Translate this page into:
Antifungal activity of Streptomyces sp. SLR03 against tea fungal plant pathogen Pestalotiopsis theae
⁎Corresponding author. elisi@ksu.edu.sa (Essam Nageh Sholkamy) essam_92003@yahoo.com (Essam Nageh Sholkamy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
The search for new biocontrol agents, especially from natural sources, to control plant pathogens is a key area in tea plant protection research.
Methods
Starch casein nitrate (SCN) agar was used to isolate the actinomycetes from the soil samples. These isolates were tested with modified version of the dual-culture method for antagonistic activity against Pestalotiopsis theae. The most bioactive isolate of actinomycets has been identified by biochemical, phisological, and morphological characterisation. Bioactivity of Streptomyces sp. SLR03 metabolites was measured in vitro and in vivo. The extract was eventually analyzed by GC–MS.
Results
For the first time, an attempt has been made to isolate actinomycete species with bio-control potential from the river soil samples. A total of one hundred and seven actinomycete strains isolated and were evaluated for antagonistic potential against Pestalotiopsis theae using a dual-culture assay. Among the strains isolated, one strain SLR03 that showed potential and it was characterized. Further, the strain SLR03 was evaluated for antagonistic activity against P. theae both in vitro and in vivo. In in vitro assay, 86.15% and 93.85% mycelial growth inhibition was observed with cell-free filtrate and ethyl acetate extract of Streptomyces sp. SLR03, respectively. The ethyl acetate extract was further evaluated for its biocontrol activity against P. theae, it exhibited 80.39% reduction of disease incidence compared to the control. Further, the ethyl acetate extract were analyzed using GC–MS. The GCMS chromatogram exhibited 24 intense peaks consistently with 19 different compounds, 10 of which contain antifungal activity.
Conclusions
This present study illustrates that Streptomyces sp. strain SLR03 is a prospective candidature for forthcoming biological control programme.
Keywords
Streptomyces sp.
Pestalotiopsis theae
Antagonistic activity
Tea plant
1 Introduction
Grey blight in tea caused by Pestalotiopsis theae has become a challenging disease in tea-cultivating countries, and 17% crop loss has been reported during monsoon seasons (Sanjay et al., 2008; Sanjay, 2017). Chemical fungicides, such as mancozeb, Companion and Carbendazim, are used to control P. theae in tea fields. The continual use of chemical fungicides leads to residue issues, disease resistance and cost constraints to the planters. To overcome these problems and effectively control plant pathogens, there is a necessity to identify antifungal compounds using a biological approach. An investigation of novel antifungal compounds from actinomycetes is of significant research around the globe.
Microbes are economically sustainable sources for the synthesis of antifungal compounds (Jiménez-Reyes et al., 2019; Janette et al., 2019). Among microorganisms, the actinobacteria are one of the most fascinating candidates of bioactive compounds that inhibit various plant pathogenic fungi (Betancur et al., 2017; Djinni et al., 2019). Actinobacteria represent an extensive array of important and renowned resources for bioactive metabolites, among the members of Streptomyces and it contributed more than 60% of the antimicrobials compounds to date (Das et al., 2018; Sholkamy et al., 2020).
Therefore, in the current era, the search for novel actinobacteria from diverse locations to explore the novel and prospective antifungal compounds have become importance. An attempt has been made to isolate actinomycetes from the fresh water river basin and evaluate in vitro and in vivo biocontrol activity of Streptomyces sp. SLR03 against P. theae it is an economically important tea pathogen.
2 Materials and methods
2.1 Collection of soil samples
Soil samples were collected on January 2012 from the Sholayar river, located at 76° 39′ 0″ E, 10° 27′ 30″ N longitude and 76° 44′ 35″ E, 10° 27′ 0″ N latitude. The Samples were procured from 4 to 5 cm of the soil profile. The soil samples were collected at different locations using clean, dry polyethylene bags and spatulas. The soil samples were stored at 4 °C temperature in refrigerator until further process it.
