Translate this page into:
Anticoccidial activities of Salvadora persica(arak), Zingiber officinale (ginger) and Curcuma longa (turmeric) extracts on the control of chicken coccidiosis
⁎Corresponding author at: Department of Biology, College of Sciences and Humanities, Prince Sattam Bin Abdulaziz University, PO Box 173, Alkharj 11942, Saudi Arabia. aljedaie@psau.edu.sa (Manei M. Aljedaie),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
To investigate the effect of Salvadora persica, Zingiber officinale andCurcuma longa plant extracts for the treatment of Eimeria tenella infection in chickens.
Methods
One hundred and fifty broiler chickens one-day-old were obtained from a commercial breeder and kept under strict hygienic conditions. The chicks were divided into five equal groups. The first group was kept as a negative control (uninfected/untreated), second group was infected with E. tenella (105) orally at 14 days of age (untreated), while the third group was infected with E. tenella (105) orally at 14 days of age, then treated with S. persica [900 mg/kg body weight (bw)] for 5 days. The fourth group was infected with E. tenella (105) orally at 14 days of age, then treated with Z. officinale (6 g/L) for 5 days and The fifth group was infected with E. tenella (105) orally at 14 days of age, then treated with C. longa (300 mg/kg bw) for 5 days. Treatment in all groups started after signs of infection appeared, i.e. from 18 to 22 days of age.
Results
There were significant decreases in Eimeria oocyst output in all treated groups from day 5 to 10 days post infection (dpi). The highest oocyst count occurred at 9 dpi and decreased on subsequent days. Red blood cell count (RBCs), hemoglobin concentration (Hb), and packed cell volume (PCV) showed significant decreases in the infection, Z. officinale and C. longa treated groups, but the S. persica treated group revealed non-significant changes in the first week after infection. The results of score lesion showed the highest lesion in the infected group with a significant decrease in the S. persica and C. longa groups; there was a non-significant decrease in the Z. officinale group. All the results were confirmed histopathologically.
Conclusion
Treatment with herbal extracts of S. persica, Z. officinale and C. longa were effective in decreasing oocyst production and reducing the cecum lesion score.
Keywords
Herbal extracts
Zingiber officinale
Curcuma longa
Salvadora persica
Chicken coccidiosis
1 Introduction
Poultry coccidiosis is a parasitic disease of the intestinal tract caused by single-celled protozoan parasite belonging to the genus Eimeria. The disease affects all kinds of domestic species and causing substantial damage of epithelial cells, which leads to hemorrhagic diarrhea, low growth and a reduced egg production (Dalloul and Lillehoj, 2005; Razzaq et al., 2011). Several drugs are available to fight against coccidiosis; however, the excessive usage of these medicines has triggered the progression of multidrug resistance and excessive presence in tissues. Hence, many researchers intended to explore the antimicrobial activity of different herbal plants to mitigate the troubles caused by coccidial infection in the poultry industry. Minimal toxicity and comparatively less expensive in drug production are the big advantages of using herbal plants based drugs against coccidiosis (Abbas et al., 2006). There are many anticoccidial agents used against eimeriosis; however, many side effects reported previously (Heinz Mehlhorn, 2014; Wunderlich et al., 2014).
Researchers efforts are now directed towards finding natural agents with reduced or with no side effects on the host infected with Eimeria spp. S. persica, commonly known as arak, is used extensively against microbes (Elvin-Lewis, 1982). The propagation and use of arak is due to the spread of Islamic culture in many countries (Bos, 1993). It has been documented that the byproducts of S. persica exhibited to have significant activity against numerous microbial agents from oral infections (Poureslami, 2007). A recent study revealed antiparasitic activity of S. persica against Echinococcus granulosus. S. persica extracts contain important phytochemicals like vitamin C, salvadorine, salvadourea, alkaloids, trimethylamine, cyanogenic glycosides, tannins, saponins and salts, mostly as chlorides (Abdel-Baki et al., 2016). It is also reported that some of the active compounds of Zingiber officinale (Z. officinale), such as gingerdoine, gengerdiol, and gingerol are having the ability to enhance digestibility and growth of broilers. Additionally, the byproducts are effective in controlling coccidial infection (Khan et al., 2012; Raza et al., 2016; Rehman et al., 2019; Zhang et al., 2009).
Curcuma longa is a yellow natural polyphenolic compound extracted from turmeric rhizome. The active compound (Curcumin) diferuloylmethane is responsible for the various pharmacological effects of C. longa; these include anticancer, anti-inflammatory and antioxidant properties (Subramanian et al., 1994). Antiprotozoal activities have also been described, such as antimalarial (Nagajyothi et al., 2012) and anti-leishmanial (Das et al., 2008) activities. C. longa (0.05%) was effective in reducing upper- and mid-small intestinal infections caused by E. acervulina and E. maxima (Allen et al., 1998). The aim of this work was to study the effect of some herbal extracts for the treatment of Eimeria tenella infection in chickens.
