Translate this page into:
Antimicrobial activities of heterocycles derived from thienylchalcones
*Tel.: +267 3552494; fax: +267 2638 mazimbaof@yahoo.com (Ofentse Mazimba)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 28 June 2014
Peer review under responsibility of King Saud University.

Abstract
Thiophene analogues of chalcones were synthesized in good yields by condensation of 2-acetylthiophene and salicylaldehydes. Solvent-free Michael addition of cyclohexanone to 2-thienylchalcones devoid of hydroxyl groups yielded 1,5-diketones. The chalcones and 1,5-diketones were utilised as synthons for flavans, 6H-benzo[c]chromen-6-ones, tetrahydro-2H-chromens, tetrahydroquinolines and diazepines. The methods utilised were short and efficient in good yields and operational simplicity. The synthesized heterocyclic compounds were characterised by IR, NMR and HR-MS spectral data and screened for their antimicrobial activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa and Candida albicans. The compounds demonstrated moderate to good antibacterial and antifungal activities. The synthesis of new heterocyclic compounds with an antimicrobial activity argument this study.
Keywords
2-Thienylchalcones
Flavans
1,5-Diketones
Tetrahydroquinolines
Diazepines
Antimicrobial
1 Introduction
Chalcones are an important group of natural products that consist of two aromatic rings joined by an α,β-unsaturated carbonyl system. The α,β-unsaturated carbonyl system enables chalcones and their heteroanalogs to undergo conjugated addition reactions in the presence of Lewis acid and basic catalysts (Al-Jaber et al., 2012; Samshuddin et al., 2012). Literature has indicated that this reaction has been exploited to obtain heterocyclic compounds of biological significance, such as pyridines, pyrazoles, pyrimidines, isoxazoles (Azab et al., 2013; Samshuddin et al., 2012), 1,5-benzodiazepines (Al-Jaber et al., 2012), flavonoids (Bano et al., 2013), thiazines (Konstantinova et al., 2007) and cyclohexenones (Sreevidya et al., 2010; Roman, 2004).
The appreciation of chalcone derived heterocyclic compounds’ diverse biological applications and the continuous application of chalcone derivatives as synthons in organic synthesis, has led to the synthesis of thiophene analogues of chalcone and their subsequent heterocyclics. The thiophene heteroaryl ring is important owing to elemental sulphur having antifungal properties, while chalcones bearing sulphur either as a thiophene or as a side chain (thiomethyl group) have been reported to exhibit biological activities such as antimicrobial, antibacterial, antifungal (Tran et al., 2012; Ranganathan et al., 2012) and anti-tumour (Rizvi et al., 2012).
Herein, the synthesis of 2-thienylchalcones as intermediates towards flavans, 6H-benzo[c]chromen-6-ones, tetrahydro-2H-chromens, tetrahydroquinolines and diazepines is reported. The new heterocyclic compounds’ chemical structures were assigned based on IR, NMR and HR-MS spectral data and were screened for antimicrobial activities against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa and Candida albicans.
2 Material and methods
2.1 General methods
Melting points were determined on a Stuart melting point apparatus SMP1 (UK) and are uncorrected. Infrared spectra were recorded neat on a Perkin Elmer FT-IR spectrophotometer 1000. 1H, 13C and 2D-NMR spectra were recorded on a Bruker Avance DPX 300 MHz NMR spectrometer in CDCl3 (or acetone-d6) with TMS as an internal standard at room temperature. Electron impact (EI) High resolution mass spectra (HR-MS) were carried out on GCT Premier Mass Spectrometer (Waters) ionisation energy 70 eV, at the Chemistry Department, University of Botswana. All reactions were monitored by TLC, which was carried out on 0.25 mm layer of Merck silica gel 60 F254 pre-coated on aluminium sheets. Laboratory grade chemicals and solvents available commercially in high purity were used. All the prepared compounds were identified by physical properties, IR, HRMS and NMR data. Yields reported are isolated yields unless indicated otherwise.
2.1.1 General procedure for the synthesis of thiophen-2-yl-chalcones (1a–d)
Chalcones, 1a and 1b were prepared using the solvent-free green protocol of hand grinding (Dev and Dhaneshwar, 2013; ZiXing et al., 2010), while conventional base-catalysed Claisen–Schmidt condensation was used for the synthesis of chalcones 1c and 1d (Mazimba et al., 2011).
2.1.1.1 (E)-3-(2-Hydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one (1a)
Yellow solid; m.p.; 156–158 °C; Yield 87%; IR (neat, cm−1): 3326 (OH), 3095 (⚌C—H), 1692 (C⚌O), 1666 (C⚌O), 2922 (C—H), 1233, 1176 (C—O), 1558, 1456, 750 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 6.98 (3H, m, H-3, 4 & 5), 7.23 (1H, dd, J = 1.2, 3.6 Hz, H-4′), 7.61 (1H, d, J = 15.6 Hz, Hα), 7.63 (1H, dd, J = 1.5, 8.1 Hz, H-6), 7.72 (1H, dd, J = 1.2, 4.5 Hz, H-5′), 7.93 (1H, dd, J = 0.9, 3.6 Hz, H-3′), 8.29 (1H, d, J = 15.6 Hz, Hβ); 13C-NMR (75 MHz, CDCl3) δ: 116.1 (C-3), 119.9 (C-5), 121.2 (Cα), 121.8 (C-1), 128.4 (C-6), 128.9 (C-4′), 131.8 (C-4), 132.0 (C-3′), 134.0 (C-5′), 138.7 (Cβ), 146.2 (C-1′), 156.9 (C-2), 181.7 (C⚌O); HRMS (EI, 70 eV): found m/z 230.0402 [M+], mol. formula C13H10O2S, needing 230.0402.
2.1.1.2 (E)-3-(2-Hydroxy-3-methoxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one (1b)
White solid; m.p.; 156–158 °C; yield 85%; IR (neat, cm−1): 3325 (OH), 3096 (⚌C—H), 2922 (C—H), 1664 (C⚌O), 1224, 1088 (C—O—C), 1558, 1457, 755 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 3.54 (3H, s, 3-OCH3), 6.68 (1H, t, J = 8.1 Hz, H-4), 7.07 (1H, dd, J = 1.2, 7.8 Hz, H-5), 7.29 (1H, dd, J = 0.9, 3.9 Hz, H-4′), 7.41 (1H, dd, J = 1.2, 7.8 Hz, H-6), 7.79 (1H, d, J = 15.6 Hz, Hα), 7.93 (1H, dd, J = 1.2, 4.8 Hz, H-4′), 8.12 (1H, dd, J = 0.9, 3.6 Hz, H-3′), 8.19 (1H, d, J = 15.6 Hz, Hβ); 13C-NMR (75 MHz, CDCl3) δ: 55.9 (3-OCH3). 112.9 (C-4), 119.3 (C-6), 120.2 (C-5), 121.3 (C-1), 121.5 (Cα), 128.4 (C-4′), 132.0 (C-3′), 134.0 (C-5′), 138.4 (Cβ), 146.2 (C-1′), 146.7 (C-2), 147.8 (C-3), 181.7 (C⚌O); HRMS (EI, 70 eV): found m/z 260.0507 [M+], mol. formula C14H12O3S, needing 260.0507.