2.2 Actinomycete isolation
Actinomycetes were isolated from the collected soil samples using standard protocols (Kuster and Williams 1964) on starch casein nitrate (SCN) agar augmented with cycloheximide (25 µg/ml) and rifampicin (50 µg/ml) and incubated at 30 °C for 5 days. Actinomycete colonies showing different morphological characteristics were picked and sub-cultured into International Streptomyces Project (ISP) 2 agar slants. Stock cultures of isolates were maintained as spore and mycelial suspensions in 20% glycerol at −20° C.
2.3 Screening of antagonistic actinomycetes
The antagonistic effects of actinomycete isolates were assessed by modified version of the dual-culture method (Landa et al., 1997). The actinomycete isolates were streaked on a one corner of the SCN agar plate and incubated for 5 days at 30° C. A mycelial disk (5 mm in diameter) of
P. theae was then inoculated on the plate, which was incubated for an additional 72 h at 30° C. The mycelial growth of P. theae toward (a) and away (b) from the actinomycetes was then measured to determine the effective antagonistic activity of actinomycete isolates and select appropriate isolates for further studies.
2.4 Characterization of prospective isolate Streptomyces sp. SLR03
The actinomycete isolate showing the most potent fungal antagonism was characterized based on morphological, biochemical and physiological characteristic features (Miyadoh et al., 1997). The morphological characteristics included colony shape and color, sporulation and pigment diffusion on ISP and TS (tryptic soy) agar (Shimizu et al., 2000). The physiological and biochemical properties of isolates were investigated according to the methods of (Shirling and Gottlieb, 1966) and Holt et al., (2000).
2.5 Production and extraction of bioactive metabolites
The seed inoculums were prepared by culturing of Streptomyces sp. SLR03 in ISP 2 medium for 24 h. The seed culture (10%) was inoculated to TS medium containing (g/l) tryptone 17.0, soybean meal 3.0, dextrose 2.5, NaCl2 5.0 and K2HPO4 2.5, pH 7.1 ± 0.2, and incubated on a rotary shaker for 5 days at 29° C. The 20 L of culture broth obtained after filtration was extracted twice with ethyl acetate and concentrated under reduced pressure to yield a crude extract that was dried in a rotary evaporator (Kavitha et al., 2009).
2.6 In vitro antagonistic activity
The agar well diffusion method was performed using the technique of Bauer et al., (1966), on double-layered PDA plates to test the non-culture filtrate (NCF) and ethyl acetate extract (EAE) of Streptomyces sp. SLR03 isolate. Three wells were made in the top layer of solidified PDA plates by punching the top agar layer 3 times with a cork borer (5 mm diameter) along a radius approximately 3 cm from the center of the plate. One agar disc (5 mm diameter) of P. theae culture was cut from the marginal colony of a 5-day-old culture cultivated in PDA plates and moved into the middle of the plate. Fifty microliters of NCF, EAE (50 µg/ml) or trypticase soy broth (TSB) was put in one of the 3 wells, and the plates were incubated at 28° C for 5 days. The standard fungicides Bavistin (0.05%), Dithane (0.3%) and Companion (1%) were used at appropriate concentrations. The results were observed and assessed the percent inhibition of colony growth (PICG), according to modified methods of Lokesha and Benagi (2007). Each test was repeated three times, and the average was calculated.
2.7 In vivo antagonistic activity
The in vivo antagonistic activity was evaluated using greenhouse conditions as reported by Kim et al., (2003). The EAE compound was dissolved in 1 ml of dimethyl sulfoxide (DMSO) and diluted with 49.5 ml of 1% Tween 80. Aliquots of 50 ml were sprayed onto one set of tea plants (consist of six plants). The treated tea saplings were maintained in a greenhouse for 1 day prior to inoculation with P. theae. The standard fungicides and 1% DMSO were used as a positive and negative control respectively. The tea plantlets were kept under greenhouse conditions suitable for establishment of disease. The disease intensity was determined according to the method described by (Wang et al., 2009). The tea saplings were spraying with spore suspension of P. theae (1X106 spores/ml) at the first stage (one plant/pot). The spore suspensions were prepared using 0.85% saline mixed with 15 days old culture spores. All the experiments were conducted in triplicate.