2 Material and methods
2.1 Preparation of herbal extracts
This extraction was prepared at the University Central Laboratory (UCL), Collage of Science, Prince Sattam bin Abdulaziz University, Saudi Arabia. S. persica L. (Salvadoraceae) roots (arak) were collected from Jizan, Saudi Arabia. The identification of plant was confirmed at the herbarium of the Botany and Microbiology Department, College of Science, King Saud University. Approximately 100 g of fine powder ground from small pieces of roots was collected and extracted using ethanol extraction method. Subsequently, the suspension was stirred for 30 min and stored for 24 h. After filtering the extract using a sterile filter paper, a rotary evaporator was used to remove the solvent (Mau et al., 2001). The final product was prepared for use at a dose of 900 mg/kg body weight in drinking water (Thagfan et al., 2016). Z. officinale (ginger) and C. longa (turmeric) products were collected from a local market in Saudi Arabia. A total of 500 g powder from each product was extracted using the ethanal extraction method. The alcohol-free residue of each extract was prepared (Almalki et al., 2017) for use at a dose of 300 mg/kg body weight for C. longa (Gogoi et al., 2019) and 6 g/L for Z. officinale in drinking water (Nidaullah et al., 2010). The extracts were given to infected chicks (18–22 days of age) after the symptoms appeared.
2.2 Isolation and propagation of E. tenella
2.2.1 Preparation of Eimeria oocysts
The oocysts of Eimeria spp. were obtained from infected ceca following the procedure of Chand et al. (Chand et al., 2016). The collected fecal samples were observed under a microscope by the direct method or using the flotation technique. After the identification procedure, the residual suspension was soaked in 2.5% potassium dichromate solution for overnight. Subsequently, the mixture was sieved, and the remaining part was centrifuged at 1500 rpm for three minutes. The precipitate was dissolved and mixed with a saturated solution of NaCl. The supernatant was discarded, and the remaining solution was centrifuged again at 1500 rpm for three minutes. The sedimented material containing oocysts was mixed in a 2.5% solution of potassium dichromate and incubated at 30 °C for 24–72 h in a petri dish and then stored at 4 °C. The sporulated oocysts were then counted by using the McMaster egg counting method. The number of oocysts was adjusted to 105 sporulated oocysts. Oocysts were administered to each bird by the oral route mixed with water (Chen et al., 2008; Jang et al., 2007).
2.2.2 E. tenella infection
Prior to experimental infection, chicks were examined to confirm they were free from coccidia infection. The oocysts were washed in distilled water and inoculated orally to chicken at a dose of 105 sporulated oocysts per bird at 14 days of age (Messaï et al., 2014). The chickens were put into a cage based on the treatment group. Clinical symptoms and the occurrence of bloody defecation observed until day 10 post infection.
2.3 Experimental design
2.3.1 Chicks
One hundred and fifty broiler chicks of either sex were aged one-day-old; all were segregated into five equal groups: first group kept as a negative control (uninfected/untreated), second group infected with E. tenella(untreated), while the remaining three groups were infected with E. tenella and then treated with S. persica, Z. officinale and C. longa, respectively. Treatment in all groups started after signs of infection appeared. The birds were weighed periodically. The birds were housed in floor pens and fed ad libitum throughout the experiment, first with starter diets provided from 1 to 14 days of age, then with a growth and finisher diet given from 15 to 45 days of age. All diets were free of coccidiostats and were prepared to ensure the essential nutritional requirements of chickens. Standard management practices of commercial broiler production were applied. Chicks were vaccinated against Newcastle’s, Gumboro and infectious bronchitis diseases.
2.3.2 Sampling
Fecal samples were collected from all groups on days 5, 7, 9 and 10 dpi and stored at 4 °C for the determination of oocysts per gram by using the McMaster egg counting method. Five birds of each group were selected to collect blood samples. Blood samples were drawn from wing vein from at 7 and 14 days post infection; samples were taken with an anticoagulant for hematological examination. Necropsy was performed and tissue specimens from the ceci was obtained and fixed in 10% formalin for histopathological examination.
2.4 Laboratory examinations
Parasitical examination
2.4.1 Oocysts counting procedure
Post coccidia infection fecal sample collection was done on days 5, 7, 9, and 10. The McMaster technique was used to count the oocyst (Chand et al., 2016). Hematological analysis such as red blood cell count (RBCs), hemoglobin concentration (Hb) and packed cell volume (PCV) was done as described previously (Jain, 2000).
2.4.2 Lesion scoring
Three birds per cage were randomly selected, weighed, euthanized by cervical dislocation and necropsied. The intestinal tract was examined for coccidial lesions by two veterinarians specializing in poultry diseases. Lesion scoring was performed on 7 dpi using the method described by Raman et al. (Raman et al., 2011). A score from 0 to 4 was given on the basis of gross lesions, degree of hemorrhage and thickness of the cecal wall.
2.4.3 Histopathological examination
A total of three birds were randomly selected from each group for slaughtering on 7, 14 and 21 dpi. The ceca were separated from each bird and prepared for histopathological examination (Chand et al., 2016). Stained sections were examined for circulatory disturbances, inflammation, degeneration, necrosis, and any other pathological changes.
3 Results
Fecal oocysts were counted from 5 dpi. The negative control group showed no fecal oocysts, while the positive control group showed the highest oocyst count per gram of fecal sample. The oocyst counts were assessed up to 10 dpi. Groups 3, 4 and 5 (S. persica 900 mg/kg body weight, Z. officinale 6 g/L and C. longa 300 mg/kg body weight) showed significant differences (Table 1). There were significant decreases in oocyst output in all treated groups from day 5 to 10 dpi. The highest oocyst count occurred at 9 dpi and decreased on subsequent days. The fecal oocyst count remained high until the last day of the experiment. Means within the same column with different letters are significantly different (p < 0.01).