2.1.1.3 (E)-3-Phenyl-1-(thiophen-2-yl)prop-2-en-1-one (1c)
White solid; m.p.; 90–92 °C; yield 87%; IR (neat, cm−1): 3082 (⚌C—H), 2948, 2863 (C—H), 1660 (C⚌O); 1H-NMR (CDCl3, 300 MHz) δ: 7.23 (1H, t, J = 5.4 Hz, H-4′), 7.44 (1H, m, H-4), 7.46 (2H, m, H-3 & 5), 7.47 (1H, d, J = 15.0 Hz, Hα), 7.69 (2H, m, H-2 & 6), 7.73 (1H, dd, J = 0.9, 4.8 Hz, H-5′), 7.88 (1H, d, J = 15.0 Hz, Hβ), 7.93 (1H, dd, J = 3.3, 4.2 Hz, H-3′); 13C-NMR (75 MHz, CDCl3) δ: 121.6 (Cα), 128.2 (C-4), 128.5 (C-3 & 5), 129.0 (C-2 & 6), 134.7 (C-1), 130.6 (C-4′), 131.9 (C-5′), 133.9 (C-3′), 144.1 (Cβ), 145.5 (C-1′), 182.0 (C⚌O); HRMS (EI, 70 eV): found m/z 214.0452 [M+], mol. formula C13H10OS, needing 214.0452.
2.1.1.4 (E)-3-(4-Methoxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one (1d)
Yellow solid; m.p.; 108–110 °C; yield 84%; IR (neat, cm−1): 3088 (⚌C—H), 2945, 2860 (C—H), 1659 (C⚌O), 1245, 1031 (C—O), 1510, 1416, 755 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 3.91 (3H, s, 4-OCH3), 7.03 (2H, dd, J = 8.4 Hz, H-3 & 5), 7.44 (2H, d, J = 8.4 Hz, H-2 & 6), 7.62 (1H, t, J = 5.0 Hz, H-4′), 7.71 (1H, dd, J = 0.9, 4.3 Hz, H-5′), 7.82 (1H, dd, J = 3.3, 4.5 Hz, H-3′), 7.90 (1H, d, J = 15.9 Hz, Hα), 8.23 (1H, d, J = 15.9 Hz, Hβ); 13C-NMR (75 MHz, CDCl3) δ: 54.8 (4-OCH3), 116.2 (C-3 & 5), 121.6 (Cα), 122.0 (C-1), 128.9 (C-2 & 6), 129.7 (C-3′), 131.7 (C-5′), 133.4 (C-4′), 140.0 (Cβ), 157.0 (C-1′), 160.0 (C-4), 189.2 (C⚌O); HRMS (EI, 70 eV): found m/z 244.0558 [M+], mol. formula C14H12O2S, needing 244.0558.
2.1.2 General procedure for the synthesis of flavans (2a,b)
To a methanolic solution of chalcone (10 mmol) at 20 °C sodium borohydride powder (50 mmol) was introduced slowly over 20 min. The reaction was quenched with 2 M HCl in an ice bath. The organic layer was extracted with ethyl acetate, dried over magnesium sulphate and concentrated to a residue. To this residue glacial acetic acid (15 ml) was added and refluxed for 20 min. The organic layer was treated as above and after purification using flash column chromatography using n-hexane–ethylacetate (5:1, v/v) a pure product was obtained.
2.1.2.1 2-(Thiophen-2-yl)chroman (2a)
Brown gum; yield 70%; IR (neat, cm−1): 3179 (⚌C—H), 2931, 2880 (C—H), 1239, 1167 (C—O—C), 1595, 1456, 755 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 2.31 & 2.40 (each 1H, m, H-3a & 3b), 2.95 & 3.04 (each 1H, m, H-4a & 4b), 5.40 (1H, dd, J = 2.4, 9.6 Hz, H-2), 6.98 (2H, m, H-6 & 8), 7.09 (1H, dd, J = 1.5, 4.8 Hz, H-5′), 7.17 (2H, m, H-5 & 7), 7.23 (1H, dd, J = 1.2, 4.2 Hz, H-4′), 7.37 (1H, dd, J = 0.9, 5.1 Hz, H-3′); 13C-NMR (75 MHz, CDCl3) δ: 24.8 (C-4), 29.8 (C-3), 73.7 (C-2), 117.1 (C-8), 120.6 (C-6), 121.6 (C-4a), 124.5 (C-3′), 125.1 (C-5′), 126.7 (C-4′), 127.4 (C-7), 129.6 (C-5), 144.8 (C-1′), 154.6 (C-8a); HRMS (EI, 70 eV): found m/z 216.0604 [M+], mol. formula C13H12OS, needing 216.0604.
2.1.2.2 8-Methoxy-2-(thiophen-2-yl)chroman (2b)
Brown gum; yield 65%; IR (neat, cm−1): 3108 (⚌C—H), 2951, 2850 (C—H), 1230, 1182, 1110 (C—O—C), 1581, 1487, 749 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 3.53 (3H, s, 8-OCH3) 2.75 & 2.82 (each 1H, m, H-3a & 3b), 2.99 & 3.01 (each 1H, m, H-4a & 4b), 4.90 (1H, dd, J = 3.9, 9.3 Hz, H-2), 6.90–6.98 (3H, m, H-5, 6 & 7), 7.13–7.18 (2H, m, H-4′ & 5′), 7.28 (1H, dd, J = 0.9, 3.6 Hz, H-3′); 13C-NMR (75 MHz, CDCl3) δ: 29.9 (C-4), 39.4 (C-3), 45.6 (8-OCH3), 68.7 (C-2), 116.1 (C-7), 120.9 (C-5), 124.1 (C-6), 124.8 (C-3′), 126.7 (C-5′), 127.7 (C-4′), 130.6 (C-4a), 147.6 (C-8a), 144.7 (C-1′), 154.3 (C-7); HRMS (EI, 70 eV): found m/z 246.0708 [M+], mol. formula C14H14O2S, needing 246.0715.
2.1.3 General procedure for synthesis of 6H-benzo[c]chromen-6-one (4a,b)
Thiophen-2-yl-chalcones (1a or 1b) (2 mmol), potassium carbonate powder (20 mol%) and ethylacetoacetate (2 mmol) were taken in ethanol and stirred at 60 °C for 30 min. Then the reaction mixture was cooled and poured into cold water. The precipitated solid was collected by filtration and recrystallized in ethanol.
2.1.3.1 7-Hydroxy-9-(thiophen-2-yl)-6H-benzo[c]chromen-6-one (4a)
White solid; m.p.; 163–165 °C; yield 60%; IR (neat, cm−1): 3105 (⚌C—H), 2974, 2860 (C—H), 1676 (C⚌O), 1276, 1208, 1108 (C—O—C), 1564, 1410, 851 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 7.22 (1H, dd, J = 1.5, 3.6 Hz, H-4′), 7.35 (1H, d, J = 1.5 Hz, H-8), 7.41 (1H, dd, J = 1.2, 8.1 Hz, H-4), 7.46 (1H, dd, J = 1.2, 8.1 Hz, H-2), 7.51 (1H, dd, J = 1.2, 5.1 Hz, H-3′), 7.54 (1H, dd, J = 1.8, 7.5 Hz, H-3), 7.59 (1H, dd, J = 1.2, 3.6 Hz, H-5′), 7.81 (1H, d, J = 1.5 Hz, H-10), 8.14 (1H, dd, J = 1.8, 7.8 Hz, H-1), 11.4 (1H, s, 7-OH); 13C-NMR (75 MHz, CDCl3) δ: 104.8 (C-6a), 109.4 (C-8), 113.0 (C-10), 117.0 (C-4), 118.1 (C-10b), 123.3 (C-2), 125.2 (C-3′), 125.8 (C-5′), 127.6 (C-4′), 128.5 (C-3), 130.8 (C-1), 135.7 (C-10a), 142.3 (C-1′), 142.9 (C-9), 150.8 (C-4a), 162.7 (C-6), 165.1 (C-7); HRMS (EI, 70 eV): found m/z 294.0351 [M+], mol. formula C17H10O3S, needing 294.0351.
