Translate this page into:
Antifungal effect of the tea tree essential oil (Melaleuca alternifolia) against Penicillium griseofulvum and Penicillium verrucosum
⁎Corresponding author at: Environmental and Food Biotechnology Research Team (EFBRT), Normal High School, BP: 209, Martil, Morocco. fchidifatima@gmail.com (Fatima Chidi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Lentil and dry grapes are foods with high nutritional value, commonly consumed in Morocco. Their microbiological quality is widely studied; however, their mycological quality was less investigated. On the other hand, the antifungal potential of essential oils against Penicillium spp. and its mycotoxins was demonstrated. The aim of this work is to investigate the antifungal activities of Melaleuca alternifolia essential oil (M. alternifolia EO) against Penicillium griseofulvum (P. griseofulvum) isolated from lentil (Lens culinaris) and Penicillium verrucosum (P. verrucosum) isolated from dry grapes (Vitis vinifera L.) in Northern Morocco.
Methods
Eight samples of lentil and nine samples of dry grapes have been analyzed. Eight strains belonging to five species of Penicillium have been isolated and identified. The identification was based on the microscopic, physiological, and cultural characteristics of growth under standard conditions. Mycotoxins production was highlighted by Thin Layer Chromatography (TLC).
Results
P. griseofulvum produced Terrestric acid and Ochratoxin A in the Yeast Extract Sucrose agar (YES) medium, while P. verrucosum produced only Terrestric acid. The major component of M. alternifolia EO was terpinene-4-ol (23.06%). This component was identified by Gas Chromatography coupled with Mass Spectrometry (GC–MS). Our results showed that the addition of M. alternifolia EO, at different concentrations to the culture medium, was completely stopped the production of Terrestric acid and Ochratoxin A by P. griseofulvum. Mycelial growth was also inhibited. The same effect was demonstrated on the growth and mycotoxins production on P. verrucosum.
Conclusions
Melaleuca alternifolia EO is suitable for control of post-harvest Penicillium contaminating lentil and dry grapes.
Keywords
P. griseofulvum
P. verrucosum
Melaleuca alternifolia
Essential oil
Mycotoxins
1 Introduction
Post-harvest fungi contaminating foodstuffs can cause the depreciation of their nutritional value as well as the deterioration of their organoleptic qualities (Tantaoui-Elaraki et al., 1994). In addition, fungi can lead to some damage because of mycotoxins production. Ingestion of contaminated food may also lead to food poisoning, fungal infections or allergic reactions (Chapeland-Leclerc et al., 2005; Codex Alimentarius, 2012; Leyral and Vierling, 2001; Nguyen et al., 2007; Zain, 2011).
Food legumes occupy the second place after cereals, as the main crops grown in Morocco (El Baghati, 1995). In hot and humid conditions due to improper or incorrect storage management, storage fungi belonging to Aspergillus and Penicillium spp. infest the seeds (Chhetri and Jha, 2016). In addition, the contamination of dried sweet fruits such as dry grapes was rarely noted, especially because of their lower activity water to allow growth and toxinogenesis by mold (Tantaoui-Elaraki et al., 1994).
Mycotoxins are stables molecules. Generally they escape antifungal treatments that act specifically on molds and not necessarily on their metabolites. They persist in contaminated foodstuffs even after elimination of mold (Boulal et al., 2011; Chapeland-Leclerc et al., 2005; Tantaoui-Elaraki et al., 1994). In order to their detoxification, food security requires the search for safe and effective postharvest treatment such as essential oils of aromatic plants (Louhibi et al., 2015; Zinedine et al., 2004).
