbial. The high temperature used to store the soap samples can decrease the water content. This is because the water will evaporate when the saponification process still running. Soap samples stored for a long time can make the saponification process perfect. The saponification process produces soap and glycerin. Glycerin can bind with water, so if the soap was stored for a long time and at a high temperature a lot of glycerin will be produced. This condition will make the water content in the soap decrease because the water binds with glycerin (Febriani et al, 2020; Idoko et al, 2018; Pramadhanti and Dianursanti, 2019).
Soap can undergo a curving process which reduces the water content in the soap through the evaporation process and make the soap harder. Furthermore, the curing process can influence the pH value of soap (Faruk et al, 2021; Widyasanti et al, 2018). Based on the result, the pH value of all samples according to the 2016 BSN standard is 8–11. The surface of the skin has slightly acidic. Soap as a cleanser can increase the pH of the skin’s surface. Increasing the skin pH can affect the skin's dehydration, and irritability, and increase the count of propionibacteria (Tarun et al, 2014).
Low heavy metal levels were detected in all samples at 0 and 1 months but at 3, 6, 9, and 12 months. The presence of heavy metal contamination in cosmetics is common, but it needs to be controlled. Contamination in cosmetics can occur during the production process or from raw materials in the formula. The cosmetics material, especially soap, which is oil, dyes, pigments such as titanium dioxide, and water can affect the levels of heavy metals. Based on the result, heavy metal levels in all soap samples are still within the standard and could be categorized as safe. However, the “safe” levels still need to be evaluated because metals can accumulate in the human body for a long time. Several heavy metals also cause varied long-term health effects, such as cancer, disorders of reproductive, cardiovascular, and problems with kidneys, and other vital organs disease. Lead, cadmium, and mercury include metals as potential impurities and have toxic or allergology concerns in the human body (Bocca et al, 2014).
The quantitative analysis for microbiology tests can use the Total Plate Count (TPC) method. TPC aims to determine the number of colonies of microbial in solid soap samples (Wenas et al, 2020). An increase in the number of microbial colonies at 9 and 12 months is possible due to environmental factors or related to heavy metal levels. Contamination of the heavy metals in the soap samples can be utilized by the microbes to grow. Microbial, such as bacteria or fungi, can process the heavy metals to be the source of energy on the cell surface and change into a non-toxic product called bioremediation. Microbial bioremediation also depends on environmental conditions suitable for their growth and metabolism. The temperature has an important role in accelerating the metabolism and enzyme activity to carry out bioremediation become fast (Rana et al, 2020; Tarekegn et al, 2020).
Storage time can affect the inhibition zone diameter of each soap sample in all microbial cultures. The saponification process has an impact on reducing the water content in the soap. The water content besides affecting the hardness of the soap, can accelerate the growth of microorganisms in the soap. That condition affects the shelf life of soap which does not last long. When the soap was stored for a long time, the saponification process will be more perfect and decrease water content, so the bacteria can be reduced (Aznury et al, 2022). In addition, the materials of the soap formulas also affect the growth of microorganisms. Caprine milk, yogurt, and kefir have bioactive compounds to inhibit the growth of pathogenic bacteria. In addition, the probiotics contained in fermented milk can produce antimicrobial peptides. The biochemical activity carried out by lactic acid bacteria (LAB) can produce peptide fragment that shows antimicrobial activity (Triprisila et al, 2016; Hanifah et al, 2016). Coconut oil that is used in the formula also has antimicrobial activity on several microorganisms is C. albicans, Gram-positive and negative bacteria such as E. vulneris, S. aureus, and S. mutans (Peedikayil et al, 2016).
5 Conclusion
The physical stability of the soaps was not affected by the temperature and time of incubations. Based on the analysis of heavy metal content and antimicrobial activity, the solid soap samples from caprine milk, yogurt, and kefir are categorized as safe and healthy for use on human skin.
Funding
This work was supported by the Brawijaya University Research Grant for HAPPU [grant numbers 959.6/UN10.C10/PN/2022].
CRediT authorship contribution statement
Fatchiyah Fatchiyah: Conceptualization, Supervision, Writing – review & editing, Investigation, Data curation, Formal analysis, Validation, Writing – original draft. Elsa Rahmania Criswahyudianti: Methodology, Software, Formal analysis. Nia Kurnianingsih: Validation, Writing – review & editing. Ema Pristi Yunita: Validation, Writing – review & editing. Regina Putri Virgirinia: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of probiotic fermented milk (kefir) on serum level of insulin and homocysteine in type 2 diabetes patients. Acta Endo. (BUC). 2017;13(4):431-436.
- [Google Scholar]
- Production of solid soap with addition of green betal leave (Piper Betle L.) extract and left lemon extract (Cymbopogon Nardus L. Rendle) as antioxidants. Atlantis Highlights in Engineering. 2022;9:148-157.
- [Google Scholar]
- Production of volatile aroma icompounds by kefir starter cultures. Int. Dairy J.. 2003;13:529-535.
- [Google Scholar]
- Goat milk CSN1S2 is able to decrease the severity scoring, TNF-α, and RAGE expression in complete Freund’s adjuvant-induced rheumatoid arthritis model of rats. Biomarkers and Genomic Medicine. 2015;7:64-71.
- [Google Scholar]
- Toxic metals contained in cosmetics: a status report. Regul. Toxicol. Pharm.. 2014;68(3):447-467.
