Translate this page into:
Antidiabetic properties of garciniacowone L, a new xanthone with an unusual 5,5,8a-trimethyloctahydro-2H-1-benzopyran moiety, and other xanthones from the twig extract of Garcinia cowa Roxb. ex Choisy
⁎Corresponding author at: Medicinal Plant Innovation Center of Mae Fah Luang University, and School of Integrative Medicine, Mae Fah Luang University, Chiang 57100, Thailand. rawiwan.cha@mfu.ac.th (Rawiwan Charoensup)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The aims of this study are to investigate chemical compounds from Garcinia cowa Roxb. ex Choisy and evaluate their antidiabetic activities, including α-glucosidase inhibitory, α-amylase inhibitory, glycation, glucose consumption, and glucose uptake. The EtOAc extract of the twigs of Garcinia cowa Roxb. ex Choisy were separated and purified by chromatographic techniques to give eight compounds (1–8). Of these, a xanthone with 5,5,8a-trimethyloctahydro-2H-1-benzopyran moiety, garciniacowone L (1), was isolated as a new compound, which was characterized by extensive spectroscopic data and high-resolution mass spectrometry. The known compounds were characterized by NMR spectroscopy techniques, and by comparisons of these data with those reported. All isolated compounds except β-mangostin (4) were evaluated for antidiabetic activities. Forbexanthone (8) exhibited good α-glucosidase inhibitory activity with an IC50 value of 85.1 ± 0.3 µM. 1-Hydroxy-7-methoxyxanthone (7) inhibited the highest glycation activity with the IC50 value of 170.3 ± 0.9 µM. From cell-based assays, mangostinone (3) showed glucose consumption and glucose uptake with the IC50 value of 18.3 ± 0.5 µM and 2.9-fold, respectively. This study revealed that some xanthones isolated from Garcinia cowa Roxb. ex Choisy might be interesting for further evaluation as a new drug candidate for diabetes mellitus.
Keywords
Garcinia cowa Roxb. ex Choisy
Xanthone
Antidiabetic activity
1 Introduction
Xanthones, known as 9H-xanthen-9-one, are one of the most important classes in natural products, as they exhibit a variety of pharmacological and health benefits (Ritthiwigrom et al., 2013; Rukachaisirikul et al., 2005; Mahabusarakam et al., 2005; Rukachaisirikul et al., 2006; Trisuwan and Ritthiwigrom, 2012; Sriyatep et al., 2015; Phukhatmuen et al., 2020; Raksat et al., 2020). Some of them have been reported to have promising antidiabetic bioactivities. α-Mangostin, the best-known xanthone from Garcinia mangostana, has several antidiabetic activities, including inhibition of insulin secretion and inhibition of protein expression of insulin signaling pathways (Lee et al., 2018), and inhibition of α-glucosidase (Sriyatep et al., 2015). In addition, mangiferin was shown to reduce blood glucose levels of KK-Ay mice after oral administration by decreasing insulin resistance (Miura et al., 2001), while γ-mangostin and smeathxanthone A displayed potent α-glucosidase inhibitory with IC50 values of 1.5 and 6.9 µM, respectively (Ryu et al., 2011). Therefore, identifying new natural products in this structural family might lead to the discovery of bioactive compounds for diabetes mellitus (DM) treatment.
Garcinia cowa Roxb. ex Choisy belonging to the Clusiaceae family have been demonstrated to be rich sources of xanthones with therapeutic properties (Santo et al., 2020). This genus contains over 300 species often distributed in tropical and subtropical countries (Raksat et al., 2019). In previous phytochemical investigations of G. cowa, a number of new xanthones were isolated and identified (Ritthiwigrom et al., 2013; Sriyatep et al., 2015; Phukhatmuen et al., 2020; Raksat et al., 2020). The different parts of the plant and the different areas of plant collection are produced the diverse structures of new xanthones. In this study, the twigs of G. cowa were collected from Chiang Rai Province, Thailand. The EtOAc extract showed good α-glucosidase inhibitory activity with an IC50 values of 23.5 ± 0.2 µg/mL. This prompted us to further investigate their phytochemicals and antidiabetic properties. This report describes the isolation and structure elucidation of a new xanthone, garciniacowone L (1), and seven known compounds, including 2-geranyl-1,3,7-trihydroxy-4-(3,3-dimethylallyl)-xanthone (2), mangostinone (3), β-mangostin (4), cochinchinone G (5), 1,7-dihydroxyxanthone (6), 1-hydroxy-7-methoxy xanthone (7), and forbexanthone (8). The anti-diabetes activities, including α-glucosidase inhibition, α-amylase inhibition, glycation, glucose consumption, and glucose uptake are also reported.