The use of essential oils, in the biological control against mold and for the detoxification of their mycotoxins, is mainly due to their richness in antimicrobial and antioxidant substances (Foganholi et al., 2015; Hmiri et al., 2011; Khaddor et al., 2007; Louhibi et al., 2015). Numerous studies have established the bactericidal and fungicidal efficacy of the essential oil of M. alternifolia, plant of family Myrtaceae from Australia (Cheng and Shao, 2011), against fungi (Brophy et al., 1989; Ebani et al., 2018). Many members of this family are useful economically for medicinal, and various commercial uses (Lang and Buchbauer, 2012). Previous studies on the essential oils of many Myrtaceae showed that these plants have a broad range of biological activities, notably their antimicrobial potential. The chemical nature of the major active ingredients was directly related to this activity (Nuzhat and Vidyasagar, 2013).
Several previous studies have focused on the chemical composition and antifungal effect of M. alternifolia EO. Thus, its richness in monoterpenes, gives it an antioxidant and antimicrobial activity. These properties promise its use as a natural food preservative (Cox et al., 2001; Zhang et al., 2018).
The present work aims to study the effect of M. alternifolia EO on the growth and the toxigenesis of two toxigenic species of Penicillium, P. griseofulvum and P. verrucosum, isolated from lentils and dry grapes marketed in Morocco.
2 Materials and methods
2.1 Samples collection
Samples were taken at random from Martil city, in northern Morocco, on batches of products offered for sale to the public. Eight samples of lentil and nine of dry grapes were analyzed in our laboratory.
2.2 Isolation of Penicillium strains
The isolation of the Penicillium strains from the samples was carried out at the Environmental and Food Biotechnology Research Team (EFBRT) at the Normal High School of Tetouan, Morocco. To do, external face of food particles were disinfected and they were placed directly on Malt Extract Agar (MEA) (Samson and Gams, 1984). The incubation was at 25 °C for 7 days.
2.3 The spore suspension
In this study, all cultured strains were prepared from a spore suspension. For each Penicillium isolate, the spores were aseptically recovered and placed in a sterile solution of 0.1% Tween 80 distilled water for dispersion. A mother solution of spores was obtained, and its concentration was adjusted to 107 spores/ml by enumeration in the Thomas cell.
2.4 Identification of isolated Penicillium
The isolation step allows separating the strains suspected to be Penicillium (Asehraou et al., 1992). For identification and confirmation steps, cultures were grown on three standard media: (MEA), Czapeck Yeast extract agar (CYA) (Samson, 1981), and 25% Glycerol Nitrate agar (G25N) (Pitt and Hocking, 2009). An inoculum, taken from the suspension of spores using a sterile loop, was cultured in petri dishes of CYA, MEA, and G25N. These were then incubated at 25 °C for 7 days (Khaddor et al., 2007).
The identification of isolates was based on the determination key of Pitt and Hocking (2009) and Visagie et al. (2014). This identification was mainly based on the morphological features and the biochemical characteristics of the isolates. The macroscopic and microscopic parameters were examined. Then, a possible production of mycotoxins by the identified strains was highlighted.
2.5 Research for mycotoxins
Two ml of 107 spore/ml suspension of each identified strain was inoculated into three culture media, namely CYA medium, Base medium (MB) and liquid YES (Khaddor et al., 2007). The media were incubated at 25 °C for 10 days (Pitt, 1988). After incubation, the culture in YES liquid medium was subjected to Wattman paper filtration to remove the mycelium and then another filtration through a Millipore filter (0.45 µm) to be sterilized. Then, 25 ml of chloroform was added to the solid media (CYA and MB), and to the YES liquid medium and stirred for 2 min. The chloroform phase was recovered and filtered through a layer of anhydrous sodium sulfate. The filtrate was concentrated in a rotavapor and then evaporated to dryness under a stream of nitrogen. The mycotoxins were sought in the concentrated filtrate, and highlighted by Thin Layer Chromatography (TLC). The TLC plates used are 60 Kieselguhr F254. This method was performed as reported by Bouhoudan et al. (2018).