Groups
Oocyst output (g) in fecal matter (days post-infection)
Day 5
Day 7
Day 9
Day 10
Infection
6200.00 ± 568.63a
6866.67 ± 233.33a
7966.67 ± 356.30a
4400.00 ± 152.77a
Salvadora persica
4190.67 ± 506.90b
3172.67 ± 363.23b
3100.00 ± 104.10c
2333.33 ± 220.47b
Zingiber officinale
3110.67 ± 220.47b
3566.67 ± 630.03b
3700.00 ± 202.07b
1866.66 ± 101.70c
Curcuma longa
4166.67 ± 220.47b
6566.67 ± 116.67a
2933.33 ± 101.70c
1466.67 ± 185.67c
RBCs, Hb% and PCV showed significant decreases in the infected Z. officinale and C. longa treated groups, but the S. persica treated group revealed non-significant changes in the first week after infection (Table 2). During the second week after infection, there were no significant changes among groups. Means within the same row with different letters are significantly different (p < 0.01).
One week after infection
Two weeks after infection
RBCs
Hb%
PCV%
RBCs
Hb%
PCV%
Control
1.73 ± 0.07a
8.67 ± 0.34a
28.60 ± 1.13a
1.72 ± 0.04a
8.63 ± 0.17a
28.49 ± 0.56a
Infection
1.29 ± 0.15b
6.47 ± 0.74b
21.34 ± 2.43b
1.62 ± 0.06a
8.13 ± 0.31a
26.84 ± 1.03a
Salvadora persica
1.68 ± 0.14ab
8.40 ± 0.69a
27.72 ± 2.29a
1.68 ± 0.12a
8.43 ± 0.63a
27.84 ± 2.05a
Zingiber officinale
1.39 ± 0.04b
6.97 ± 0.22b
22.99 ± 0.70b
1.61 ± 0.06a
8.03 ± 0.28a
26.51 ± 0.94a
Curcuma longa
1.48 ± 0.06b
7.40 ± 0.28b
24.42 ± 0.91b
1.81 ± 0.08a
9.07 ± 0.38a
29.92 ± 1.24a
Body weight showed a significant decrease in the infected groups at 21, 28 and 35 days of age. Treated groups reveled a significant decrease at 21 days only and returned to normal at 28 and 35 days of age (Table 3). Values are means ± standard error. Mean values with different letters in the same column differ significantly (p < 0.05).
Body weight (g) at 21 days
Body weight (g) at 28 days
Body weight (g) at 35 days
Control
640.00 ± 43.76a
1264.00 ± 67.65a
1832.00 ± 174.33a
Infection
457.50 ± 58.66c
1056.00 ± 69.95b
1614.00 ± 82.23b
Salvadora persica
573.33 ± 25.33b
1248.00 ± 40.45a
1828.00 ± 80.09a
Zingiber officinale
560.00 ± 82.12bc
1284.00 ± 22.23a
1716.00 ± 61.64a
Curcuma longa
613.33 ± 40.93b
1290.00 ± 46.55a
1752.00 ± 78.00a
At 7 dpi, three birds from each group were randomly selected, weighed, euthanized by cervical dislocation and necropsied. The intestinal tract was examined for coccidial lesions and the scores were recorded as 0, 1, 2, 3, or 4, from no lesions to most severe. The results showed the highest lesion. The burden in the affected group with a significant decrease in the S. Persica group, the C. longa group was negligible high, and the Z. officinale group was the highest treatment groups (Table 4). Values are means ± standard error. Mean values with different letters in the same column differ significantly (p < 0.05).
Groups
Lesion score at day 7 after infection
Infection
3.50 ± 0.26a
Salvadora persica
2.00 ± 0.50c
Zingiber officinale
3.00 ± 0.50ab
Curcuma longa
2.67 ± 0.29b
Histopathological results one-week post-infection showed mucosal crypts and glands massively invaded by various coccidial developmental stages. Some of the developmental changes, especially schizonts, sporocysts and oocytes, were seen invading the histiocytes of the lamina propria. The tips of the mucosal folds were massively desquamated, denuded and necrotic with the presence of shedding sporocysts and oocysts in affected cells. Massive leukocytic infiltration (mostly heterophils and lymphocytes) was seen in large areas of the lamina propria and submucosa. A large amount of desquamated epithelial cells, leukocytes, necrotic debris and mucin threads were seen impacting the cecal lumen Fig. 1(A&B). Two and three weeks post-infection, moderate to severe cellular involvement of the mucosal crypts and glands by the developmental stages was seen, with massive tissue damage and severe congestion of the mucosal and submucosal blood vessels with the extravasation of erythrocytes and the presence of a bloody exudate in the ceca Fig. 1(C&D).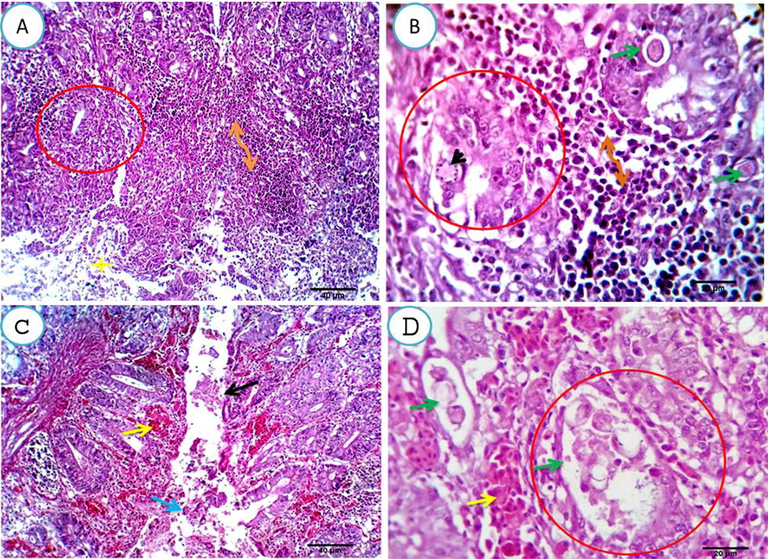
A, B. Cecal mucosa and submucosa of the infected group, one-week post-infection, showing mucosal crypts and glands massively invaded by coccidial developmental stages (merizoites, schizonts, gametes, sporocysts and oocysts) (green arrows and black arrowhead). Some of the developmental changes, especially (schizonts, sporocysts and oocytes) were seen invading the histiocytes of the lamina properia (B, green arrow) The tips of the mucosal folds were massively desquamated, denuded and necrotic with presence of shedding sporocysts and oocysts in the affected cells. Massive leucocytic infiltrations (mostly heterophils and lymphocytes) were seen replacing large areas from the lamina properia and submucosa (A, B double head arrows). Large amount of desquamed epithelial cells, leucocytes, necrotic debris and mucin threads were seen impacting the cecal lumina (A, yellow star). Scale Bars 40, 20 μm. C, D. Two and three weeks post-infection, showing moderate to severe cellular involvement of mucosal crypts and glands by developmental stages (D, red circle and green arrows), massive tissue damage (C, black arrow), beside severe congestion of the mucosal and submucosal blood vessels with extravasation of erythrocytes (yellow arrows) and presence of blood exudate in the cecal lumen (C, blue arrow). Scale bars 40, 20 μm.