2 Materials and methods
2.1 Materials and instruments
Materials for chromatography and instruments were the same as in previous reports (Phukhatmuen et al., 2020; Raksat et al., 2020; Raksat et al., 2019).
2.2 Extraction and isolation
The twigs of G. cowa were collected in January 2019 from Chiang Rai Province, Thailand. Herbarium specimen number MFU-NPR0186 was deposited at the Natural Products Research Laboratory, School of Science, Mae Fah Luang University.
Air-dried twigs of G. cowa (3.8 kg) were extracted with EtOAc for 3 days at room temperature and concentrated under reduced pressure to give the EtOAc extract (103.6 g). This extract was subjected to QCC over silica gel and eluted with a gradient of hexanes-acetone (100% hexanes to 100% acetone) to obtain eight fractions (GCT1-GCT8). Fraction GCT5 (1.1 g) was isolated by CC over silica gel (1:9 v/v, acetone-hexanes) to give compounds 2 (9.5 mg) and 5 (12.4 mg). Fraction GCT6 (2.3 g) was further purified by CC over silica gel (3:17 v/v, EtOAc-hexanes) to obtain compounds 1 (2.8 mg) and 7 (3.7 mg). Compounds 4 (1.3 mg) and 8 (2.6 mg) were afforded from fraction GCT7 (3.3 g) by repeated CC over silica gel (1:9 v/v, acetone-hexanes). Fraction GCT8 (4.13 g) was purified by CC over silica gel (1:4 v/v, acetone-hexanes) yielded compounds 3 (4.6 mg) and 6 (4.5 mg).
Garciniacowone L (1). Light yellow viscous oil. [α]D25 + 9 (c 0.1, MeOH); UV λmax (log ε): 212 (2.94), 242 (3.46), 259 (3.61), 286 (2.90), 318 (3.55), and 368 (3.38) nm; IR (KBr) vmax: 3354, 2932, 2162, 1712, 1478, 1285, and 1173 cm−1; 1H and 13C NMR spectral data, see Table 1; HRESITOFMS m/z 411.1785, [M + H]+ (calcd for C24H26O6,411.1802).
Position
δC
Carbon type
δH (mult J in Hz)
HMBC
1
160.5
C
–
–
2
105.5
C
–
–
3
160.1
C
–
–
4
94.7
CH
6.31 (s)
C-1, C-3, C-4a, C9a
4a
155.7
C
–
–
5
102.7
CH
6.93 (s)
C-6, C-7, C-10a
6
152.7
C
–
–
7
144.2
C
–
–
8
104.5
CH
7.59 (s)
C-7, C-8a, C-9, C10a
8a
113.3
C
–
–
9
180.0
C
–
–
9a
103.6
C
–
–
10a
152.4
C
–
–
1′
17.1
CH2
2.85 (dd, 16.5, 4.9);2.36
(dd, 16.6, 13.4)C-1, C-2, C-2′, C-3′
2′
47.4
CH
1.68 (m)
C-1′, C-3′, C-7′, C-8′, C-9′
3′
79.4
C
–
–
4′
39.7
CH2
2.01 (dd, 10.7, 2.4);1.65
(m)C-2′, C-3′, C-5′, C-7′
5′
19.7
CH2
1.67 (m)
C-4′, C-6′, C-7′
6′
41.4
CH2
1.52, 1.33 (m)
C-5′, C-7′
7′
33.6
C
–
–
8′
20.6
CH3
0.96 (s)
C-2′, C-6′, C-7′, C-9′
9′
32.1
CH3
1.06 (s)
C-2′, C-6′, C-7′, C-8′
10′
19.9
CH3
1.26 (s)
C-2′, C-3′, C-4′
1-OH
–
–
13.39 (brs)
–
7-OMe
56.5
CH3
4.01 (s)
C-7
2.3 α-Glucosidase inhibitory assay
The α-glucosidase inhibitory assay was performed in triplicate using the previous reports (Phukhatmuen et al., 2020; Raksat et al., 2020). Positive controls were acarbose, voglibose, and quercetin.