2.6 Chemical characterization of EO
Essential oil of M. alternifolia L. was supplied, in May 2015, by the Ain Lahjer women’s cooperative - Nouara - Dar Ben Karrich - Tetouan. The EO was obtained by hydrodistillation of dried aerial parts for 90 min in a device of Clevenger type. The resulting oil was dehydrated with anhydrous sodium sulfate and stored at 4 °C in the dark (Benayad et al., 2012). The chemical composition analysis of this EO was carried out by Gas Chromatography coupled with Mass Spectrometry (GC–MS). This method was done as described by Louhibi et al. (2015) and Derwich et al. (2009). The chemical characterization of EO M. alternifolia L. was performed on a gas chromatograph Trace GC Ultra-ITQ900 thermoscientific, equipped with a polar capillary column TG-SQC thermoscientific (60 m × 0.25 mm, 0.5 μm film thickness). Oven temperature program was from 50 °C to 240 °C at 5 °C/min. Injector temperature, 250 °C. Carrier gas Helium, flow rate 1 ml/min. The volume of injected sample was 1 μl (20 μl of EO diluted in 1000 μl of hexane). These analyses were conducted at the National Institute of Agricultural Research (INRA) of Tangier.
2.7 Antifungal effect of M. alternifolia EO on P. griseofulvum and P. verrucosum
In this study, two toxigenic isolates of Penicillium such as P. verrucosum and P. griseofulvum, isolated respectively from dry grapes and lentil, were subjected to the effect of different concentrations (Table 1) of M. alternifolia EO. The different concentrations of EO were prepared from a stock solution. The stock solution was obtained by dilution (1/10: v/v) of EO in distilled water containing 0.2% agar (Khaddor et al., 2007, 2006). Volumes corresponding to the concentrations (Table 1) were placed, each, in a 100 ml erlenmeyer flask. A volume of culture medium (MB, CYA and, liquid and solid YES) was added to each flask until a final volume of 25 ml. Then, each medium was inoculated with 0.5 ml of the spore solution. The control consists of 25 ml of culture medium added with 0.5 ml of distilled water at 0.2% agar. All these cultures were incubated at 25 °C for 10 days. Experiments were conducted in triplicate.
Percentage of EO
0%
0.08%
0.16%
0.63%
1.25%
2.50%
2.75%
Volume of EO (ml)
0
0.2
0.4
1.57
3.12
6.25
6.87
The media then was filtered by Buchner vacuum filter and Whatman N°1 filter to separate the mycelia from the liquid medium. The mycelia were then rinsed with hot distilled water to remove any trace of the culture medium. The dry weight was measured after placing the mycelia in an oven set at 80 °C for 24 h. The effect of the EO on the Penicillium growth was assessed by comparing the dry weight of the mycelium and the control.
2.8 Effect of M. alternifolia EO on mycotoxins production by P. griseofulvum and P. verrucosum
Culture media prepared at different concentrations of M. alternifolia EO inoculated with both Penicillium species were also used to study the effect of EO on production of mycotoxins. They were incubated at 25 °C for 10 days. The extraction of any mycotoxins was carried out by adding 25 ml of chloroform to each erlenmeyer flask followed by stirring for 10 min. The chloroform phase was recovered and filtered through a layer of anhydrous sodium sulfate. The chloroform recovered was then evaporated under vacuum in the rotary evaporator. The resulted extracts were supplemented with 0.5 ml of the chloroform-methanol extraction solvent (2/1: V/V) to be analyzed by TLC.
Toxigenesis study was made according to the method reported by Khaddor et al. (2007) and Bouhoudan et al. (2018). Mycotoxins standards used by the reference of migration forehead (Rf) were patulin (P), citrinin (C), ochratoxin A (OTA), penicillic acid (PA), and griseofulvin (Gi) (Cunniff and Association of Official Analytical Chemists, 1995). Fifty µl of each extract and standard solutions (1 mg/ml) were spotted on TLC plates. Elution systems used were: toluene-ethyl acetate - formic acid (5/4/1, v/v/v) and chloroform - acetone - 2-propanol (85/15/20, v/v/v). The plates were examined in daylight and by ultraviolet 365 and 254 nm after spraying the spots by ANIS (p-anisaldehyde solution) and 8 min heating to 120 °C.