2.8 Gas chromatography-mass spectrometry (GC–MS) analysis
Partially purified Streptomyces extract was analyzed by using GC–MS with a fused silica capillary column (C18, 30 × 0.25 mm ID, film thickness 0.5 µm). The data were processed with GC–MS ChemStation (Agilent Technologies, 6890-N series GC with 5990 series II). The column conditions were as follows: column oven temperature 150° C (4 min) – 4° C/min, injection port temperature 250° C and detector port temperature 280° C (Roy et al., 2006). The sample peaks detected with gas chromatography were subjected to mass spectral analysis. The spectra were analyzed using available library data and with National Institute of Standards and Technology (NIST) MS search (version 2.0; included with NIST ’02 mass spectral library and accessed with Agilent p/n G1033A).
3 Results
3.1 Isolation and screening of antagonistic actinomycetes
A total of 107 actinomycetes isolated from 15 different soil samples, of 107 isolates, the 6 most prominent isolates (isolates that were found abundantly, produced pigments and inhibited adjacent colonies on SCN agar plates) were further screened for their antagonistic potential against P. theae using an in vitro dual-culture assay. Among, six actinomycete isolates, only one strain (SLR03) was found to have antagonistic potential against P. theae (Fig. 1).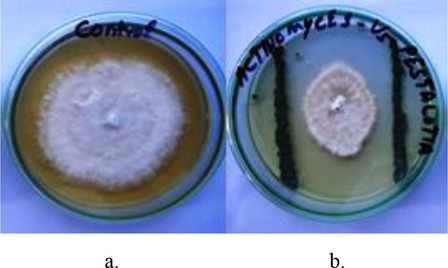
P. theae using cross plug method incubated for 7 days at 28° C on Potato Dextrose agar medium; (a) control plate of P. theae; (b) antagonism activity of Streptomyces sp. SLR03 against P. theae.
The strain SLR03 grew on SCN agar media and showed the typical morphology of Streptomyces sp. The color of the aerial and substrate mycelium tended to be green and light green respectively. The surface of the mycelium dusty with rough in nature (Table 1). The isolate SLR03 not produced any diffusible pigments. Further, isolate SLR03 Gram positive with filamentous in nature aerial hyphae were differentiated into long looped chains of spores with spiral spore morphology (Fig. 2). The biochemical and physiological results as follows; indole negative, methyl red negative, Voges Proskauer negative, citrate positive, starch and casein positive. The sugar utilization results showed with positive for glucose and sucrose, negative for lactose and mannitol (Table 2). The strain Streptomyces sp. SLR03 was evaluated further for its antagonistic activity against P. theae under in vitro and in vivo conditions. ‘+’ - positive; ‘−’ – negative.