2.1.3.2 7-Hydroxy-4-methoxy-9-(thiophen-2-yl)-6H-benzo[c]chromen-6-one (4b)
White solid; m.p.; 148–150 °C; yield 52%; IR (neat, cm−1): 3130 (⚌C—H), 2952, 2850 (C—H), 1678 (C⚌O), 1271, 1268 (C—O—C), 1556, 1407, 817 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 3.57 (3H, s, 4-OCH3), 6.84 (1H, dd, J = 0.9, 7.8 Hz, H-3), 7.14 (1H, dd, J = 1.5, 3.6 Hz, H-4′), 7.27 (1H, dd, J = 1.2, 7.8 Hz, H-2), 7.33 (1H, d, J = 1.5 Hz, H-8), 7.42 (1H, dd, J = 1.5, 3.6 Hz, H-3′), 7.92 (1H, dd, J = 1.2, 3.6 Hz, H-3), 7.99 (1H, dd, J = 1.2, 7.8 Hz, H-1), 8.12 (1H, d, J = 1.5 Hz, H-10), 12.6 (1H, s, 7-OH); 13C-NMR (75 MHz, CDCl3) δ: 55.7 (4-OMe), 105.7 (C-6a), 108.4 (C-8), 111.3 (C-10), 113.0 (C-3), 119.4 (C-1), 121.2 (C-10b), 121.9 (C-2), 127.5 (C-5′), 127.9 (C-3′), 128.0 (C-4′), 134.4 (C-10a), 140.0 (C-1′), 140.3 (C-9), 148.1 (C-4a), 150.0 (C-4), 161.9 (C-6), 165.2 (C-7); HRMS (EI, 70 eV): found m/z 324.0456 [M+], mol. formula C18H12O4S, needing 324.0456.
2.1.4 General procedure for synthesis of tetrahydroquinolines (7a,b)
A mixture of chalcones (1c or 1d) (1 mmol), cyclohexanone (2 mmol) and NaOH (2 mmol) were ground with an agate mortar and a pestle for 25 min until the reaction mixture became a solid. The solid was dissolved in acetone (10 mL) and cold water was added (20 mL). The separated solid was collected by filtration, washed with water and ethanol. The 1,5-diketone (1 mmol) and ammonium acetate (5 mmol) in glacial acetic acid (20 mL) were heated to reflux (30 min). The crude product was precipitated out of solution by the addition of cold water (10 mL), collected and washed with water and ethanol.
2.1.4.1 5,6,7,8-Tetrahydro-4-phenyl-2-(thiophen-2-yl)quinoline (7a)
Brown solid; m.p.; 175–177 °C; yield 90%; IR (neat, cm−1): 3057 (⚌C—H), 2928, 2854 (C—H), 1655, 1544 (pyridine ring), 1584, 1453, 853 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 1.66 (2H, m, H-6), 1.82 (2H, m, H-7), 2.52 (2H, m, H-5), 2.94 (2H, t, J = 4.5 Hz, H-8), 6.96 (1H, m, H-4′), 6.98 (1H, m, H-4″), 7.22 (2H, m, H-2″ & 6″), 7.25 (1H, s, H-3), 7.31 (1H, m, H-5′), 7.34 (2H, m, H-3″ & 5″), 7.45 (1H, dd, J = 0.9, 3.6 Hz, H-3′); 13C-NMR (75 MHz, CDCl3) δ: 23.0 (C-7), 27.3 (C-6), 29.7 (C-5), 33.1 (C-8), 117.6 (C-3), 124.1 (C-3′), 127.6 (C-4′), 127.8 (C-4a), 128.0 (C-5′), 128.4 (C-2″ & 6″), 128.5 (C-3″ & 5″), 128.6 (C-4″), 139.5 (C-1″), 145.1 (C-1′), 149.3 (C-4), 150.3 (C-2), 157.2 (C-8a); HRMS (EI, 70 eV): found m/z 291.1080 [M+], mol. formula C19H17NS, needing 291.1082.
2.1.4.2 5,6,7,8-Tetrahydro-4-(4-methoxyphenyl)-2-(thiophen-2-yl)quinoline (7b)
Brown solid, m.p.; 212–214 °C; yield 90%; IR (neat, cm−1): 3025 (⚌C—H), 2931, 2860 (C—H), 1658, 1537 (pyridine ring), 1515, 1450, 834 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 1.82 (2H, m, H-7), 1.93 (2H, m, H-6), 2.68 (2H, m, H-5), 3.07 (2H, t, J = 6.6 Hz, H-8), 3.91 (3H, s, 4″-OCH3), 7.04 (2H, d, J = 8.4 Hz, H-3″ & 5″), 7.12 (2H, dd, J = 1.2, 3.6 Hz, H-4′ & 5′), 7.33 (2H, d, J = 8.4 Hz, H-2″ & 6″), 7.37 (1H, s, H-3), 7.56 (1H, d, J = 3.6 Hz, H-3′); 13C-NMR (75 MHz, CDCl3) δ: 23.1 (C-7), 27.4 (C-6), 29.7 (C-5), 33.1 (C-8), 55.3 (4″-OCH3), 113.8 (C-3″ & 5″), 117.7 (C-3), 124.0 (C-3′), 127.8 (C-3′ & 4′), 129.0 (C-2″ & 6″), 129.1 (C-4), 131.7 (C-4a), 145.2 (C-1′), 149.3 (C-2), 149.9 (C-4), 157.6 (C-8a), 159.3 (C-4′); HRMS (EI, 70 eV): found m/z 321.1185 [M+], mol. formula C20H19NOS, needing 321.1187.
2.1.5 General procedure for synthesis of 5-oxotetrahydro-2H-chromenes
To a methanolic solution of the 1,5-diketone (1 mmol) sodium borohydride powder (5 mol) was added over 10 min. Then cold 2 M HCl was added. The organic layer was extracted with chloroform, dried over magnesium sulphate and concentrated to a residue. The residue was taken in glacial acetic acid (10 ml) and refluxed for 30 min. The organic layer was extracted using ethyl acetate and dried over magnesium sulphate. After being concentrated it was purified using flash column chromatography eluted by petroleum ether-ethylacetate (v/v, 4:1) to obtain a pure product.
2.1.5.1 Octahydro-4-phenyl-2-(thiophen-2-yl)-2H-chromene (9a)
White gum; yield 85%; IR (neat, cm−1): 3059 (⚌C—H), 2923, 2856 (C—H), 1598, 1450, 757 (Aromatic), 1239, 1077 (C—O); 1H-NMR (CDCl3, 300 MHz) δ: 1.17–1.87 (8H, m, H-5, 6, 7 & 8), 2.05 (1H, dt, J = 2.4, 9.0 Hz, H-4a), 2.17 (2H, m, H-3), 3.09 (1H, dt, J = 3.3, 12.3 Hz, H-4), 4.06 (1H, q, J = 2.4, 12.3 Hz, H-8a), 5.05 (1H, dd, J = 2.8, 8.4 Hz, H-2), 6.83 (1H, m, H-4′) 6.86 (1H, m, H-3′), 7.10 (1H, m, H-5′), 7.14 (2H, m, H-2″ & 6″), 7.19 (1H, m, H-4″), 7.20 (2H, m, H-3″ & 5″); 13C-NMR (75 MHz, CDCl3) δ: 20.3 (C-7), 25.4 (C-5), 25.5 (C-6), 26.8 (C-8), 39.3 (C-4), 40.4 (C-3), 42.8 (C-4a), 68.4 (C-2), 76.5 (C-8a), 123.2 (C-3′), 124.3 (C-5′), 126.4 (C-4″), 126.5 (C-4′), 127.5 (C-2″ & 6″), 128.6 (C-3″ & 5″), 143.9 (C-1″), 146.4 (C-1′); HRMS (EI, 70 eV): found m/z 298.1391 [M+], mol. formula C19H22OS, needing 298.1391.