- [Google Scholar]
- Physicochemical and sensory characteristics of fat-free goat milk yogurt with added stabilizers and skim milk powder fortification. J. Dairy Sci.. 2016;99:3316-3324.
- [Google Scholar]
- Determination of lead and cadmium in cosmetic products. J. Chem. Pharm. Res.. 2010;2(6):92-97.
- [Google Scholar]
- CSN1S2 protein of goat milk inhibits the decrease of viability and increases the proliferation of MC3T3E1 pre-osteoblast cell in methyl glyoxal exposure. Asian. Pac. J. Trop. Dis.. 2015;5(3):219-223.
- [Google Scholar]
- The determination of heavy metals level: lead in cosmetic soap preparation by atomic absorption spectrophotometer (AAS). J. Phys.: Conf. Ser.. 2019;1179:1-6.
- [Google Scholar]
- Comparative studies of the curing and hardening process of soaps produced from locally processed saturated and unsaturated fatty acids. Algerian Journal of Engineering and Technology. 2021;5:1-8.
- [Google Scholar]
- The utilization of oil palm leaves (Elaeis guineensis Jacq.) waste as an antibacterial solid bar soap. IOP Conf. Ser.: Earth Environ. Sci.. 2020;572:1-10.
- [Google Scholar]
- Antimicrobial activity of goat milk yogurt with addition of a probiotic Lactobacillus acidophilus IIA-2B4 and roselle (Hibiscus sabdariffa L) Extract. Int. Food Res. J.. 2016;23(6):2638-2645.
- [Google Scholar]
- Quality assessment on some soaps sold in Nigeria. Niger. J. Technol.. 2018;37(4):1137-1140.
- [Google Scholar]
- Arsenic and mercury content determination in commercial cosmetics products by atomic absorption spectroscopy. Quality of Life. 2017;8(1–2):23-26.
- [Google Scholar]
- Designing a suitable stability protocol in the face of a changing retail landscape. MDPI. Cosmetics. 2021;8(64):1-8.
- [Google Scholar]
- Nutritional and nutraceutical properties of goat milk. Indian Journal Dairy Sci.. 2016;69(5):513-518.
- [Google Scholar]
- The effects of postexercise consumption of kefir beverage on performance and recovery during intensive endurance training. J. Dairy Sci.. 2015;98:7446-7449.
- [Google Scholar]
- Profile of volatile compounds in dessert yogurts prepared from cow and goat milk using different starter cultures and probiotics. Foods. 2021;10:1-15.
- [Google Scholar]
- Comparison of antibacterial efficacy of coconut oil and chlorhexidine on Streptococcus mutans: an in vivo study. J. Int. Soc. Prev. Community Dent.. 2016;6:447-452.
- [Google Scholar]
- Effect of increasing reaction temperature on quality of VCO and microalgae Spirulina platensis-based anti-bacterial soap. AIP Conference Proceedings. 2019;2193:1-6.
- [Google Scholar]
- Environmentally safe technology with the conversion of used cooking oil into soap. J. Phys. Conf. Ser... 2021;1869:1-7.
- [Google Scholar]
- A novel strategy: microbes in relation to heavy metals toxicity and future prospects. J. Soil Plant Biol.. 2020;1:113-128.
- [Google Scholar]
- Peraturan Badan Pengawas Obat dan Makanan Nomor 12 Tahun 2019 Tentang Cemaran Dalam Kosmetika. Jakarta: BPOM RI; 2019.
- Study of goat milk and goat milk yogurt to decrease parasitemia index on malaria-infected mice. Food Res.. 2020;4(3):127-133.
- [Google Scholar]
- Sant’Ana, A. M. S., Ribeiro, J. E. S., Bezerril, F. F., Silva, F. L. H., Madruga, M. S., Queiroga, R. C. R. E., 2017. Volatile compound characterization of caprine milk by multivariate optimization of headspace solid phase micro-extraction (HS-SPME). Revista Mexicana de Ingeniería Química 16 (3), 781-791.
- Kefir: a powerful probiotics with anticancer properties. Med. Oncol.. 2017;34(183):1-7.
- [Google Scholar]
- Kajian kualitas fisik susu kambing peranakan ettawa (PE) dengan metode pasteurisasi yang berbeda. Jurnal Ilmu dan Teknologi Peternakan. 2016;4(3):130-134.
- [Google Scholar]
- The physicochemical, microbiology, and sensory characteristics of kefir goat milk. Tropical Animal Science Journal. 2019;32(2):152-158.
- [Google Scholar]
- Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food & Agriculture. 2020;6(1):1-19.
- [Google Scholar]
- Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J. Dermatol.. 2014;59(5):442-444.
- [Google Scholar]
- The comparing of antimicrobial activity of CSN1S2 protein of fresh milk and yogurt goat breed ethawah inhibited the pathogenic bacteria. Mater Sociomed. 2016;28(4):244-248.
- [Google Scholar]
- Short-term effect of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS One. 2015;10(12):1-17.
- [Google Scholar]
- Effects of fermented dairy products on skin: a systematic review. J. Altern. Complement. Med.. 2015;21(7):380-385.
- [Google Scholar]
- The production of paper soaps from coconut oil and Virgin Coconut Oil (VCO) with the addition of glycerine as plasticizer. IOP. Conf. Series: Earth and Environmental Science. 2018;141:1-13.
- [Google Scholar]