2.4 α-Amylase inhibitory assay
The α-amylase inhibitory assay was performed in triplicate using a modified previous report (Kusano et al., 2011).
2.5 Glycation inhibitory assay
The glycation inhibition assay was performed in triplicate using the same procedure as in the previous report (Justino et al., 2016). The standard control was quercetin. The procedure for the glycation inhibition assay was performed.
2.6 Glucose uptake assay
The glucose uptake assay was carried out in triplicate using the same procedure as in the previous report with slight alteration (Phukhatmuen et al., 2020), and metformin was used as the standard control.
2.7 Glucose consumption assay
The glucose consumption assay was performed in triplicate, using the same procedure as in our previous report (Phukhatmuen et al., 2020), and metformin was used as standard control. Cell viability was carried out by MTT assay, as previously described (Phukhatmuen et al., 2020).
3 Results and discussion
3.1 Isolation and structure elucidation
Phytochemical investigation of the EtOAc extract of G. cowa twigs led to the isolation and identification of a new xanthone, garciniacowone L (1), together with seven known xanthones (Fig. 1). The known xanthones were identified 2-geranyl-1,3,7-trihydroxy-4-(3,3-dimethylallyl)-xanthone (2) (Bennett et al., 1993), mangostinone (3) (Asai et al., 1995), β-mangostin (4) (Trisuwan and Ritthiwigrom, 2012), cochinchinone G (5) (Boonnak et al., 2009), 1,7-dihydroxyxanthone (6) (Mak et al., 1999), 1-hydroxy-7-methoxy xanthone (7) (Dharmaratne et al., 2009), and forbexanthone (8) (Harrison et al., 1993) by comparisons made with the literature reported spectroscopic data.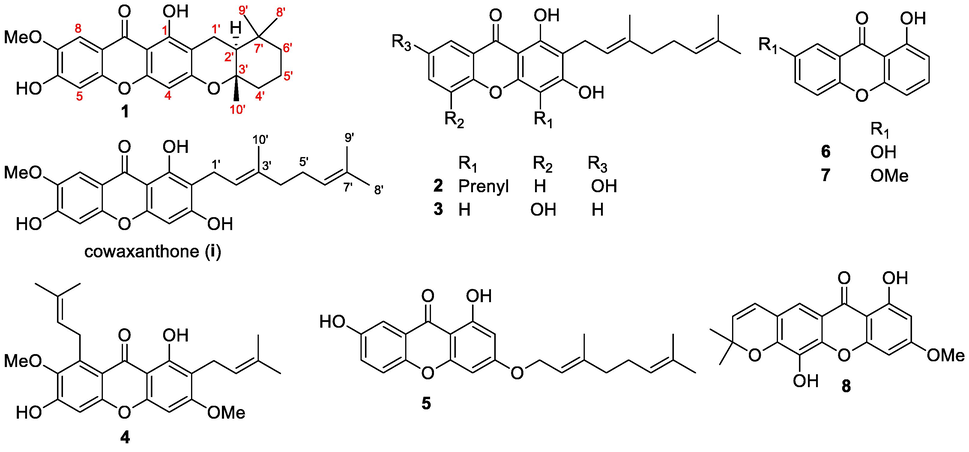
Isolated compounds from the twigs extract of G. cowa.