The S. persica treated group, one-week post-infection, showed a heavy infection of the crypts and glands by the different developmental stages; almost all the cells were infected. The lamina propria showed dilated capillaries, extravasated erythrocytes and a moderate number of lymphocytes, histiocytes and heterophils Fig. 2(A&B). Two weeks post- infection, the previously parasitized cells appeared apparently normal with a healthy epithelial lining and moderate infiltration of the lamina propria by lymphocytes, macrophages and heterophils with the presence of a remnant of extravasated erythrocytes Fig. 2(C&D). Three weeks post-infection, the necrotic debris had been cleared from the cecal lumen together with a variable number of the glandular cells with regenerative attempts and round cell infiltration of the lamina propria. The submucosa and muscular coat appeared normal Fig. 2(E&F).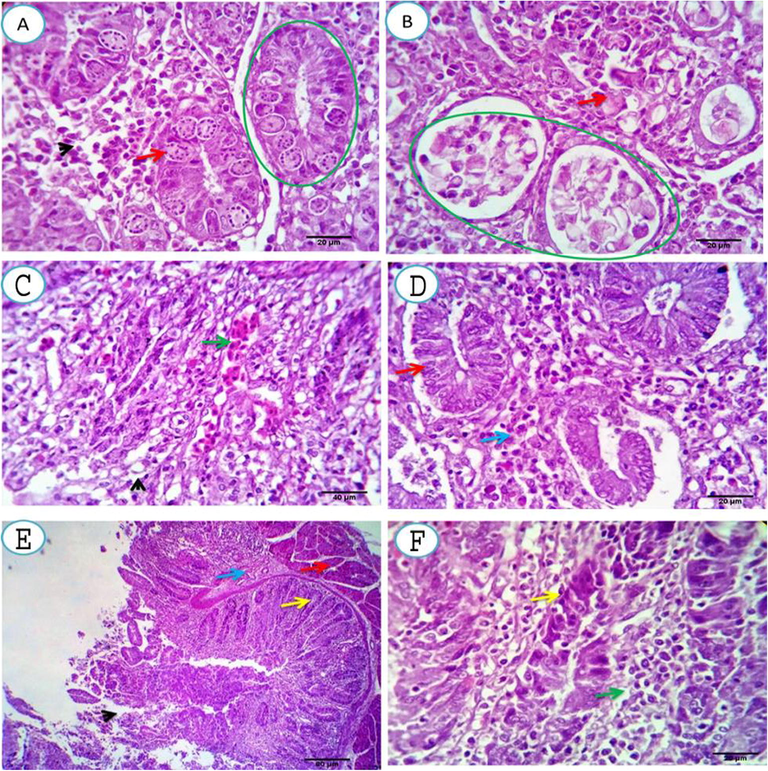
A, B. Cecal mucosal and submucosal tissue of S. persica treated group, one-week post-infection, showing heavy infection of the crypts and glands by the different developmental stages, almost all the cells are infected (green circles and red arrows). The lamina propria shows dilated capillaries, extravasated erythrocytes and moderate number of lymphocytes, histiocytes and heterophils (arrowhead). Scale bars 20, 20 μm. C, D. Two weeks post- infection, showing the previously parasitized cells appears apparently normal with healthy epithelial lining (D, red arrow) and moderate infiltration of the lamina propria by lymphocytes, macrophages and heterophils (D, blue arrow) with presence of a remnant from the extravasated erythrocytes. (C, green arrow). Scale bars 40, 20 μm. E, F. Three weeks post-treatment, showing washing up of the necrotic debris from the cecal lumina together with a variable number of the glandular cells with regenerative attempts (yellow arrows) and round cell infiltration of the lamina propria (F, green arrow). The submucosa and muscular coat appear normal (E, blue and red arrows). Scale bars 80, 20 μm.