2.1.5.2 Octahydro-4-(4-methoxyphenyl)-2-(thiophen-2-yl)-2H-chromene (9b)
Brown gum; yield 86%; IR (neat, cm−1): 3025 (⚌C—H), 2923, 2857 (C—H), 1510, 1444 (Aromatic), 1240, 1171 (C—O); 1H-NMR (CDCl3, 300 MHz) δ: 1.19–1.99 (8H, m, H-5, 6, 7 & 8), 2.04 (1H, dt, J = 3.9, 11.4 Hz, H-4a), 2.17 (2H, m, H-3), 3.05 (1H, dt, J = 3.3, 7.5 Hz, H-4), 3.68 (3H, s, 4″-OCH3), 4.05 (1H, q, J = 3.9, 7.5 Hz, H-8a), 5.01 (1H, dd, J = 2.1, 11.4 Hz, H-2), 6.78 (2H, d, J = 8.4 Hz, H-3″ & 5″), 6.85 (2H, brs, H-4′ & 5′), 7.06 (2H, d, J = 8.4 Hz, H-2″ & 6″), 7.11 (1H, dd, J = 1.8, 4.8 Hz, H-3′); 13C-NMR (75 MHz, CDCl3) δ: 20.3 (C-7), 25.4 (C-5), 26.8 (C-6), 29.7 (C-8), 38.4 (C-4), 40.7 (C-3), 43.1 (C-4a), 55.2 (4″-OCH3), 68.4 (C-2), 76.5 (C-8a), 114.0 (C-3″ & 5″), 123.2 (C-3′), 124.3 (C-5′), 126.4 (C-4′), 128.3 (C-2″ & 6″), 135.8 (C-1″), 146.8 (C-1′), 158.2 (C-4″); HRMS (EI, 70 eV): found m/z 328.1500 [M+], mol. formula C20H24O2S, needing 328.1497.
2.1.6 General procedure for the preparation of diazepine (10a,b)
A mixture of 1,5-diketone (10 mmol) and hydrazine hydrate (10 mmol) in glacial acetic acid (20 mL) was refluxed for 3 h. The reaction mixture was cooled and poured into ice-cold water. The precipitate formed was collected by filtration and recrystallized from ethanol.
2.1.6.1 5-Phenyl-3-(thiophen-2-yl)-5,5a,6,7,8,9-hexahydro-4H-benzo[c][1,2]diazepine (10a)
White solid; m.p.; 126–128 °C; yield 66 %; IR (neat, cm−1): 2931, 2854, (CH), 1607 (C⚌N), 1510, 1450 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 1.14–1.78 (8H, m, H-6, 7, 8 & 9), 2.05–2.17 (2H, m, H-4), 2.24 (1H, m, H-5a), 2.34 (1H, m, H-5), 6.72–6.84 (5H, m, H-2″, 3″, 4″, 5″ & 6″), 6.98 (1H, t, J = 3.1 H-4′), 7.12 (1H, dd, J = 2.4, 3.8 Hz, H-5′), 7.15 (1H, dd, J = 1.5, 6.0 Hz, H-3′); 13C-NMR (75 MHz, CDCl3) δ: 24.9 (C-7), 26.0 (C-8), 28.9 (C-6), 33.7 (C-9), 43.8 (C-4), 42.1 (C-5), 46.9 (C-5a), 125.0 (C-3′), 126.0 (C-4″), 126.6 (C-4′), 126.9 (C-5′), 127.6 (C-1′), 135.0 (C-1″), 128.8 (C-2″ & 6″), 129.2 (C-3″ & 5″), 157.9 (C-3), 158.0 (C-9a); HRMS (EI, 70 eV): found m/z 308.1350 [M+], mol. formula C19H20N2S, needing 308.1347.
2.1.6.2 5-(4-Methoxyphenyl)-3-(thiophen-2-yl)-5,5a,6,7,8,9-hexahydro-4H-benzo[c] [1,2]diazepine (10b)
White solid, m.p; 126–128 °C; yield 68 %; IR (neat, cm−1): 2930, 2856 (CH), 1610 (C⚌N), 1570, 1458 (Aromatic); 1H-NMR (CDCl3, 300 MHz) δ: 1.16–1.82 (8H, m, H-6, 7, 8 & 9), 2.29 (2H, m, H-4), 2.54 (1H, t, J = 6.6 Hz, H-5a), 2.99 (1H, t, J = 6.0 Hz, H-5), 3.54 (3H, s, 4″-OCH3), 6.94 (2H, d, J = 8.4 Hz, H-3″ & 5″), 7.09 (1H, t, J = 2.4 Hz, H-4′), 7.13 (1H, dd, J = 1.2, 2.4 Hz, H-5′), 7.22 (2H, d, J = 8.4 Hz, H-2″ & 6″), 7.31 (1H, dd, J = 1.5, 5.4 Hz, H-3′); 13C-NMR (75 MHz, CDCl3) δ: 21.9 (C-7), 23.0 (C-8), 29.1 (C-6), 33.4 (C-9), 39.1 (C-4), 42.3 (C-5), 43.1 (C-5a), 60.0 (4″-OCH3), 118.0 (C-3″ & 5″), 124.1 (C-1′), 125.6 (C-3′), 127.8 (C-4′), 127.9 (C-5′), 128.4 (C-2″ & 6″), 134.9 (C-1″), 159.8 (C-4″), 168.7 (C-9a & C-3); HRMS (EI, 70 eV): found m/z 338.1461 [M+], mol. formula C20H22N2OS, needing 338.1453.
2.2 Antimicrobial activity
The synthesized compounds were tested in vitro for their antibacterial and antifungal profile using Dilution Method (Ragavan et al., 2010; Danielle, 2006) against S. aureus (ATCC 9144), E. coli (ATCC 11229), B. subtilis (ATCC 6633), P. aeruginosa (NCTC 10332) and C. albicans (ATCC 10231). The test microorganisms were obtained from the Department of Biological Sciences, University of Botswana. Ciprofloxacin and Fluconazole were used as standards for bacteria and fungi respectively. The tested solutions were serially diluted to give concentrations of 5, 2.5, 1.25, 0.625 and 0.313 mg/mL. All tests were done in triplicate. The antimicrobial activity was expressed as minimum inhibitory concentration (MIC) of sample that prevented the visible growth of a microorganism.