Compound 1 was obtained as a light-yellow viscous oil. The molecular formula of C24H26O6 was deduced from HRESITOFMS data, which showed a [M+H]+ ion peak at m/z 411.1785, (calcd 411.1802). The IR spectrum showed the hydroxy and carbonyl functionalities at 3354 and 1712 cm1, respectively, while the UV spectrum showed absorption bands at λmax 212, 242, 259, 286, 318, and 368 nm. The 13C NMR and DEPT spectroscopic data (Table 1) displayed resonances for 24 carbons, including four methyls (δC 19.9, 20.6, 32.1, and 56.5), four methylenes (δC 17.1, 19.7, 39.7, and 41.4), four methines (δC 94.7, 102.7, 104.5, and 47.7), and 12 quaternary carbons (δC 33.6, 79.4, 103.6, 105.5, 113.3, 144.2, 152.4, 152.7, 155.7, 160.1, 160.5, and 180.0). The 1H NMR spectroscopic data (Table 1) indicated that this compound showed the characteristics of a xanthone (Trinh et al., 2017) with a hydrogen-bonded hydroxy proton [δH 13.39 (1H, s, OH-1)], three singlet aromatic protons [δH 6.31 (1H, s, H-4), 6.93 (1H, s, H-5), and 7.59 (1H, s, H-8)], and a methoxy group [δH 4.01 (3H, s, OMe-7)]. The methoxy group was placed at C-7 due to the HMBC correlation (Fig. 2) between H-8 (δH 7.59), H-5 (δH 6.93), and methoxy protons (δH 4.01) with C-7 (δC 144.2). The methoxy at C-7 (δC 144.2) was also confirmed by the NOESY cross peak between H-8 (δH 7.59) and 7-OMe (δH 4.01). The low field 13C NMR resonance of C-6 (δC 152.7) suggested that a hydroxy group was attached to this carbon. Furthermore, the main interest of this molecule is a cyclization of the geranyl side chain and cowaxanthone (i) was proposed as a precursor. The concerted cyclization of the geranyl side chain of cowaxanthone (i) would give the 5,5,8a-trimethyloctahydro-2H-1-benzopyran moiety of compound 1 (Fig. 2), which showed the 1H and 13C resonances at δH 2.85 (dd, 16.5, 4.9 Hz, H-1′)/δC 17.1, 2.36 (dd, 16.6, 4.8 Hz, H-1′)/δC 17.1, 1.68 (1H, m, H-2′)/δC 47.4, 2.01 (1H, dd, 10.7, 2.4, H-4′)/δC 39.7, 1.65 (2H, m, H-4′)/δC 39.7, 1.67 (1H, m, H-5′)/δC 19.7, 1.52, 1.33 (2H, m, H-6′)/δC 41.4, 1.26 (3H, s, H-10′)/δC 19.9, 0.96 (3H, s, H-8′)/δC 20.6, 1.06 (3H, s, H-9′)/δC 32.1, δC 79.4 (C-3′), and δC 33.6 (C-7′). The 13C NMR resonances of the geminal dimethyl group [C-8′ (δC 20.6) and C-9′ (δC 32.1)] on C-7 were different because they were diastereotropic methyl groups. The following HMBC correlations (Fig. 3, Supplementary Material, Fig. S5 and Fig. S6) supported the 5,5,8a-trimethyloctahydro-2H-1-benzopyran moiety: H-1′ (δH 2.85 and 2.36) with C-2′ (δC 47.4), C-3′ (δC 79.4), and C-7′ (δC 33.6); H-2′ (δH 1.68) with C-1′ (δC 17.1), C-3′ (δC 79.4), and C-7′ (δC 33.6); Me-8′ (δH 0.96) and Me-9′ (δH 1.06) with C-2′ (δC 47.4), C-6′ (δC 41.4), and C-7′ (δC 33.6), and Me-10′ (δH 1.26) with C-2′ (δC 47.4), C-3′ (δC 79.4), and C-4′ (δC 39.7). In addition, the 1H–1H COSY correlations between H-1′ (δH 2.85 and 2.36) with H-1′ (δH 2.85 and 2.36) with H-2′ (δH 1.68) and H-4′ (δH 1.33) with H-5′ (δH 1.67) and H-5′ (δH 1.67) with H-6′ (δH 1.33) supported the connections of C-1′−C-2′ and C-4′−C-5′−C-6′, respectively (Fig. 3). The ring junction at C-2′/C-3′ was proposed to be a trans-ring junction because there is no NOESY cross peak between H-2′ (δH 1.68) and Me-10′ (δH 1.26) (Supplementary Material, Fig. S7). The 5,5,8a-trimethyloctahydro-2H-1-benzopyran moiety was placed at C-2/C-3 due to HMBC correlations between H-1′ (δH 2.85 and 2.36) with C-2 (δC 105.5). Finally, the C-3 of the xanthone skeleton was an oxygenated carbon due to the low field 13C NMR resonance of this carbon (δH 160.1). Accordingly, compound 1 was characterized as garciniacowone L. The full assignments of 1H and 13C NMR spectroscopic data were shown in Table 1.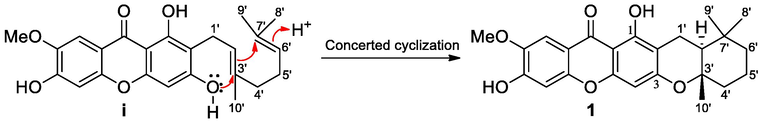
The propose of concerted cyclization of garciniacowone L (1) from cowaxanthone (i).