After one-week of infection, strong infection of the crypts and glands by different developmental stages was observed among the Z. officinale treated birds; maximum cells were infected Fig. 3(A&B). Two weeks post-infection, a variable number of degenerated schizonts was seen in the glandular epithelium. Meanwhile, other cells appeared free of infection. The mucosal epithelium was apparently normal and the lamina propria and submucosa were heavily infiltrated by round cells Fig. 3(C&D). Three weeks post-infection, an apparently normal mucosal and glandular epithelium was observed. The lamina propria and submucosa were heavily infiltrated by mononuclear cells, predominantly around cecal mucosal glands Fig. 3(E&F).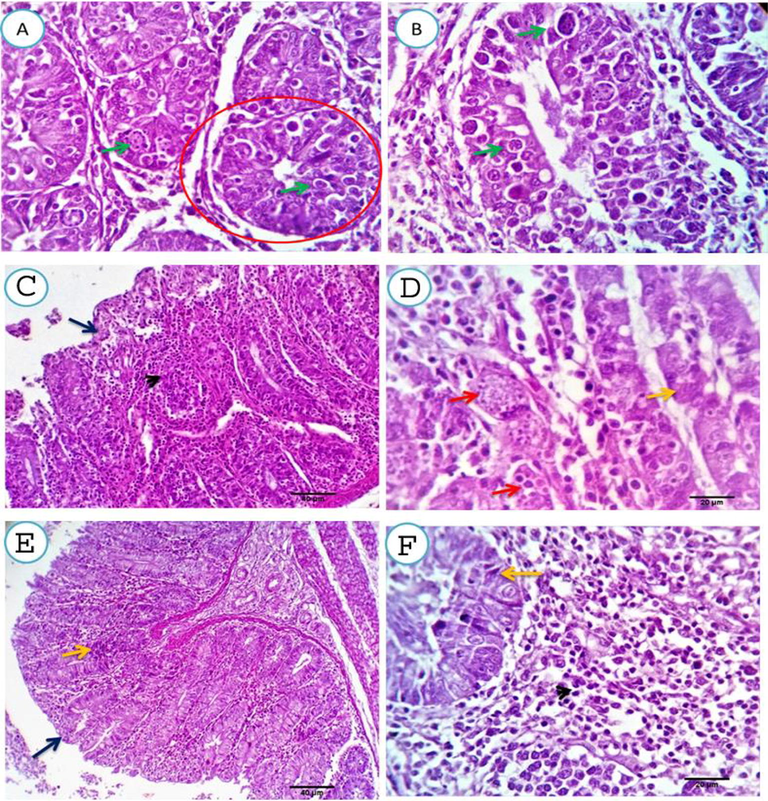
A, B. Cecal mucosal and submucosal tissue of Z. officinale treated. group, one-week post- infection, showing heavy infection of the crypts and glands by the different developmental stages, almost all the cells are infected (red circles and green arrows). Scale bars 20, 20 μm. C, D. two weeks post- infection, showing variable number of degenerated schizonts in some e glandular epithelium (red arrows), meanwhile other cells appears free of infection (yellow arrow). The mucosal epithelium is apparently normal (dark blue arrow) and the lamina propria and submucosa are heavy infiltrated by round cells (arrowhead). Scale bars 40, 20 μm. E, F three weeks post-treatment, showing apparently normal mucosal and glandular epithelium. The lamina propria and submucosa are heavy infiltrated by mononuclear cells which are seen predominantly around the cecal mucosal glands. Scale bars 40, 20 μm.
In the C. longa treated group, one-week post-infection, cecal submucosal crypts and glands were heavy infected, degenerated and necrotized by the developmental stages of test coccidia Fig. 4(A&B). Two weeks post-infection, the infection was nearly totally eliminated, apart from a very small number of degenerated schizonts. The remaining epithelium appeared healthy and focally regenerated Fig. 4(C&D). Three weeks post-infection, the infection had been completely cleared from the submucosal glands with an absolutely healthy epithelium which showed marked regeneration with moderate submucosal round cell infiltration Fig. 4(E&F).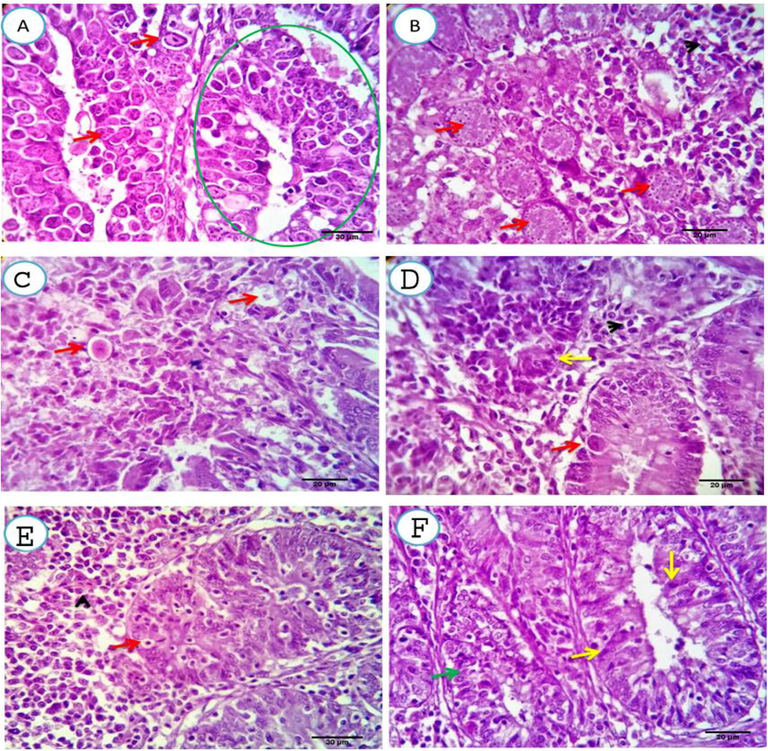
A, B. Cecal mucosal and submucosal tissue of C. longa treated group, one-week post- infection, showing cecal submucosal crypts and glands heavy infected, degenerated and necrotized by the developmental stages of coccidia (green circle and red arrows). Scale bars 20, 20 μm. C, D. Two weeks post- infection, showing nearly totally eliminated infection spars a very few numbers of degenerated schizonts (red arrows). The remaining epithelium appears healthy and focally regenerated (yellow arrows). Scale bars 20, 20 μm. E, F. Three weeks post-treatment, showing completely discarded infection from the submucosal glands with absolutely healthy epithelium (red and yellow arrows). The later shows marked regeneration (green arrow) with moderate submucosal round cell infiltration (arrowhead). Scale bars 30, 20 μm.