3 Result and discussions
Initially, the synthesis of trans-2-thienylchalcones was attempted using the efficient, economical and a green protocol of grinding the reactants together in the presence of catalytic amount of sodium hydroxide. This method worked only for chalcones devoid of hydroxyl groups (1c–d, 90% yield) while grinding 2-hydroxybenzaldehydes did not afford chalcones. The presence of hydroxyl groups on aryl aldehydes or ketones is a limiting factor for the green protocol of chalcone synthesis. Literature survey shows solvent-free synthesis of chalcones and heterocyclic analogous of chalcones that excludes hydroxyl groups as substituents (Dev and Dhaneshwar, 2013; ZiXing et al., 2010; Rateb and Hussein, 2009). Hence, the conventional base-catalysed Claisen–Schmidt condensation reaction was adopted (Mazimba et al., 2011) and the thiophene chalcone analogues (1a–d) were obtained in 84–87% yields. The thienyl chalcones have been previously reported in good yields (81–90%) using the base catalysed condensation method (Roman, 2004; Tran et al., 2012; Yin et al., 2012), the literature spectroscopic data were also used to confirm the identity of reported chalcones. Next, the chalcones (Fig. 1) were used for the synthesis of the desired heterocyclic compounds.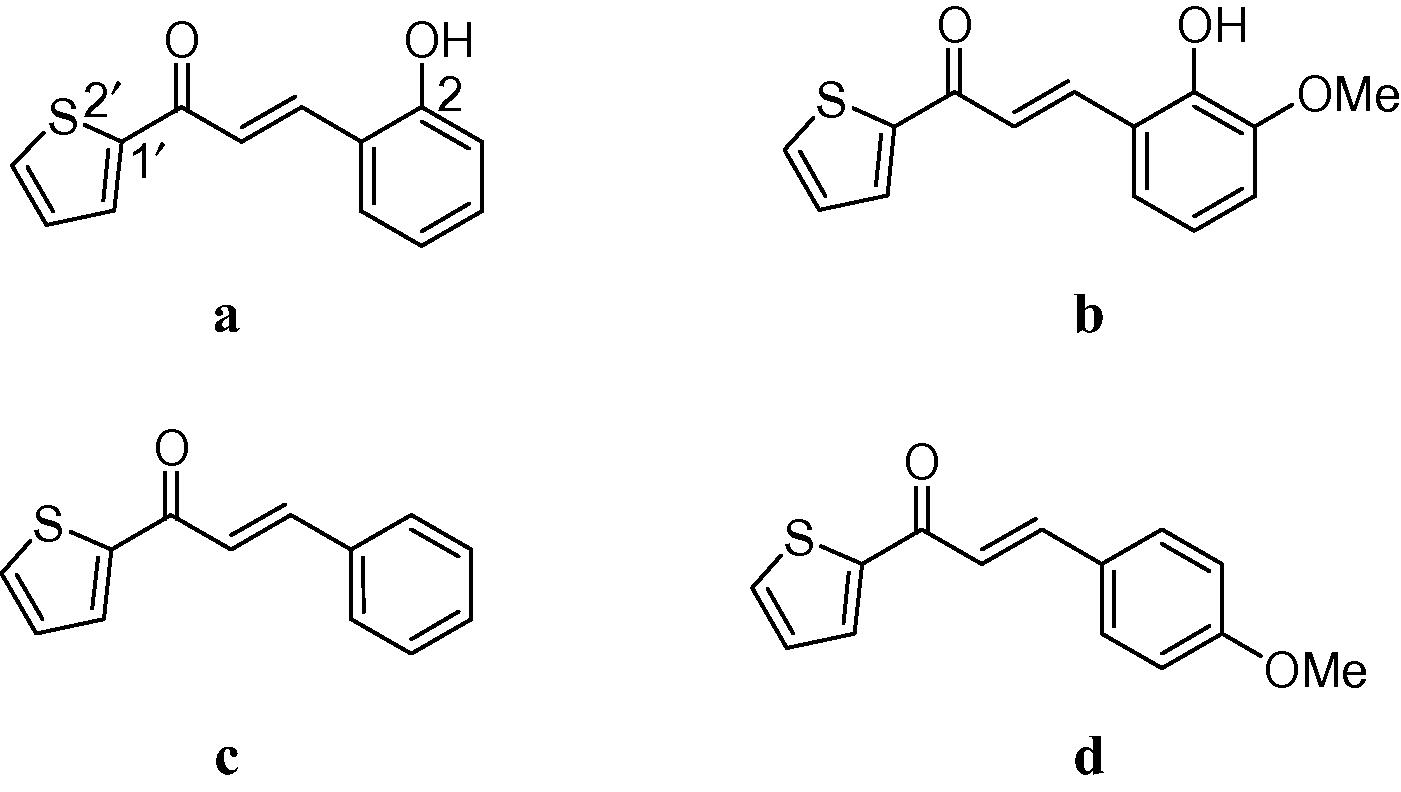
Structures of thienylchalcones.
3.1 Synthesis of flavans
Flavans (2-(thiophen-2-yl)chroman, 2a,b) were derived by the reduction of the α,β-unsaturated ketone function of the chalcones (1a,b) using NaBH4. The ring closure to form a chroman ring was achieved by refluxing the resulting 2-(3-hydroxy-3-(thiophen-2-yl)propyl)phenol (2c) in glacial acetic acid (Scheme 1) as previously described (Mazimba et al., 2011). The substitution of the chalcone benzene ring with the electron rich thiophene ring did not affect the reaction path but resulted in reduced yields. The 2-phenylchroman yield was 88% (Mazimba et al., 2011) while the yield for 2-thiophenylchroman (2a) was found to be 70%. The electron donating MeO-group led to the corresponding flavan (2b) in 65% yields.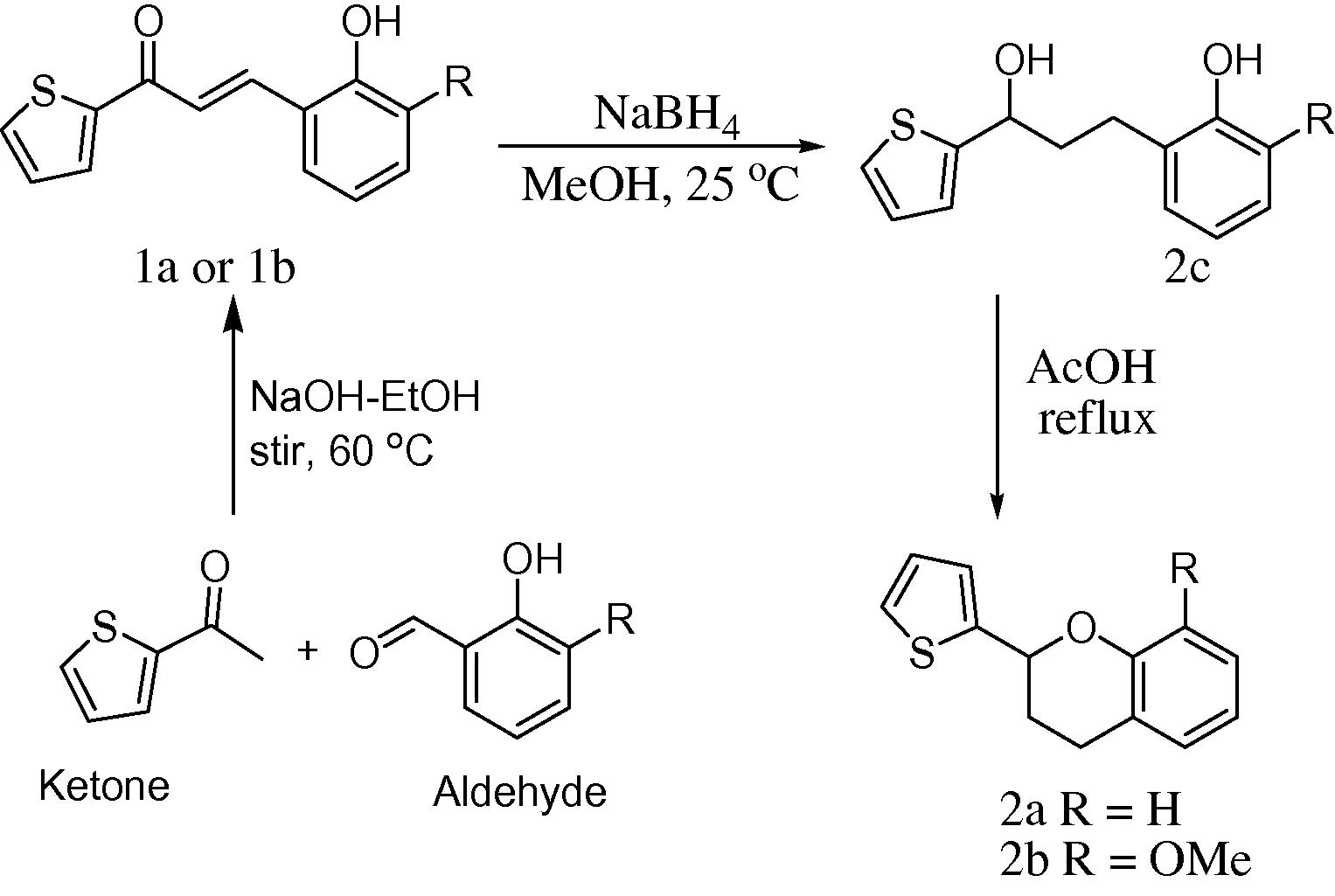
Synthesis of thienylchalcones and flavans.