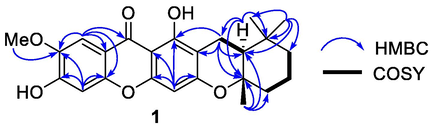
1H−1H COSY and selected HMBC correlations of garciniacowone L (1).
The known xanthones (2–8) displayed a resonance of a hydrogen-bonded hydroxy proton (ca. δH 13.7–12.6) at C-1 (Figs. S9–S15, Supplementary Material). Compounds 2 (2-geranyl-1,3,7-trihydroxy-4-(3,3-dimethylallyl)-xanthone) and 3 (mangostinone) contained a geranyl group at C-2. These two compounds have differed in the substituent groups of R1, R2, and R3 at C-4, C-5, and C-7, respectively. Compound 2 displayed an isoprenyl unit, a hydrogen atom, and a hydroxy group at R1, R2, and R3, respectively, whereas compound 3 was two hydrogen atoms at R1 and R3 and a hydroxy group at R2. Compound 4 (β-mangostin) was identified as a tetraoxygenated xanthone containing two isoprenyl groups at C-2 and C-8, two methoxy groups at C-3 and C-7, and a hydroxy group at C-6. In the case of compound 5 (cochinchinone G), an oxygeranyl unit and a hydroxy group were observed at C-3 and C-7, respectively. Compounds 6 (1,7-dihydroxyxanthone) and 7 (1-hydroxy-7-methoxy xanthone) were the simplest xanthone, which containing a hydroxy group (6) or a methoxy group (7) at C-7. In contrast, compound 8 (forbexanthone) displayed a chromene ring, a methoxy group, and a hydroxy group at C-6/C-7, C-3, and C-5, respectively.
Xanthones are the principal chemotaxonomic markers in Garcinia genus (Ritthiwigrom et al., 2013; Rukachaisirikul et al., 2005; Mahabusarakam et al., 2005; Rukachaisirikul et al., 2006; Trisuwan and Ritthiwigrom, 2012; Sriyatep et al., 2015; Phukhatmuen et al., 2020; Raksat et al., 2020). Xanthones 2, 5, 7, and 8 were found in G. cowa for the first time. However, they have been reported from other Garcinia species and the related Clusiaceae family. 2-Geranyl-1,3,7-trihydroxy-4-(3,3-dimethylallyl)-xanthone (2) was previously isolated from two species of Cratoxylum genus (Hypericaceae): C. cochinchinense (Bennett et al., 1993) and C. formasum (Chailap and Nuanyai, 2019), while mangostinone (3) widely distributed in several Garcinia species: G. cowa (Mahabusarakam et al., 2005; Raksat et al., 2020), G. parvifolia (Rukachaisirikul et al., 2006), G. mangostana (Asai et al., 1995), and G. xipshuanbannaensis (Na and Xu, 2010). β-Mangostin (4) and 1,7-dihydroxyxanthone (6) were also found in various Garcinia species, including G. cowa (Phukhatmuen et al., 2020), G. malaccensis (Taher et al., 2012), Garcinia sp. (Siridechakorn et al., 2014), G. schomburgkiana (Vo et al., 2012), G. dulcis (Likhitwitayawuid et al., 1998), and G. griffithii (Nguyen et al., 2005) and Cratoxylum species, including C. glaucum (Sim et al., 2011), C. arborescens (Syam et al., 2014). Cochinchinone G (5), 1-hydroxy-7-methoxy xanthone (7), and forbexanthone (8) are commonly found in other Clusiaceae genus and another family related to the Clusiaceae, including C. cochinchinense (Hypericaceae) (Boonnak et al., 2009), C. formasum (Hypericaceae) (Duan et al., 2011), Hypericum laricifolium (Hypericaceae) (Ramírez-González et al., 2013), H. petiolulatum (Hypericaceae) (Rui et al., 2017), H. przewalskii (Hypericaceae) (Zhang et al., 2021), Allanblackia gabonensis (Clusiaceae) (Azebaze et al., 2008), G. edulis (Magadula, 2010), G. vieillardii (Hay et al., 2004), G. nigrolineata (Rukachaisirikul et al., 2005).