2.9 Statistical analysis
The Student’s t-test was used to determine the statistical significance of experiments performed in triplicates. Statistical values including means and standard deviation from the obtained data were calculated using Statistical Tools for High-Throughput Data Analysis; STHDA (http://www.sthda.com/french/rsthda/rsthda.php). Differences were considered significant if p < 0.05.
3 Results
3.1 Identification of Penicillium strains and their mycotoxins
Color conidia (shades between blue, green and/or gray), roughness or smoothness of stipes on CYA and MEA, spherical mature conidia or ellipsoidal, colony diameter on CYA and MEA, structure and number of branches of penicil on MEA, were the characters analyzed and compared to the identification keys of Pitt and Hocking (2009). In this study, we isolated 14 strains of Penicillium. These strains belong to seven species (Table 2). *: Toxigenic species.
Penicillium species
Lentil
Dry grapes
P. simplicissimum
–
1
P. viridicatum
3
–
P. italicum
1
–
P. verrucosum*
–
3
P. griseofulvum*
2
2
P. citrinum
1
–
P. brevicompactum
1
–
Our results showed that food samples of lentil were contaminated with eight strains belonging to five species of Penicillium. While, six strains belonging to three species of Penicillium were isolated from the samples of dry grapes. The isolated toxigenic species were P. verrucosum and P. griseofulvum.
Our findings showed that mycotoxins were produced only in YES medium. The metabolic profile showed that the Terrestric acid was produced by both Penicillium species. The Ochratoxin A production was revealed in the profile of P. griseofulvum (Table 3).
Strain
Mycotoxins
Revelation TLC
Food source
P. verrucosum
Terrestric acid
ANIS
Dry grapes
P. griseofulvum
Terrestric acidOchratoxin A
ANIS366 nm
Dry grapes and lentil
As P. verrucosum and P. griseofulvum were, in this work, mycotoxin-producing species, they were subject to the study of the effect of M. alternifolia EO.
3.2 Antifungal effect of M. alternifolia EO on P. verrucosum and P. griseofulvum
The CG-SM essays showed that the major component of the M. alternifolia EO was the terpinene-4-ol with a percentage of 23.06%. The identification of this EO component was done by comparing the mass spectrum of each peak separated by GC with those reported as references such in specialized bibliographies of mass spectrometry (Brophy et al., 1989; Hammer et al., 2006).
3.2.1 Effect of M. alternifolia on mycelial growth of P. verrucosum and P. griseofulvum
A significant decrease in dry weight (p < 0.05) relative to the control was noted for the mycelia of both species: P. verrucosum and P. griseofulvum. The graphic representation of the dry weight of P. verrucosum in relation to the dry weight of control (Fig. 1) showed that the lowest concentration (0.08%) significantly reduced the dry weight of mycelium to 60% of control dry weight. The decrease in dry weight was proportional to the increase in the concentration of EO. At 2.75%, the EO completely inhibits growth of P. verrucosum in the liquid YES.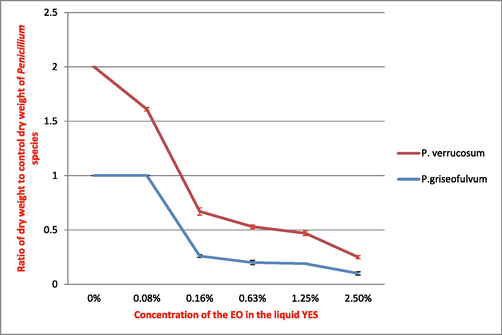
Effect of M. alternifolia EO on the growth of P. verrucosum and P. griseofulvum.