Characteristic features
Observation
Cultural
Mycelium
Colony nature
Colony colour
Pigmentation
Aerial
Dusty with spores
Green
No pigment
Substrate
Rough surface
Light green
Microscopic
Gram staining
Mycelial nature
Gram positive filamentous
Long looped chain of spore
Biochemical
Indole
MR
VP
Citrate
Negative
Negative
Negative
Positive
Physiological
Oxidase
Catalase
Starch
Casein
Negative
Negative
Positive
Positive
Sugar Utilization
Glucose
Sucrose
Lactose
Mannitol
Positive
Positive
Negative
Negative
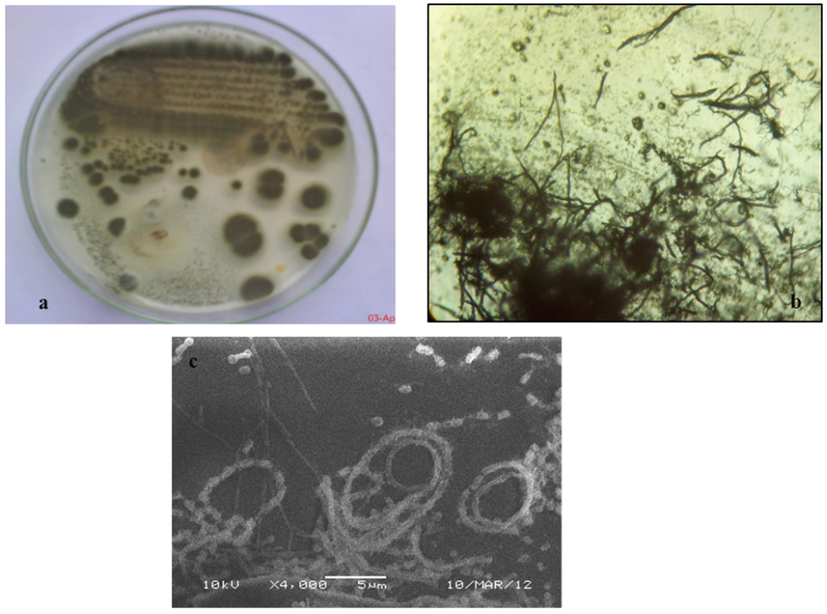
Streptomyces SLR03 a) colony morphology on starch casein nitrate medium (SCN) in a 5 days old culture, b) micrograph of branched aerial mycelium 7 days old culture (x 1000), c) SEM image of isolate SLR03 spore morphology.
Biochemical tests
SLR03
Carbohydrate utilization
SLR03
Biopolymer hydrolysis
SLR03
Indole
−
Dextrose
+
Gelatin
−
Methyl red
−
Fructose
+
Starch
+
VP
−
Sucrose
+
Casein
+
Citrate
+
Galactose
−
TSI
A/K
Lactose
−
Oxidase
−
Mannitol
−
Catalase
−
3.2 In vitro antagonistic activity
The antagonistic activity of Streptomyces sp. SLR03 was measured by testing SLR03-derived NCFs and EAEs against P. theae. After 5 days, EAEs showed the maximum inhibition of P. theae colony growth (93.08%), whereas NCFs showed minimum inhibition (93.85%). The commercial fungicides Bavistin (0.05%), Dithane (0.3%) and Companion (1.0%) inhibited colony growth by 97.08%, 96.11% and 95.15%, respectively, and the control showed no inhibition of fungal colony growth (0.0%) (Fig. 3 and Table 3).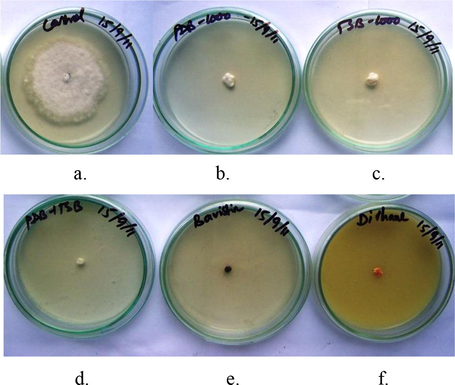
Antagonistic activity of Streptomyces sp. SLR03 against P. theae grown on PDA plates; (a) control plate of P. theae on PDA plates, (b) PDA plate mixed with 1ml of non-culture filtrate, (c) PDA plate mixed with 1ml of ethyl acetate extract, (d) PDA plate mixed with 0.05 % of bavistin, (e) PDA plate mixed with 0.3 % of dithane, (f) PDA plate mixed with 1.0 % of companion.