3.2 Antidiabetic activities
3.2.1 α-Glucosidase inhibition activity
All isolated compounds, except compound 4, were further evaluated for their α-glucosidase inhibition activity. Compound 8 displayed moderated inhibitory effect with an IC50 value of 85.1 ± 0.3 µM, which is better than that of the voglibose (127.4 ± 1.2 µM). However, it was less active than those of acarbose (76.7 ± 1.4 µM) and quercetin (30.6 ± 0.9 µM). Other compounds were found to have weak α-glucosidase inhibition activity or inactive. This study is the first report of the α-glucosidase inhibitory activity of compounds 2, 5, and 8. The IC50 values of compounds 3 (188.8 ± 0.6 µM) and 7 (156.9 ± 1.4 µM) were consistent with the previous study, which had been reported their IC50 values of > 100 µM (Phukhatmuen et al., 2020; Raksat et al., 2020). In 2011, Ryu and co-workers have reported the α-glucosidase inhibitory activity of β-mangostin (4) with the IC50 value of 14.4 ± 0.1 µM. In this study, the α-glucosidase inhibitory activity of β-mangostin (4) was not evaluated due to the small isolation of β-mangostin (4).
3.2.2 α-Amylase inhibition activity
The inhibition of carbohydrate hydrolyzing enzymes (α-amylase) can be a practical therapeutic approach for diabetes by preventing the breakdown of long-chain polysaccharides to glucose and decreasing high blood glucose levels (Ojah et al., 2020). The isolated xanthones (1–8) were assayed for inhibition of α-amylase as indicated in Table 2. Unfortunately, they showed no α-amylase inhibition activity at 100 µg/mL. Inactive at >200 µM.
Compounds
α-Glucosidase inhibition
α-Amylase inhibition
Glycation inhibition
IC50, µM
1
117.2 ± 1.5
Inactive
Inactive
2
111.7 ± 0.1
Inactive
Inactive
3
188.8 ± 0.6
Inactive
Inactive
5
162.6 ± 0.3
Inactive
Inactive
6
Inactive
Inactive
Inactive
7
156.9 ± 1.4
Inactive
170.3 ± 0.9
8
85.1 ± 0.3
Inactive
Inactive
Acarbose
76.7 ± 1.4
105.8 ± 1.1
Not tested
Voglibose
127.4 ± 1.2
198.3 ± 0.8
Not tested
Quercetin
30.6 ± 0.9
180.1 ± 1.4
62.4 ± 1.5
3.2.3 Glycation inhibition activity
The formation of advanced glycation end products (AGEs) contributes to the development and progression of diabetic complications, including nephropathy, retinopathy, and neuropathy (Singh et al., 2014). Xanthones have been reported to have the ability to inhibit the formation of AGEs (Abdallah et al., 2017). The inhibition of glycation by xanthones 1–8 is summarized in Table 2. Only xanthone 7 displayed glycation inhibition activity with the IC50 value of 170.3 ± 0.9 µM, which was less active than that of the positive control (quercetin, IC50 value of 62.4 ± 1.5 µM). All remaining tested compounds were inactive. These findings may lead to further investigation and clarification of other mechanisms of AGEs properties of xanthones.