4 Discussion
Recently, several studies have used plant extracts as antiparasitic agents (Alzahrani et al., 2016; Metwaly et al., 2013). In the present study, a significant decrease in oocyst output in all treated groups from 5 to 10 days after infection was confirmed. The results of diminished oocyst output suggest that herbal extracts can inhibit or impair the invasion, replication and development of Eimeria parasite species in the gut tissues of chickens. Such a damage and similar restriction of the intracellular progression of parasitic organism is also observed with many anticoccidial drugs. The anticoccidial effect of these tested plant extracts may be attributed to their antioxidant effect. Antioxidant-rich plants can be toxic to parasitic organisms by stimulating oxidative stress and neutralizing reactive oxygen species; this may have beneficial effects in treating coccidial infections. According to Allen et al. (1998), antioxidant compounds are known to reduce the severity of Eimeria tenella infections by ameliorating the degree of intestinal lipid peroxidation. Moreover, the lower oocyst counts recorded in the infected groups given the herbal powders was probably due to the presence of phenolic compounds in the tested herbal extracts. Phenolic compounds can react with cytoplasmic membranes and modify their cation absorption, leading to damage of vital activities in coccidia cells and, ultimately, their death (Sikkema et al., 1995).
The Z. officinale and C. longa treated groups showed a significant reduction of RBCs, Hb% and PCV. This reduction in blood components may be due to severe bleeding and tissue damage in the mucosa following invasion by Eimeria tenella. The highest lesion score was observed in the infected group, with significant decreases in the S. persica and C. longa groups; there was a negligible reduction in the Z. officinale group. Infection with Eimeria mainly damages intestine at the site of infection as of parasites at the primary stages, especially merozoites, breaking out of gut cells and invading other cells of the gut. Infection is linked with oxidative loss in the intestine and severe local and systemic inflammatory responses (Al-Quraishy et al., 2019; Dkhil et al., 2015). This oxidative damage is linked with a decrease in the activity of antioxidant enzymes. Intestinal infection with Eimeria causes possibly oxidative and cytotoxic destruction within infected mucosal tissue. Natural antioxidants obtained from S. persica have medicinal value in infection. Several medicinal plants have significant antioxidant potential (Krishnaiah et al., 2011). Al-Quraishy et al., (2019) & Mohamed and Khan (2013) reported a high therapeutic potential of S. persica reflecting the anti-inflammatory and antioxidant properties of the plant extracts. The active compounds in turmeric and ginger are phenolic compounds, which have been shown to have antioxidative, anti-inflammatory and immunomodulatory properties (Allen et al., 1998). It is also reported that the aqueous extract. S. persica inhibited stress-induced abnormalities in hematological parameters, demonstrating its defensive effect against stress (Ramadan and Alshamrani, 2015).
Body weight underwent a significant decrease in the infected group at 21, 28 and 35 days of age. This weight loss was previously studied by others (Dkhil et al., 2014; Metwaly et al., 2013) who explained this loss due to a reduction of water and food intake (Anwar et al., 2008). Also, Metwaly et al. (2013) related this weight loss to the consumption of carbohydrates by parasitic stages inside the intestinal villi and to structural alterations to the villi. The most important structural change is the disruption of the host epithelium due to the discharge of developed oocysts to the intestinal lumen; this was confirmed histopathologically. The treated groups showed a significant decrease in body weight at 21 days only and returned to normal at 28 and 35 days of age.
The histopathological results of the S. persica treated group, one-week post-infection, showed heavy infection of the crypts and glands by the different developmental stages; almost all the cells are infected. At two weeks post-infection, the previously parasitized cells appeared normal with a healthy epithelial lining, and at three weeks post-infection, the necrotic debris was been cleared from the cecal lumen and a variable number of glandular cells with regenerative attempts were observed. Also, the results of the present study revealed the strong anti-Eimeria activity of S. persica against intestinal E. papillata infection. This effect may be due to the active ingredients present in the extract. Khan et al. (Khan et al., 2010) found that S. persica contains flavonoids, glycosides, alkaloids, saponins, carbohydrates, tannins and steroids. S. persica reportedly has beneficial therapeutic properties.
In the Z. officinale treated group, one-week post-infection, heavy infection of the crypts and glands by the different developmental stages was observed; almost all the cells were infected. Two weeks post-infection, a variable number of degenerated schizonts was seen in the glandular epithelium. This result may be due to the bioactive components of Z. officinale, such as oleoresin and gingerol. Gingerol is a phenol derivative that interacts with parasite through an adsorption involving hydrogen bonding. Low levels of phenol interact with proteins and form a phenol protein complex. Since the linkage between protein and phenol is not very strong and immediately collapses. The free phenol infiltrates into the parasite, causing precipitation and protein denaturation. The high levels of phenol cause the coagulation of proteins such that cell membranes undergo lysis (Nasution et al., 2018).