S. No.
Treatment
% of inhibition fungal colony growth
1
Control
0.00
2
1 ml of Non-culture filtrate
93.85
3
1 ml of Ethyl acetate filtrate
93.08
4
Bavistin (0.05%)
97.08
5
Dithane (0.3%)
96.11
6
Companion (1.0%)
95.15
SE
3.84
CD
7.88
3.3 In vivo antagonistic activity
The results of in vivo studies in a greenhouse with infected plants are displayed in Table 4 and Fig. 4. The partially purified compound and ethyl acetate extract from Streptomyces sp. SLR03 efficiently controlled gray blight disease and was exhibited maximum 89.39 and 81.09% of disease control efficiency. The maximum percentage of disease control efficiency was observed at 7 days compared to the P. theae infected tea plants. The maximal reduction of disease incidence was observed with partially purified compound (89.39%), followed by EAEs (81.09%), but the commercial fungicides such as Bavistin, Dithaneand Companion showed reduction in disease incidence by 62.28%, 75.73% and 76.72%, respectively (Table 4 and Fig. 4).
S.No
Treatments
Average disease increased in shoots (mm)
% disease control
1.
Un-inoculated (disease free)
–
2.
Inoculated (control)
16.57
–
3.
Ethyl acetate extract
3.25
81.09
4.
Partially purified compound
5.33
89.39
5.
Bavistin (0.05%)
6.25
62.28
6.
Dithane (0.3%)
7.83
52.73
7.
Companion (1.0%)
3.86
76.72
SE
3.64
CD
7.47
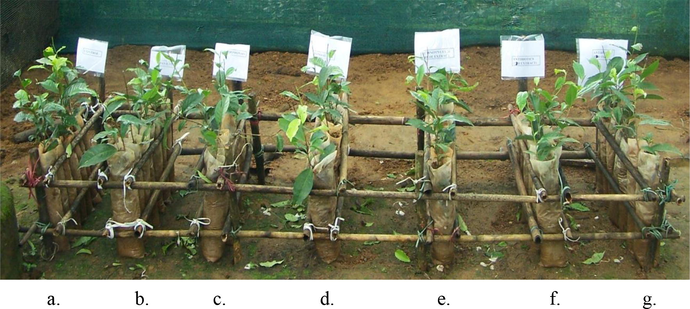
In vivo Biocontrol activity of Streptomyces sp. SLR03 against P. theae; (a) control tea plants (un-inoculated, disease free), (b) inoculated with P. theae (diseased), (c) P. theae inoculated plants with ethyl acetate extract, (d) P. theae inoculated plants with partially purified compound, (e) P. theae infected tea plants with Bavistin (0.05 %), (f) P. theae infected tea plants with Dithane (0.3 %), (g) P. theae inoculated plants with companion (1.0 %).
3.4 GC–MS analysis
GC–MS analysis of the partially purified Streptomyces extract revealed 19 different compounds. Only 10 of these compounds have antimicrobial activities such as tetradecane (C14H30), nonadecane (C19H40), 10-Henicosene (C21H42), 3-Eicosene (C20H40), 1-Hexadecanol (C16H34O), 1-iodo-2-methylundecane (C12H25I), Tetradecane, 2,6,10- trimethyl (C17 H36), Decane – 2,3,5,8 trimethyl (C14, H30), Pterin-6-carboxylic acid (C7H5N5O3) and 1-octadecanesulfonylchloride (C18H37ClO2S) as in Table 5. The nature and activity of each chemical compound are also depicted in Table 5. The GC–MS spectrum confirmed the presence of 10 major components with retention times of 4.10, 5.09, 6.05, 7.00, 8.04, 9.06, 10.08, 11.00, 12.05, 14.02, 15.05 and 16.00 min (Fig. 5). Mass spectral data obtained from GC–MS were interpreted using the NIST database.
S.No.
Compounds
Bioactive propertiesAs per literatures
1.
Hexadecane (C16 H34)
2.
Tetradecane (C14 H30)
Antifungal and antibacterial
3.
Decane-2,4,6 trimethyl (C13 H28)
4.
Nonadecane (C19 H40)
Antifungal
5.
3-Trifluroacetoxytetradecane (C16H29F3O2)
6.
10-Henicosene (C21 H42)
Anti-phytopathogenic fungal pathogen
7.
Trichloroacetic acid (C16 H29Cl3O2)
8.
3-Eicosene (C20 H40)
Antifungal
9.