3.2 Synthesis of 7-hydroxy-9-(thiophen-2-yl)-6H-benzo[c]chromen-6-ones
It is well reported that the reaction of chalcones with ethylacetoacetate affords cyclohexanones (Samshuddin et al., 2012; Roman, 2004), while the Knoevenagel reaction affords coumarins from the condensation of ethylacetoacetate with 2-hydroxybenzaldehydes (Sashidhara et al., 2010). Therefore, it was expected that 2-hydroxy-2′-thienylchalcones would react with ethylacetoacetate to furnish a 6H-benzo[c]chromene-6,7(6aH)-dione (4c) ring system. But the product of the reaction that was assisted by K2CO3, was identified to be 6H-benzo[c]chromen-6-ones (4a,b) instead of the expected 9-(thiophen-2-yl)-10,10a-dihydro-6H-benzo[c]chromene-6,7(6aH)-dione (4c), Scheme 2. The reaction is thought to proceed by the Michael addition of ethylacetoacetate to chalcone, followed by the ring forming intramolecular Aldol condensation of the ester methyl group with the chalcone keto-group to form cyclohexenones (Roman, 2004). The presence of 2′-OH enables a trans-esterification reaction to form intermediate 4c, which undergoes oxidative aromatization to form 6H-benzo[c]chromen-6-ones (4a,b).![Synthesis of thiophen-2-yl-6H-benzo[c]chromen-6-one.](/content/185/2015/27/1/img/10.1016_j.jksus.2014.06.003-fig3.png)
Synthesis of thiophen-2-yl-6H-benzo[c]chromen-6-one.
3.3 Synthesis of tetrahydroquinolines
2,4-Diaryltetrahydroquinolines were targeted owing to their pharmacological activities (Chabert et al., 2006) and ease of the availability of 1,5-diketones from chalcones. The required 1,5-diketones (6a–b) were obtained via the green protocol involving the Michael addition of cyclohexanone (5) to chalcones (1c,d) under solventless conditions. The reaction exclusively affords a single Michael addition product due to the unfavourable intramolecular self-condensation of the 1,5-diketones (ZiXing et al., 2010). Treatment of 1,5-diketones with ammonium acetate in acetic acid (Scheme 3) yielded tetrahydroquinolines (7a,b) in good yields, 90%. This green protocol is an efficient ‘1 + 5’ strategy that involves double condensation of an amino group with 1,5-diketones to afford six membered ring 1,4-dihydropyridine (4-phenyl-2-(thiophen-2-yl)-1,4,5,6,7,8-hexahydroquinoline, 7c). The 1,4-dihydropyridine is subsequently oxidised to 4-phenyl-2-(thiophen-2-yl)-5,6,7,8-tetrahydroquinoline, Scheme 3 (Gezegen et al., 2010). The quinolyl ring singlet proton NMR signal (7.25 ppm) was diagnostic.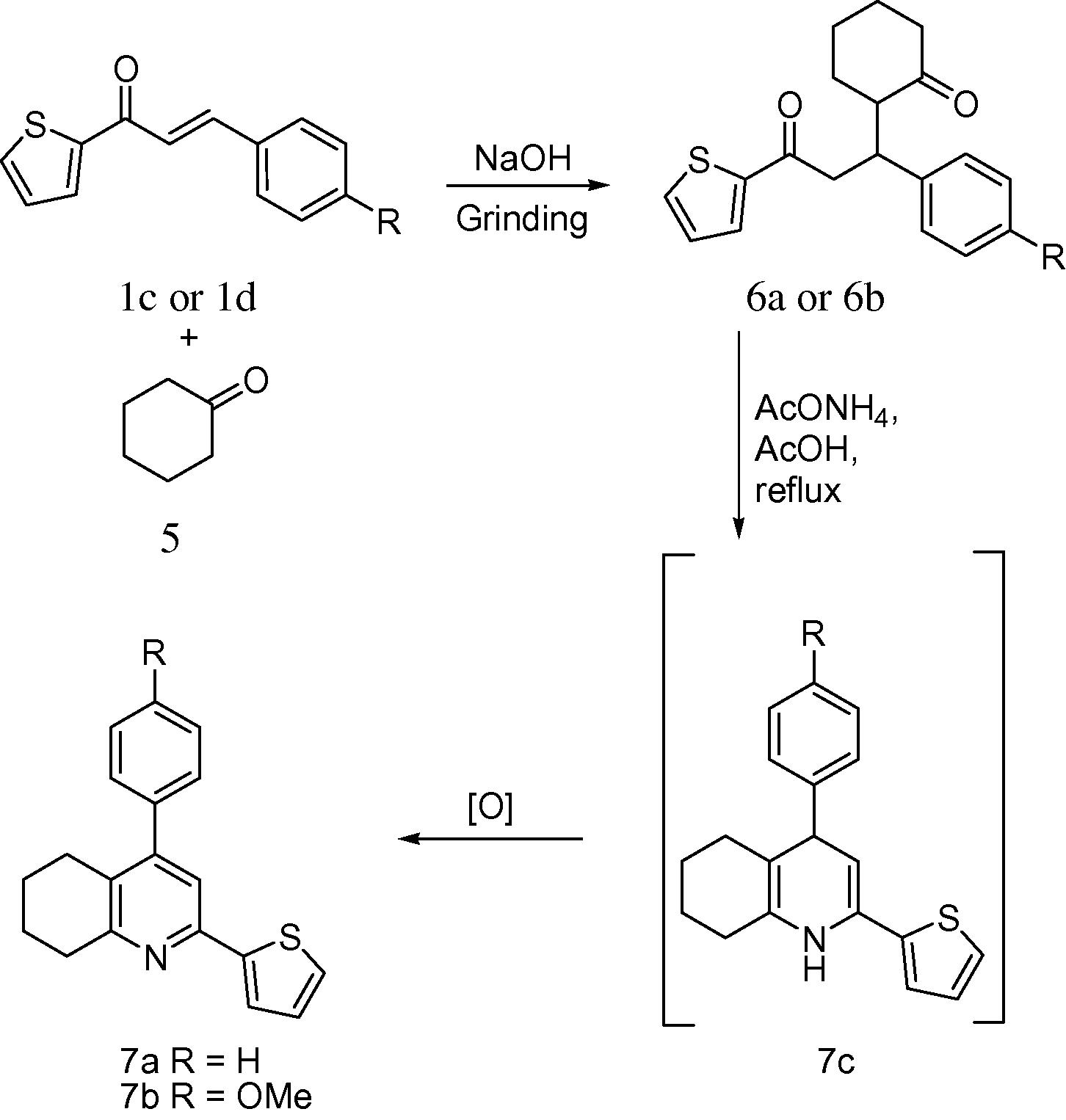
Synthesis of tetrahydroquinolines.