In the C. longa treated group, one-week post-infection, cecal submucosal crypts and glands were heavy infected, degenerated and necrotized by the developmental stages of test coccidia. Two weeks post-infection, the infection was nearly totally eliminated, apart from a very small number of degenerated schizonts. The remaining epithelium appeared healthy and focally regenerated. Three weeks post-infection, the infection had been completely cleared. Plants of the genus Curcuma, including C. longa (turmeric), have anti-oxidant and anti-inflammatory properties, which could be detrimental for disease outcome, although C. longa is used to eliminate intracellular parasites (Chan et al., 2005; Policegoudra et al., 2007). The active compound of C. longa from turmeric is also phenolic compound, which has been shown to have antioxidative, anti-inflammatory and immunomodulatory activities (Allen et al., 1998).
5 Conclusion
In the present study, the potential of effect of selected medicinal plants [S. persica (Arak), Z. officinale (ginger) and C. longa (turmeric)] ethanolic extract was tested against E. tenella in chicks. The inhibition of oocytes led to preserving the body weight, reducing the cecum lesion score and improving the histopathology of the cecum obtained due to high concentration of bio-active compounds. It was also noted that the S. persica shown anticoccidial effects at low concentration and more effective followed by other Z. officinale (ginger) and C. longa (turmeric).
Treatment with herbal extracts of S. persica, Z. officinale and C. longa were effective in decreasing oocyst production, reducing the cecum lesion score and improving the histopathology of the cecum. However, further studies are needed to identify molecules that are responsible for anticoccidial activities and to evaluate the mechanism of natural products and parasitic interaction against Eimeria parasite which is a common problem in poultry industry.
Acknowledgment
This project was supported by the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University under the research project 2020/01/16593.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anticoccidial screening of Azedarach Indica (Neem) in broilers. Pharmacology Online. 2006;3:365-371.
- [Google Scholar]
- Abdel-Baki, A.A.S., Almalki, E., Mansour, L., Al-Quarishy, S., 2016. In vitro scolicidal effects of salvadora persica root extract against protoscolices of Echinococcus granulosus. Korean J. Parasitol. https://doi.org/10.3347/kjp.2016.54.1.61.
- Almalki, E., Al-Shaebi, E.M., Al-Quarishy, S., El-Matbouli, M., Abdel-Baki, A.A.S., 2017. Ibn vitro effectiveness of Curcuma longa and Zingiber officinale extracts on Echinococcus protoscoleces. Saudi J. Biol. Sci. https://doi.org/10.1016/j.sjbs.2016.05.007.
- Al-Quraishy, S., Thagfan, F.A., Al-Shaebi, E.M., Qasem, M., Abdel-Gaber, R., Dkhil, M.A.M., 2019. Salvadora persica protects mouse intestine from eimeriosis. Rev. Bras. Parasitol. Vet. https://doi.org/10.1590/s1984-29612019068.
- Alzahrani, F., Al-Shaebi, E.M., Dkhil, M.A., Al-Quraishy, S., 2016. In vivo Anti-Eimeria and in vitro anthelmintic activity of Ziziphus Spina-christi leaf extracts Pakistan J. Zool. 48, 409–413.
- Anwar, M.I., Akhtar, M., Hussain, I., Haq, A.U., Muhammad, F., Hafeez, M.A., Mahmood, M.S., Bashir, S., 2008. Field evaluation of Eimeria tenella (local isolates) gametocytes vaccine and its comparative efficacy with imported live vaccine, LivaCox®. Parasitol. Res. https://doi.org/10.1007/s00436-008-1171-5.
- Bos, G., 1993. The miswāk, an aspect of dental care in Islam. Med. Hist. https://doi.org/10.1017/S0025727300057690.
- Chan, M.M.Y., Adapala, N.S., Fong, D., 2005. Curcumin overcomes the inhibitory effect of nitric oxide on Leishmania. Parasitol. Res. https://doi.org/10.1007/s00436-005-1323-9.
- Chand, N., Faheem, H., Khan, R.U., Qureshi, M.S., Alhidary, I.A., Abudabos, A.M., 2016. Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-016-6600-x.
- Chen, T., Zhang, W., Wang, J., Dong, H., Wang, M., 2008. Eimeria tenella: Analysis of differentially expressed genes in the monensin- and maduramicin-resistant lines using cDNA array. Exp. Parasitol. https://doi.org/10.1016/j.exppara.2008.02.010.
- Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis 2005
- [CrossRef] [Google Scholar]
- Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 2008
- [CrossRef] [Google Scholar]
- Dkhil, M.A., Abdel-Baki, A.A.S., Wunderlich, F., Sies, H., Al-Quraishy, S., 2014. Dietary selenium affects intestinal development of Eimeria papillata in mice. Parasitol. Res. https://doi.org/10.1007/s00436-013-3653-3.
- Dkhil, M.A., Metwaly, M.S., Al-Quraishy, S., Sherif, N.E., Delic, D., Al Omar, S.Y., Wunderlich, F., 2015. Anti-Eimeria activity of berberine and identification of associated gene expression changes in the mouse jejunum infected with Eimeria papillata. Parasitol. Res. https://doi.org/10.1007/s00436-015-4344-z.
- The therapeutic potential of plants used in dental folk medicine. Odontostomatol. Trop.. 1982;5:107-117.