3.2.4 Glucose consumption and glucose uptake activities
It has been reported that xanthones from Garcinia species displayed glucose consumption and glucose uptake activities (Li et al., 2017). Xanthones 1–8 were evaluated for their glucose consumption in 3T3-L1 cells. Of these, xanthones 3, 6, and 7 displayed glucose consumption (Table 3) with IC50 values in the range of 18.3–57.5 µM. Xanthones 3 and 7 showed the glucose consumption activity better than that of positive control (metformin, IC50 = 50.3 ± 0.9) with the IC50 values of 18.3 ± 0.5 and 25.3 ± 0.7 µM. To confirm the glucose consumption activity, xanthones 3, 6, and 7 were further evaluated for glucose uptake induced by L6 myotube cells. As summarized in Table 3, xanthones 3, 6, and 7 enhanced the glucose uptake stimulation in adipocyte L6 myotube cells by 2.9, 1.2, and 1.6-fold, respectively, compared to the positive control (metformin, 3.8-fold). This information suggested that xanthones 3 showed potential glucose transportation into cells and provide energy in adenosine triphosphate (ATP) and play a crucial part in other cellular operations.
Compounds
Glucose consumption (IC50, µM)
Glucose uptake (fold)
3
18.3 ± 0.5
2.9
6
57.5 ± 1.3
1.2
7
25.3 ± 0.7
1.6
Metformin
50.3 ± 0.9
3.8
4 Conclusions
The chemical investigation of G. cowa twigs led to the isolation and identification of a new xanthone, garciniacowone L (1), together with seven known xanthones. Xanthone 8 exhibited moderate α-glucosidase inhibitory activity, while xanthones 1–8 showed no α-amylase inhibitory activity. Xanthone 7 showed the best inhibition of glycation activity, whereas xanthone 3 displayed the best glucose consumption and glucose uptake activities without cell toxicity. Based on these findings, xanthone derivatives from various species of Garcinia genus might be interesting lead compounds for developing drug candidates with therapeutic potential for the treatment of diabetes mellitus.
Funding
This work was supported by Thailand Science Research and Innovation (DBG6280007).
Acknowledgements
We would like to thank Mae Fah Luang University for their laboratory facilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mangostanaxanthones III and IV: advanced glycation end-product inhibitors from the pericarp of Garcinia mangostana. J. Nat. Med.. 2017;71:216-226.
- [Google Scholar]
- Antimicrobial and antileishmanial xanthones from the stem bark of Allanblackia gabonensis. Chem. Nat. Compd.. 2008;44:582-587.
- [Google Scholar]
- Triterpenoids, tocotrienols and xanthones from the bark of Cratoxylum cochinchinense. Phytochemistry. 1993;32:1245-1251.
- [Google Scholar]
- Anti-Pseudomonas aeruginosa xanthones from the resin and green fruits of Cratoxylum cochinchinense. Tetrahedron. 2009;65:3003-3013.
- [Google Scholar]
- Antioxidant activities and electrochemical behaviors of xanthones from Cratoxylum cochinchinense and Cratoxylum formasum. Naresuan Univ. J.: Sci. Technol. (NUJST). 2019;27:35-42.
- [Google Scholar]
- Xanthones from roots of Calophyllum thwaitesii and their bioactivity. Nat. Prod. Res.. 2009;23:539-545.
- [Google Scholar]
- Xanthone and benzophenone glycosides from the stems of Cratoxylum formosum ssp. pruniflorum. Chem. Pharm. Bull.. 2011;59:231-234.
- [Google Scholar]
- Peel of araticum fruit (Annona crassiflora Mart.) as a source of antioxidant compounds with α-amylase, α-glucosidase, and glycation inhibitory activities. Bioorg. Chem.. 2016;69:167-182.
- [Google Scholar]
- α-Amylase and lipase inhibitory activity and structural characterization of Acacia bark proanthocyanidins. J. Nat. Prod.. 2011;74:119-128.
- [Google Scholar]
- α-Mangostin improves insulin secretion and protects INS-1 cells from streptozotocin-induced damage. Int. J. Mol. Sci.. 2018;19:1484.
- [Google Scholar]
- Depsidone and xanthones from Garcinia xanthochymus with hypoglycemic activity and the mechanism of promoting glucose uptake in L6 myotubes. Bioorg. Med. Chem.. 2017;25:6605-6613.