3.4 Synthesis of 5-oxotetrahydro-2H-chromenes
The strategy towards 2H-chromene involved the reduction of 1,5-diketones (6a,b) using NaBH4 followed by the cyclization of the resulting 1,5-diols (8a,8b) in glacial acetic acid which afforded 5-oxotetrahydro-2H-chromenes (9a,b) (Scheme 4). The heterocyclization of 1,5-diketones has been reported under CF3CO2H/PtCl2–H2 at 200 °C, Rh/C-H2, Raney Nickel–AcOH at 150 °C (Kharchenko et al., 2000) and TiCl4–Et3N (Sergeeva et al., 2010). The reported reactions were carried out at elevated temperatures and suffered from low yields (34–50%), but the current protocol offers higher yields (85–86%) and operational simplicity.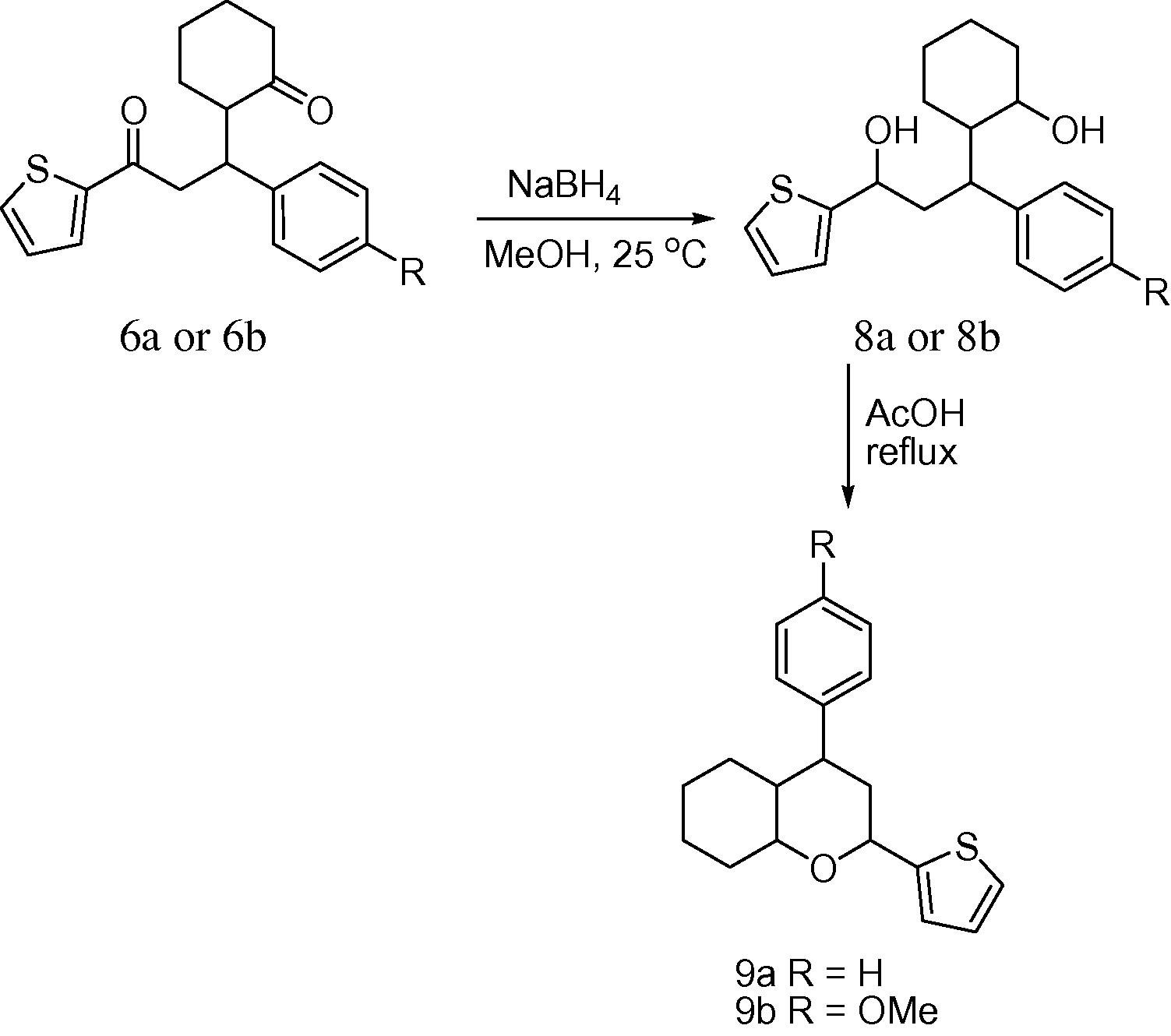
Synthesis of 5-oxotetrahydro-2H-chromenes.
3.5 Synthesis of diazepines
The condensation reaction between amines and ketones is known to yield imines or Schiff bases (Shaikh et al., 2013). Thus, 1,5-diketones bears a good synthon for heterocyclization reactions with N-nucleophiles to form six or seven membered rings. This conventional reaction was applied to obtain diazepines (10a,b, Scheme 5) from the reaction of hydrazine hydrate with 1,5-diketones (6a,b) derived from 2-thienylchalcone. The reaction was found to be simple and short affording moderate yields (66–68%) and a single product. The synthesis and pharmacological importance of diazepines are widely reported (Samshuddin et al., 2012; Luszczki, 2009; Rodriguez et al., 2004).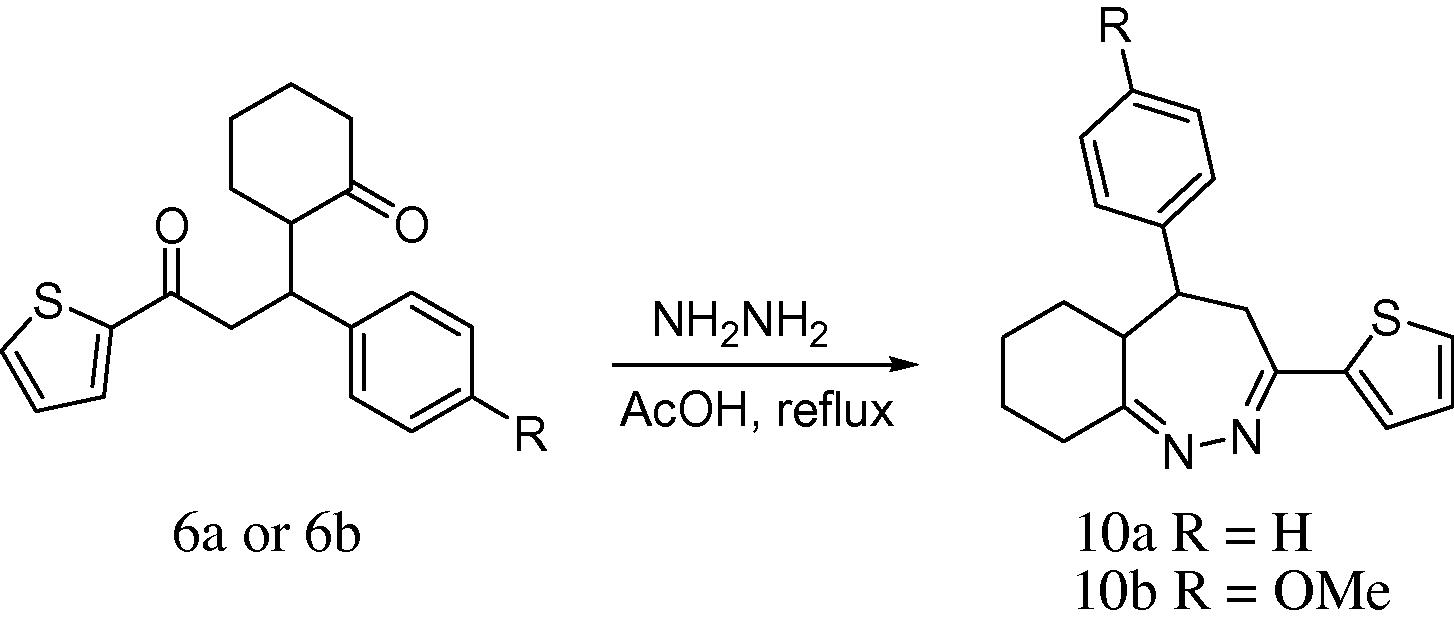
Synthesis of diazepines.