1-Hexadecanol (C16 H34 O)
Antifungal/Antimicrobial
10.
1-iodo -2-methylundecane (C12 H25I)
Antifungal/Antimicrobial
11.
Tetradecane, 2,6,10-trimethyl (C17 H36)
Anti-inflammatory and Antifungal
12.
Decane-2,3,5,8 trimethyl (C14 H30)
Anti-phytopathogenic fungal pathogen
13.
4-Trifluroacetoxypentadecane(C17H31F3O2)
14.
3-Trifluroacetoxytetradecane (C16H29F3O2)
15.
2-Trifluroacetoxytetradecane (C16H29F3O2)
16.
5-Eicosene (E)(C20 H40)
17.
Pterin 6 carboxylic acid (C7H5N5O3)
Antifungal
18.
1octadecanesulfonylchloride(C18H37 ClO2S)
Antifungal
19.
Ethanol-2(hexadecyloxy) (C18 H38 O2)
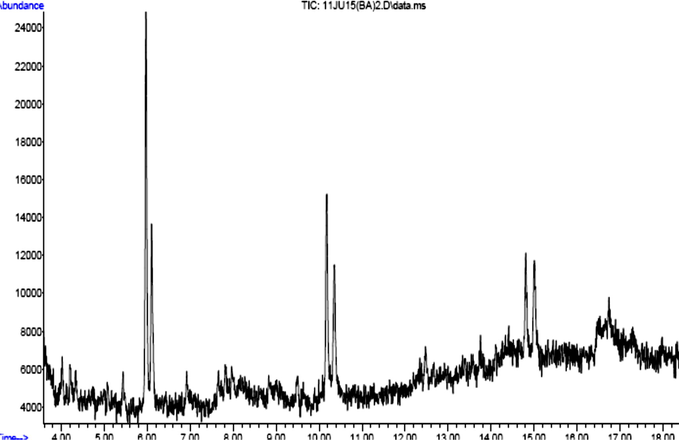
GC MS analysis of Streptomyces sp. SLR03 partially purified extracts.
4 Discussion
The overuse of chemical fungicides and fertilizers is common in agricultural farming systems of most parts of the world which affects, it threatening the environments and health of individuals. In general pesticides persists in the environment and also destroys the beneficial microorganisms which have positive effects on soil fertility and plant growth. Biological controls with use of microorganisms are an appropriate substitute for such negative impacts on environment and also disease management. Such biological control measures with use of microorganisms are well adapted to the soil ecology and also determinedly exert effective antagonistic activity against soil-dwelling plant pathogens (Kohl et al., 2019).
Over the years, the use of microbial secondary metabolites for crop protection received greater attention, and these metabolites are used as an alternative to chemical compounds (Singh et al., 2017; Cardoso et al., 2019). Since, these metabolites are biologically synthesized, it contains specific activity against the plant pathogens and also environment friendly (Heydari and Pessarakli, 2010; Masi et al., 2018). Thus, worldwide interest has been renewed, and approximately 40 different plant diseases and associated pathogenic organisms are currently managed with microbial metabolites (Pal and Gardener, 2006; Panth et al., 2020). Several microbial bioactive secondary metabolites have also been discovered as agrochemicals, and many of them are currently commercially available (Sanjai, 2014).
The current investigation on in vitro and in vivo using an existing natural resource, a Streptomyces strain that showed effective antagonistic characteristics against P. theae. Ara et al., (2012) reported a similar antifungal activity of actinomycetes against P. mangifera, a causal agent of mango brown rot. The results of Ponmurugan et al., (2011) revealed that most isolates of Streptomyces spp. from tea rhizosphere soil showed potential antagonists activity against tea pathogens. Several other researchers have reported satisfactory results using Streptomyces spp. against plant pathogens in vitro (Kim and Song, 2016; Lyu et al., 2017; Kim et al., 2019).