As for P. griseofulvum, inhibition of mycelial growth was significant from the 0.16% concentration (Fig. 1). Inhibition of mycelial growth was greater at the highest concentration of EO. Total inhibition of mycelial growth resulted in 2.75% of M. alternifolia EO for both species Penicillium in liquid YES.
3.2.2 Effect of M. alternifolia EO on mycotoxins production of P. verrucosum and P. griseofulvum
As we mentioned before, both species of Penicillium produce their mycotoxins only in YES medium. TLC performed to demonstrate the effect of EO on mycotoxin production by both fungal species also revealed that these mycotoxins were not produced in CYA and MB media. The production of Terrestric acid by both Penicillium species, and Ochratoxin A by P. Griseofulvum was inhibited from the lowest concentration of M. alternifolia EO.
The TLC plate (Fig. 2) showed that the production of the terrestric acid by P. verrucosum was inhibited at increasing concentrations of the EO of M. alternifolia represented respectively by B, C, D, E, F, and G. The yellow spot, in position A, represents the terrestric acid produced in the control culture in liquid YES medium.
Picture of a TLC plate showing the disappearance of the production of terrestric acid by P. verrucosum at increasing concentrations of M. alternifolia EO.
4 Discussion
In this study, we identified 14 Penicillium strains contaminating commercialized lentil and dry grapes in northern Morocco. This identification was based on the determination key of Pitt and Hocking (2009). The identified species were P. simplicissimum, P. viridicatum, P. italicum, P. citrinum, and P. brevicompactum. In addition, two toxinogenics species; P. verrucosum and P. griseofulvum were identified. The characterisation of toxigenic molds is of great importance. However, this procedure based on the determination of cultural, macroscopic and microscopic traits remains a complicated method and requires particular precision. Penicillium commonly present in food are for the most part similar in color and appearance of colony. The reproductive structures are small and often ephemeral. It is therefore essential that the cultures of the isolates are performed under standardized conditions.
According to Nguyen et al. (2007) and Frisvad et al. (2004), the method used in this study was suitable for identification and classification of foodstuffs fungal contaminants and also for determination of phenotypic data.
P. verrucosum is ochratoxigenic specie. However, Pitt (1987) and Hajjaji et al. (2004) reported that there are non-ochratoxigenic strains of this specie. Our results showed that P. verrucosum produced Terrestric acid. Indeed, mycotoxins production was controlled by environmental factors such as water activity, temperature, and nature of the substrate. Moreover, the production of a mycotoxin in a species does not mean that this metabolite was expressed by all the strains belonging to this specie (Pitt and Hocking, 2009). This could explain the difference between the metabolic profile of our isolates and those reported in the literature for the same species.
In this work, the antifungal activity of M. alternifolia EO has been demonstrated against Penicillium species as validated by Cheng and Shao (2011) and Bishop and Thornton (1997). Total inhibition of mycelial growth of both Penicillium species resulted in 2.75% of this EO. In this context, Zhang et al. (2018) reported the antifungal effect of M. alternifolia EO by inhibiting the growth of P. italicum and P. digitatum. The results obtained by Yonghua et al. (2017) showed that M. alternifolia EO exhibits antifungal activity against P. expansum.
In fact, EO impaired ergosterol, component of the cell membranes of molds, synthesis and induced cell membrane rupture (Lang and Buchbauer, 2012). In the other hand, Cox et al. (2001) interprets the action of EO by the penetration of terpinene-4-ol through the cell wall and the structures of the cell membrane leading to cell death.
The international standard regulation for M. alternifolia EO sets a minimum content of 30% terpinen-4-ol (Hammer et al., 2006). While, in our study terpinene-4-ol represents 23.06% of the chemical composition. This data is in agreement with those reported by Carson and Riley (1995) who demonstrated that the concentration of each component could vary widely depending on the oil sample. Our results revealed that M. alternifolia EO has completely stopped the production of Terrestric acid by P. griseofulvum and P. verrucosum, and Ochratoxin A by P. griseofulvum. This demonstrated the inhibitory effect of the EO on P. griseofulvum and P. verrucosum toxigenesis.