4 Antimicrobial studies
The synthesized new heterocyclic compounds were tested in vitro for their antimicrobial profile using the dilution method (Ragavan et al., 2010; Danielle, 2006) against S. aureus, E. coli, B. subtilis, P. aeruginosa and C. albicans. The tested compounds showed moderate to good antibacterial and antifungal activities. 6H-benzo[c]chromen-6-ones (4a,b) show good activities (0.625 mg/mL) against both bacteria and fungi, and the conjugation of hydroxy and ester groups may favour the uptake of these compounds by microbial cells. Tetrahydroquinolines (7a,b) and diazepines’ (10a,b) excellent (0.313 mg/mL) results show the importance of the carbon–nitrogen bond in biological systems (Yadav and Purohit, 2013). The presence of the MeO-group does not seem to have a significant effect on the activities of compounds 7a,b and 10a,b, Table 1. These activities are in comparison with other reports which show antimicrobial activities for these N-containing compounds (Samshuddin et al., 2012; Dodiya et al., 2011). Flavans (2a,b) and 5-oxotetrahydro-2H-chromenes (9a,b) indicate moderate (1.25 mg/mL) activities. The data on antimicrobial activities of the target compounds are given in Table 1.
Compound
Minimum inhibitory concentration (MIC; mg/mL)
S. aureus
E. coli
B. subtilis
P. aeruginosa
C. albicans
2a
1.25
1.25
1.25
1.25
0.625
2b
1.25
1.25
1.25
1.25
1.25
4a
0.625
0.625
0.625
0.625
0.625
4b
0.625
0.625
0.625
0.625
0.625
7a
0.625
0.625
0.625
0.313
0.625
7b
0.625
0.625
0.625
0.313
0.625
9a
1.25
2.5
1.25
1.25
2.5
9b
1.25
1.25
1.25
1.25
1.25
10a
0.313
0.625
0.625
0.313
0.313
10b
0.313
0.625
0.625
0.313
0.313
Ciprofloxacin
0.625
0.625
0.625
0.625
–
Fluconazole
–
–
–
–
0.625
5 Conclusion
Thiophene analogues of chalcone (1a–d) were synthesized in good yields. 1,5-diketones were obtained utilising green Michael addition of cyclohexanone to 2-thienylchalcones devoid of hydroxyl groups. The chalcones and 1,5-diketones were a good synthon for the synthesis of heterocyclic compounds, shown here by the synthesis of new flavans, 6H-benzo[c]chromen-6-ones, tetrahydro-2H-chromens, tetrahydroquinolines, and diazepines. The methods utilised were short and efficient in good yields and operational simplicity. The heterocyclic compounds were screened for their antimicrobial activity against S. aureus, E. coli, B. subtilis, P. aeruginosa and C. albicans, showing moderate to good antibacterial and antifungal activities. Diazepines (10a,b) exhibited excellent antibacterial (S. aureus and P. aeruginosa) and antifungal (C. albicans) activities. The preliminary data on the antimicrobial activities of the new scaffolds should serve as an indicator for structural modulation to improve activities and explain the structure activity relationships. The exhibition of chalcone synthon potential and the antimicrobial activity of the target heterocyclic compounds validates the significance of this study.
Acknowledgments
The author thanks Mr. D. Mosimaneathebe and Dr. K. Sichilongo for HR-Mass Spectrometry data.
References
- Study of Michael addition on chalcones and or chalcone analogues. J. Saudi Chem. Soc.. 2012;16(1):45-53.
- [Google Scholar]
- Synthesis and antibacterial evaluation of novel heterocyclic compounds containing a sulfonamido moiety. Molecules. 2013;18(1):832-844.
- [Google Scholar]
- Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur. J. Med. Chem.. 2013;65:51-59.
- [Google Scholar]
- Benzo[b]thiophene as a template for substituted quinolines and tetrahydroquinolines. Tetrahedr. Lett.. 2006;47(6):1015-1018.
- [Google Scholar]
- Danielle, L.D., 2006. Optimization and characterization of the growth of the photosynthetic bacterium Blastochloris viridis and a brief survey of its potential as a remediative tool. PhD dissertation, University of Notre Dame, Indiana, USA.
- A solvent-free protocol for the green synthesis of heterocyclic chalcones. Der Pharm. Lett.. 2013;5(5):219-223.
- [Google Scholar]
- An efficient, microwave-assisted, one-pot synthesis of novel 5,6,7,8-tetrahydroquinoline-3-carbonitriles. J. Serb. Chem. Soc.. 2011;76(6):823-830.
- [Google Scholar]
- Three step synthesis of 2,4-diaryl-5,6,7,8-tetrahydroquinoline. J. Heterocycl. Chem.. 2010;47(5):1017-1024.
- [Google Scholar]
- Oxygen-containing heterocyclic compounds from 1,5-diketones. Chem. Heterocycl. Comp.. 2000;36(9):1007-1025.
- [Google Scholar]
- Selective synthesis of bis[1,2]dithiolo[1,4]thiazines from 4-isopropylamino-5-chloro-1,2-dithiole-3-ones. Tetrahedr. Lett.. 2007;48(44):5851-5854.
- [Google Scholar]
- Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions. Pharmacol. Rep.. 2009;61(2):197-216.
- [Google Scholar]
- An efficient synthesis of flavans from salicylaldehyde and acetophenone derivatives. Tetrahedr. Lett.. 2011;52(50):6716-6718.
- [Google Scholar]
- Synthesis and antimicrobial activities of novel 1,5-diaryl pyrazoles. Eur. J. Med. Chem.. 2010;45(3):1173-1180.
- [Google Scholar]
- Silica-H2SO4 catalyzed environmentally benign crossed aldol condensation: synthesis, spectral studies and biological activities of some 5-chloro-2-thienyl chalcones. Int. J. Pharm. Med. Biol. Sci.. 2012;1(1):62-85.
- [Google Scholar]
- Atom-efficient, solvent-free, green synthesis of chalcones by grinding. Synth. Commun.. 2009;39(15):2789-2794.
- [Google Scholar]
- Preparation of some light-sensitive 2-nitrophenyl-2,3-dihydro-1H-benzodiazepines. ARKIVOC. 2004;2004(13):67-71.
- [Google Scholar]
- Discovery and molecular docking of quinolyl-thienyl chalcones as anti-angiogenic agents targeting VEGFR-2 tyrosine kinase. Bioorg. Med. Chem.. 2012;22(2):942-944.
- [Google Scholar]
- Cyclohexanone through addition of ethyl acetoacetate to chalcones derived from 2-acetylthiophene. Acta Chim. Slov.. 2004;51(3):537-544.
- [Google Scholar]
- Synthesis, characterization and biological evaluation of functionalized derivatives of versatile synthon 4,4′-difluoro chalcone. Der Pharm. Chem.. 2012;4(4):1445-1457.
- [Google Scholar]
- Synthesis and in vitro evaluation of novel coumarin–chalcone hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett.. 2010;20(24):7205-7211.
- [Google Scholar]
- Stereochemistry of products of Aldol condensation of 2-(3-oxo-1,3-diphenylpropyl)cyclohexanone with aromatic aldehydes. Russ. J. Org. Chem.. 2010;46(9):1348-1354.
- [Google Scholar]
- Ecofriendly synthesis of Schiff bases. Int. J. Pharm. Pharm. Sci. Res.. 2013;3(3):100-102.
- [Google Scholar]
- Synthesis and characterization of some chalcones and their cyclohexenone derivatives. Cent. Eur. J. Chem.. 2010;8(1):174-181.
- [Google Scholar]
- Synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics. Molecules. 2012;17(6):6684-6696.
- [Google Scholar]
- Synthesis and evaluation of some bioactive compounds having oxygen and nitrogen heteroatom. J. Chem. Sci.. 2013;125(1):165-173.
- [Google Scholar]
- Synthesis of functionalized 2-aryl-4-(indol-3-yl)-4H-chromenes via iodine-catalyzed domino Michael addition-intramolecular cyclization reaction. Org. Biomol. Chem.. 2012;10(44):8877-8883.
- [Google Scholar]
- New observation on a class of old reactions: chemoselectivity for the solvent-free reaction of aromatic aldehydes with alkylketones catalyzed by a double-component inorganic base system. Sci. China Chem.. 2010;53(5):1095-1101.
- [Google Scholar]