- [Google Scholar]
- Xanthones with antimalarial activity from Garcinia dulcis. Planta Med.. 1998;64:281-282.
- [Google Scholar]
- A bioactive isoprenylated xanthone and other constituents of Garcinia edulis. Fitoterapia. 2010;81:420-423.
- [Google Scholar]
- Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine. 2001;8:85-87.
- [Google Scholar]
- A new prenylated xanthone from Garcinia xipshuanbannaensis YH Li. Nat. Prod. Res.. 2010;24:1648-1653.
- [Google Scholar]
- Xanthones and benzophenones from Garcinia griffithii and Garcinia mangostana. Phytochemistry. 2005;66:1718-1723.
- [Google Scholar]
- α-Amylase and α-glucosidase antidiabetic potential of ten essential oils from Calophyllum inophyllum Linn. Iberoam. J. Med.. 2020;2:253-260.
- [Google Scholar]
- Bioassay-guided isolation and identification of antidiabetic compounds from Garcinia cowa leaf extract. Heliyon. 2020;6:e03625.
- [Google Scholar]
- A tocotrienol quinone dimer and xanthones from the leaf extract of Garcinia nigrolineata. Fitoterapia. 2019;136:104175.
- [Google Scholar]
- Phloroglucinol benzophenones and xanthones from the leaves of Garcinia cowa and their nitric oxide production and α-glucosidase inhibitory activities. J. Nat. Prod.. 2020;83:164-168.
- [Google Scholar]
- Xanthones from aerial parts of Hypericum laricifolium Juss. Nat. Prod. Commun.. 2013;8
- [Google Scholar]
- Chemical constituents and biological activities of Garcinia cowa Roxb. Maejo Int. J. Sci. Technol.. 2013;7:212-231.
- [Google Scholar]
- Chemical constituents of Hypericum petiolulatum. Chem. Nat. Compd.. 2017;53:457-462.
- [Google Scholar]
- Phloroglucinols, depsidones and xanthones from the twigs of Garcinia parvifolia. Tetrahedron. 2006;62:8578-8585.
- [Google Scholar]
- Benzopyran, biphenyl, and tetraoxygenated xanthone derivatives from the twigs of Garcinia nigrolineata. J. Nat. Prod.. 2005;68:1218-11122.
- [Google Scholar]
- α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry. 2011;72:2148-2154.
- [Google Scholar]
- Medicinal potential of Garcinia species and their compounds. Molecules. 2020;25:4513.
- [Google Scholar]
- Alpha-mangostin and beta-mangostin from Cratoxylum glaucum. Res. J. Chem. Environ.. 2011;15:62-66.
- [Google Scholar]
- Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol.. 2014;18:1-14.
- [Google Scholar]
- Biphenyl and xanthone derivatives from the twigs of a Garcinia sp. (Clusiaceae) Phytochem. Lett.. 2014;8:77-80.
- [Google Scholar]
- Bioactive prenylated xanthones from the young fruits and flowers of Garcinia cowa. J. Nat. Prod.. 2015;78:265-271.
- [Google Scholar]
- Cytotoxicity and oral acute toxicity studies of β-mangostin isolated from Cratoxylum arborescens. Pharmacogn. J.. 2014;6:47-56.
- [Google Scholar]
- Apoptosis, antimicrobial and antioxidant activities of phytochemicals from Garcinia malaccensis Hk. f. Asian Pac. J. Trop. Med.. 2012;5:136-141.
- [Google Scholar]
- Xanthones from the twigs of Garcinia oblongifolia and their antidiabetic activity. Fitoterapia. 2017;118:126-131.
- [Google Scholar]
- Benzophenone and xanthone derivatives from the inflorescences of Garcinia cowa. Arch. Pharm. Res.. 2012;35:1733-1738.
- [Google Scholar]
- Cytotoxic tetraoxygenated xanthones from the bark of Garcinia schomburgkiana. Phytochem. Lett.. 2012;5:553-557.
- [Google Scholar]
- Hyperprzeone A, a new benzophenone with cytotoxicity from Hypericum przewalskii Maxim. Nat. Prod. Res.. 2021;35:4960-4968.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102201.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