- [Google Scholar]
- Gogoi, C., Sarma, J., CC, B., Tamuly, S., TN, U., S, I., Sonowal, J., Borthakur, U., Banerjee, D.K., N, B., 2019. Evaluation of nano-curcumin on experimentally induced coccidiosis in broiler chicks. Int. J. Chem. Stud. 7, 4514–4520.
- Jain, N.C., 2000. Schally’s Veterinary Hematology, 8th ed. Philadelphia, U.S.A.
- Jang, S.I., Jun, M.H., Lillehoj, H.S., Dalloul, R.A., Kong, I.K., Kim, S., Min, W., 2007. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet. Parasitol. https://doi.org/10.1016/j.vetpar.2006.09.005.
- Khan, R.U., Naz, S., Nikousefat, Z., Tufarelli, V., Javdani, M., Qureshi, M.S., Laudadio, V., 2012. Potential applications of ginger (Zingiber officinale) in poultry diets. Worlds. Poult. Sci. J. https://doi.org/10.1017/S004393391200030X.
- Pharmacognostic and preliminary phytochemical investigation of Salvadora persica Linn (Salvadoraceae) Res. J. Pharmacogn Phytochem. 2010
- [Google Scholar]
- Krishnaiah, D., Sarbatly, R., Nithyanandam, R., 2011. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. https://doi.org/10.1016/j.fbp.2010.04.008.
- Antimicrobial effect of extracts from Chinese chive, cinnamon, and corni, fructus. J. Agric. Food Chem. 2001
- [CrossRef] [Google Scholar]
- Mehlhorn Heinz, ed. Encyclopedia of Parasitology (4th ed.). Heidelberg: Springer, Berlin Heidelberg, Berlin; 2014.
- Effects of white wormwood (Artemisia herba-alba Asso), during an experimental coccidiosis in broilers. Ann. Biol. Res.. 2014;5:61-66.
- [Google Scholar]
- Metwaly, M.S., Dkhil, M.A., Gewik, M.M., Al-Ghamdy, A.O., Al-Quraishy, S., 2013. Induced metabolic disturbance and growth depression in rabbits infected with Eimeria coecicola. Parasitol. Res. https://doi.org/10.1007/s00436-013-3485-1.
- Mohamed, S.A., Khan, J.A., 2013. Antioxidant capacity of chewing stick miswak Salvadora persica. BMC Complement. Altern. Med. https://doi.org/10.1186/1472-6882-13-40.
- Nagajyothi, F., Zhao, D., Weiss, L.M., Tanowitz, H.B., 2012. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol. Res. https://doi.org/10.1007/s00436-011-2790-9.
- Nasution, E.Z.J., Tafsin, M., Hanafi, N.D., 2018. The response of red ginger (Zinggiber officinalle var rubra) with various processing in broilers were infected by Eimeria tenella, in: IOP Conference Series: Earth and Environmental Science. https://doi.org/10.1088/1755-1315/122/1/012117.
- Nidaullah, H., Durrani, F.R., Ahmad, S., Jan, I.U., Gul, S., 2010. Aqueous extract from different medicinal plants as anticoccidial, growth promotive and immunostimulant in broilers. J. Agric. Biol. Sci.
- Isolation and characterization of antioxidant and antibacterial compound from mango ginger (Curcuma amada Roxb.) rhizome. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007
- [CrossRef] [Google Scholar]
- Paraclinical effects of Miswak extract on dental plaque. Dent. Res. J. 2007;4(2):106-110.
- [Google Scholar]
- Effects of Salvadora persica extract on the hematological and biochemical alterations against immobilization-induced rats. Scientifica (Cairo) 2015
- [CrossRef] [Google Scholar]
- Raman, M., Banu, S.S., Gomathinayagam, S., Raj, G.D., 2011. Lesion scoring technique for assessing the virulence and pathogenicity of Indian field isolates of avian Eimeria species. Vet. Arh.
- Raza, T., Chand, N., Khan, R.U., Shahid, M.S., Abudabos, A.M., 2016. Improving the fatty acid profile in egg yolk through the use of hempseed (Cannabis sativa), ginger (Zingiber officinale), and turmeric (Curcuma longa) in the diet of Hy-Line White Leghorns. Arch. Anim. Breed. https://doi.org/10.5194/aab-59-183-2016.
- Training needs assessment of poultry farmers in tehsil Faisalabad. J. Anim. Plant Sci. 2011
- [Google Scholar]
- Serum biochemical profile of two broiler strains supplemented with vitamin E, raw ginger (Zingiber officinale) and L-carnitine under high ambient temperatures. S. Afr. J. Anim. Sci. 2019
- [CrossRef] [Google Scholar]
- Sikkema, J., De Bont, J.A.M., Poolman, B., 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. https://doi.org/10.1128/mmbr.59.2.201-222.1995.
- Subramanian, M., Sreejayan, Devasagayam, T.P.A., Singh, B.B., 1994. Diminution of singlet oxygen-induced DNA damage by curcmin and related antioxidants. Mutat. Res. Regul. Pap. https://doi.org/10.1016/0027-5107(94)90183-X
- In vivo Anticoccidial Activity of Salvadora persica Root Extracts. Pak. J. Zool.. 2016;49:53-57.
- [Google Scholar]
- Wunderlich, F., Al-Quraishy, S., Steinbrenner, H., Sies, H., Dkhil, M.A., 2014. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol. Res. https://doi.org/10.1007/s00436-014-4101-8.
- Zhang, G.F., Yang, Z.B., Wang, Y., Yang, W.R., Jiang, S.Z., Gai, G.S., 2009. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. https://doi.org/10.3382/ps.2009-00165.