Finally, Hammer et al. (2006) suggested that the administration of M. alternifolia EO in diluted form is without adverse effects. The toxicity of this EO was demonstrated in high doses for humans and animals.
Our study showed some limitations. It would be more appropriate to use of dry weight measurement to estimate fungal biomass. This parameter was found to be indirectly proportional to fungal growth. Future researches should use some biochemical indices. A correlation was found between those indices and linear extension of mycelium.
Furthermore, our team is working on antifungal effect directly on food samples to better understand their behavior and better define the optimal antifungal dose in vivo. In the other hand, we will introduce the molecular approach for the identification of Penicillium.
5 Conclusions
Lentils and dry grapes are staples of the human diet. The conditions of foodstuffs storage, including humidity and darkness, are appropriate for toxigenic Penicillium growth. Hence, biological control should take place. The strains belonging to the Penicillium genus were called storage molds. These molds can cause significant losses by reducing the quality and/or quantity of food stored.
Our results reveal the presence of different species of Penicillium in analyzed foodstuffs, among these fungal contaminants, two species are toxigenic. The effect of M. alternifolia EO on growth and mycotoxins production of these molds makes it possible to develop an effective and healthy method for foodstuffs preservation. This EO was suitable for in vitro control of post-harvest Penicillium of lentil and dry grapes, but the specific mechanism of this control needs be further studied.
References
- Physico-chemical properties and the microflora of Moroccan table black olives. Grasas Aceites. 1992;43:130-133.
- [Google Scholar]
- Chemical characterization and insecticidal evaluation of the essential oil of Mentha suaveolens L. and Mentha pulegium L. growing in Morocco. Sci. Study Res.. 2012;1:27-32.
- [Google Scholar]
- Evaluation of the antifungal activity of the essential oils of Monarda citriodora var. citriodora and Melaleuca alternifolia on post-harvest pathogens. J. Essent. Oil Res.. 1997;9:77-82.
- [Google Scholar]
- The effect of carbon source concentration on toxigenesis and lipase activity of Penicillium aurantiogriseum. Agric. Forest.. 2018;64:59-70.
- [CrossRef] [Google Scholar]
- Isolement et identification de souches de moisissures réputées toxinogènes dans le blé local stocké traditionnellement dans la région d’Adrar. Rech. Agron. 2011:49-60.
- [Google Scholar]
- Gas chromatographic quality control for oil of melaleuca terpinen-4-01 type (Australian Tea Tree) J. Agric. Food Chem.. 1989;37:1330-1335.
- [Google Scholar]
- Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol.. 1995;78:264-269.
- [CrossRef] [Google Scholar]
- Moisissures et risques alimentaires (mycotoxicoses) Rev. Frangaise Lab. 2005:61-66.
- [Google Scholar]
- In vivo antifungal activities of the tea tree oil vapor against Botrytis cinerea. In: 2011 International Conference on New Technology of Agricultural. Presented at the 2011 International Conference on New Technology of Agricultural Engineering (ICAE). Zibo, China: IEEE; 2011. p. :949-951.
- [CrossRef] [Google Scholar]
- Seed mycoflora of Lens culinaris Meddik. from different storage conditions and in-vitro inhibition using plant extracts. Ecoprint Int. J. Ecol.. 2016;23:19.
- [CrossRef] [Google Scholar]
- Prévention et Réduction de la Contamination des Produits de Consommation Humaine et Animale. Rome: Organisation mondiale de la santé organisation des nations unies pour l’alimentation et l’agriculture; 2012.
- The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) J. Appl. Microbiol.. 2001;88:170-175.
- [CrossRef] [Google Scholar]
- GC–MS analysis of the leaf essential oil of Mentha rotundifolia, a traditional herbal medicine in Morocco. Chem. Bull. “POLITEHNICA” Univ. (Timisoara). 2009;54:85-88.