Streptomyces sp. strain SLR03 suppressed fungal disease in inoculated tea saplings under greenhouse condition. Greenhouse experiments showed that the biocontrol efficacy of Streptomyces sp. strain SLR03 correlated with anti-P. theae activity. The results confirm the importance of the fresh water Streptomyces isolates as biocontrol agents and emphasize the importance of indigenous Streptomyces sp. as biocontrol agents against tea fungal phytopathogens. The present results corroborate with the previous studies which determined the biocontrol potential of microorganisms under artificial conditions in greenhouse experiments (Lia et al., 2012; Zhou et al., 2014; Besset-Manzoni et al., 2019).
GC–MS analysis of Streptomyces sp. strain SLR03 EAE showed 24 retention peaks, most of which were reported to have antifungal metabolites. Due to a lack of authentic samples and library data for the corresponding compounds, some of the GC–MS peaks remained unidentified. The chemical constituents of extracts and the important functional groups they contribute are characteristic of bioactive compounds and validate the use of Streptomyces sp. strain SLR03 as an important candidate antifungal agent. Most of the compounds were aromatic, and has varied biological activity, including antifungal activity (Sholkamy, 2014). The investigation confirmed the potential richness of antifungal agents from Streptomyces sp. strain SLR03. Further investigation is needed to determine the structure of an active compound and scale up its production. From the results of primary screening, a phytopathogenic study and GC–MS analysis, it is evident that strain SLR03 has great potential for secondary metabolite production.
5 Conclusion
The present investigation reports the presence of bioactive actinomycetes in the Sholayar river basin (fresh water environment), which is surrounded by reserve forest and tea plantations and originates from the Western Ghats, South Asia. Locally available strains of bioactive actinomycetes can be explored and tapped as one of the potential source of novel antifungal antibiotics. The strain Streptomyces sp. SLR03 exhibited effective antifungal activities against P. theae. Further, the ethyl acetate extract showed presence of antifungal compound, when analyzed with GC–MS chromatography spectrum. This biological agent could be combined with fungicides for the effective control of foliar pathogens, such as P. theae, that cause grey blight leading dieback disease in tea. The future, the potential candidature of Streptomyces sp. SLR03 characterized further with NGS approach and bioactive principle compound was identified.
Acknowledgments
The authors thank the Deanship of Scientific Research at King Saud University for funding this work through research group RG-1439-025. The authors are thankful to the management team of Parry Agro Industries Ltd. for providing facilities to carry out this investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antagonism of actinomycete against Pestalotiopsis mangifera, causal agent of mango brown rot in post-harvest storage. Afr. J. Microbiol. Res.. 2012;6:1782-1789.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45:493-496.
- [Google Scholar]
- Does in vitro selection of biocontrol agents guarantee success in planta? A study case of wheat protection against Fusarium seedling blight by soil bacteria. PLoS One.. 2019;14:e0225655
- [Google Scholar]
- Marine Actinobacteria as a source of compounds for phytopathogen control: An integrative metabolic-profiling/bioactivity and taxonomical approach. PLoS One.. 2017;12:e0170148
- [Google Scholar]
- Advances and challenges on the in vitro production of secondary metabolites from medicinal plants. Hortic. Bras.. 2019;37:124-132.
- [Google Scholar]
- Antimicrobial potentiality of actinobacteria isolated from two microbiologically unexplored forest ecosystems of Northeast India. BMC Microbiol.. 2018;18:17.
- [Google Scholar]
- Actinobacteria Derived from Algerian Ecosystems as a Prominent Source of Antimicrobial Molecules. Antibiot. (Basel, Switzerland). 2019;8:172.
- [Google Scholar]
- A Review on Biological Control of Fungal Plant Pathogens Using Microbial Antagonists. J. Biol. Sci.. 2010;10(4):273-290.
- [Google Scholar]
- Bergey’s Manual of Determinative Bacteriology. Baltimore, MD (9th ed.,). USA: Williams and Wilkins; 2000.
- A Review of the Microbial Production of Bioactive Natural Products and Biologics. Front. Microbiol.. 2019;10:1404.