- [Google Scholar]
- Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Vet. Sci.. 2018;5:62-74.
- [CrossRef] [Google Scholar]
- La production des légumineuses alimentaires au Maroc. Dir. Prod. Végétale MAMVA 1995:77-82.
- [Google Scholar]
- Chemical composition and antifungal activity of pennyroyal essential oil in different stages of development. Semina Ciênc. Agrár. Londrina. 2015;36:3091-3100.
- [Google Scholar]
- Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol.. 2004;49:201-241.
- [Google Scholar]
- Evaluation de la production de l’ochratoxine A par quelques espèces fongiques d’origine marocaines isolées à partir de graines de céréales. Biotechnologies.. 2004;1:482-486.
- [Google Scholar]
- A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol.. 2006;44:616-625.
- [CrossRef] [Google Scholar]
- Évaluation du potentiel antifongique des huiles essentielles de Mentha pulegium et d’Eucalyptus camaldulensis dans la lutte biologique contre les champignons responsables de la détérioration des pommes en conservation. Bull. Société R. Sci. Liège. 2011;80:824-836.
- [Google Scholar]
- Antibacterial effects and toxigenesis of Penicillium aurantiogriseum and P. viridicatum. Afr. J. Biotechnol.. 2007;6:2314-2318.
- [Google Scholar]
- Antifungal activity of three essential oils on growth and toxigenesis of Penicillium aurantiogriseum and Penicillium viridicatum. J. Essent. Oil Res.. 2006;18:586-589.
- [Google Scholar]
- A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr. J.. 2012;27:13-39.
- [Google Scholar]
- Microbiologie et toxicologie des aliments. Hygiène et sécurité alimentaires (third ed.). Paris: Doin; 2001.
- Chemical composition and antimicrobial activity of essential oils, Thymus vulgaris L. and Mentha L. pulegium against the major post harvest diseases of citrus in Morocco. Int. J. Sci. Res.. 2015;4:1181-1184.
- [Google Scholar]
- Occurrence of aflatoxin B1, citrinin and ochratoxin A in rice in five provinces of the central region of Vietnam. Food Chem.. 2007;105:42-47.
- [Google Scholar]
- Antifungal investigations on plant essential oils. A review. Int. J. Pharm. Pharm. Sci.. 2013;5:19-28.
- [Google Scholar]
- Pitt, J.I., 1988. A Laboratory Guide to Common Penicillium Species, second ed., North Ryde Australia.
- Penicillium viridicatum, Penicillium verrucosum, and production of ochratoxin A. Appl. Environ. Microbiol.. 1987;53:266-269.
- [Google Scholar]
- Fungi and Food Spoilage (third ed.). Springer Science & Business Media; 2009.
- Review of the genus Penicillium and its teleomorphic states EuPenicillim and Talaromyces. Mycologia. 1981;73:582-584.
- [Google Scholar]
- The taxonomic situation in the hyphomycete genera Penicillium, Aspergillus and Fusarium. Antonie Van Leeuwenhoek. 1984;50:815-824.
- [Google Scholar]
- Recherche de mycotoxines dans des denrées alimentaires distribuées au Maroc. Actes Inst. Agron. Vet.. 1994;14:11-16.
- [Google Scholar]
- Identification and nomenclature of the genus Penicillium. Stud. Mycol.. 2014;78:343-371.
- [CrossRef] [Google Scholar]
- Effects and possible mechanism of tea tree oil against Botrytis cinerea and Penicillium expansum in vitro and in vivo test. Can. J. Microbiol.. 2017;63:219-227.
- [Google Scholar]
- In vitro evaluation of antioxidant and antimicrobial activities of Melaleuca alternifolia essential oil. BioMed Res. Int.. 2018;2018:8.
- [CrossRef] [Google Scholar]
- Ochratoxin A determination in dried fruits and black olives from Morocco. Alimentaria. 2004;359:73-76.
- [Google Scholar]