- [Google Scholar]
- Natural Compounds: A Sustainable Alternative to the Phytopathogens Control. J. Chil. Chem. Soc.. 2019;64:2019.
- [Google Scholar]
- Production of bioactive metabolites by Nocardia levis MK-VL -113. Lett. Appl. Microbiol.. 2009;49:484-490.
- [Google Scholar]
- Antifungal activity of Streptomyces costaricanus HR391 against some plant-pathogenic fungi. Korean J. Microbiol.. 2016;52:437-443.
- [Google Scholar]
- Fungicidal Property of Curcuma longa L. Rhizome-Derived Curcumin against Phytopathogenic Fungi in a Greenhouse. J. Agric. Food Chem.. 2003;51(6):1578-1581.
- [Google Scholar]
- Antifungal Activities of Streptomyces blastmyceticus Strain 12-6 Against Plant Pathogenic Fungi. Mycobiology. 2019;47(3):329-334.
- [Google Scholar]
- Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci.. 2019;10:845.
- [Google Scholar]
- Antagonistic activity of bacteria from chickpea rhizosphere against Fusarium oxysporum f. sp. ciceris. Phytoparasitica. 1997;25:305-318.
- [Google Scholar]
- Biocontrol management of pigeon pea dry root caused by Macrophomina phaseolina. Karnataka J. Agri. Sci.. 2007;20:54-56.
- [Google Scholar]
- Reveromycins A and B from Streptomyces sp. 3–10: Antifungal Activity against Plant Pathogenic Fungi In vitro and in a Strawberry Food Model System. Front. Microbiol.. 2017;8:550.
- [Google Scholar]
- Fungal Metabolite Antagonists of Plant Pests and Human Pathogens: Structure-Activity Relationship Studies. Molecules (Basel, Switzerland).. 2018;23(4):834.
- [Google Scholar]
- Atlas of Actinomycetes. Japan: SAJ; 1997. p. :233.
- Methods for Management of Soilborne Diseases in Crop Production. Agri.. 2020;10:16.
- [Google Scholar]
- Evaluation of actinomycetes isolated from southern Indian tea plantations for the biological control of tea pathogens. J. Plant. Crops. 2011;39:239-243.
- [Google Scholar]
- Dibutyl phthalate, the bioactive compound produced by Streptomyces albidoflavus 321.2. Microbiol. Res.. 2006;161(2):121-126.
- [Google Scholar]
- Microbial metabolites for development of ecofriendly agrochemicals. Allelopathy J.. 2014;33:1-24.
- [Google Scholar]
- Studies on Pestalotiopsis spp. affecting tea in southern India: Management of grey blight disease in tea. Latvia: Lambert Academic Publishing; 2017.
- Evaluation of fungicides and biocontrol agents against grey blight disease of teas in the field. J. Crop Prot.. 2008;27:689-694.
- [Google Scholar]
- Studies on Endophytic Actinomycetes (I ) Streptomyces sp. Isolated from Rhododendron and Its Antifungal Activity. J. Gen. Plant Pathol.. 2000;66(4):360-366.
- [Google Scholar]
- Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol.. 1966;16:313-340.
- [Google Scholar]
- Microbial metabolites in nutrition, Singh healthcare and agriculture. 3 Biotech.. 2017;7:15.
- [Google Scholar]
- Antimicrobial quercetin 3-O-glucoside derivative isolated from Streptomyces antibioticus strain ess_amA8. J. KSU-Sci.. 2020;3:1838-1844.
- [Google Scholar]
- Gas chromatography-mass spectrometry (GC-MS) analysis of bioactive compounds extracted from streptomyces antibioticus by ethyl acetate. J. Pure Appl. Microbiol.. 2014;8:761-767.
- [Google Scholar]
- Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. B-141 of Azadirachta indica. Chem. Biodiversity. 2009;6:79-85.
- [Google Scholar]
- Isolation and characterization of bacterial isolates for biological control of clubroot on Chinese cabbage. Eur. J. Plant Pathol.. 2014;140:159-168.
- [Google Scholar]







